-
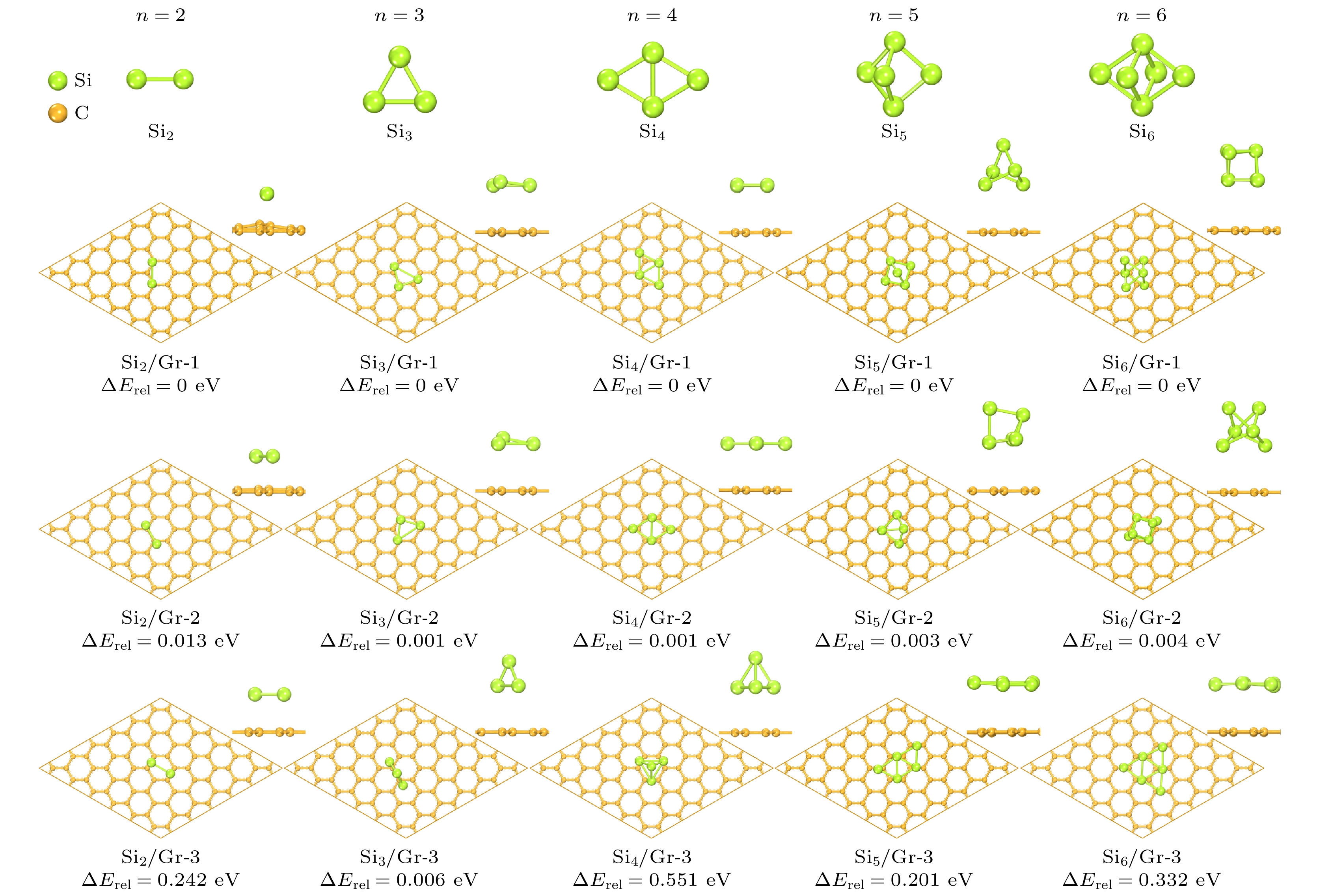
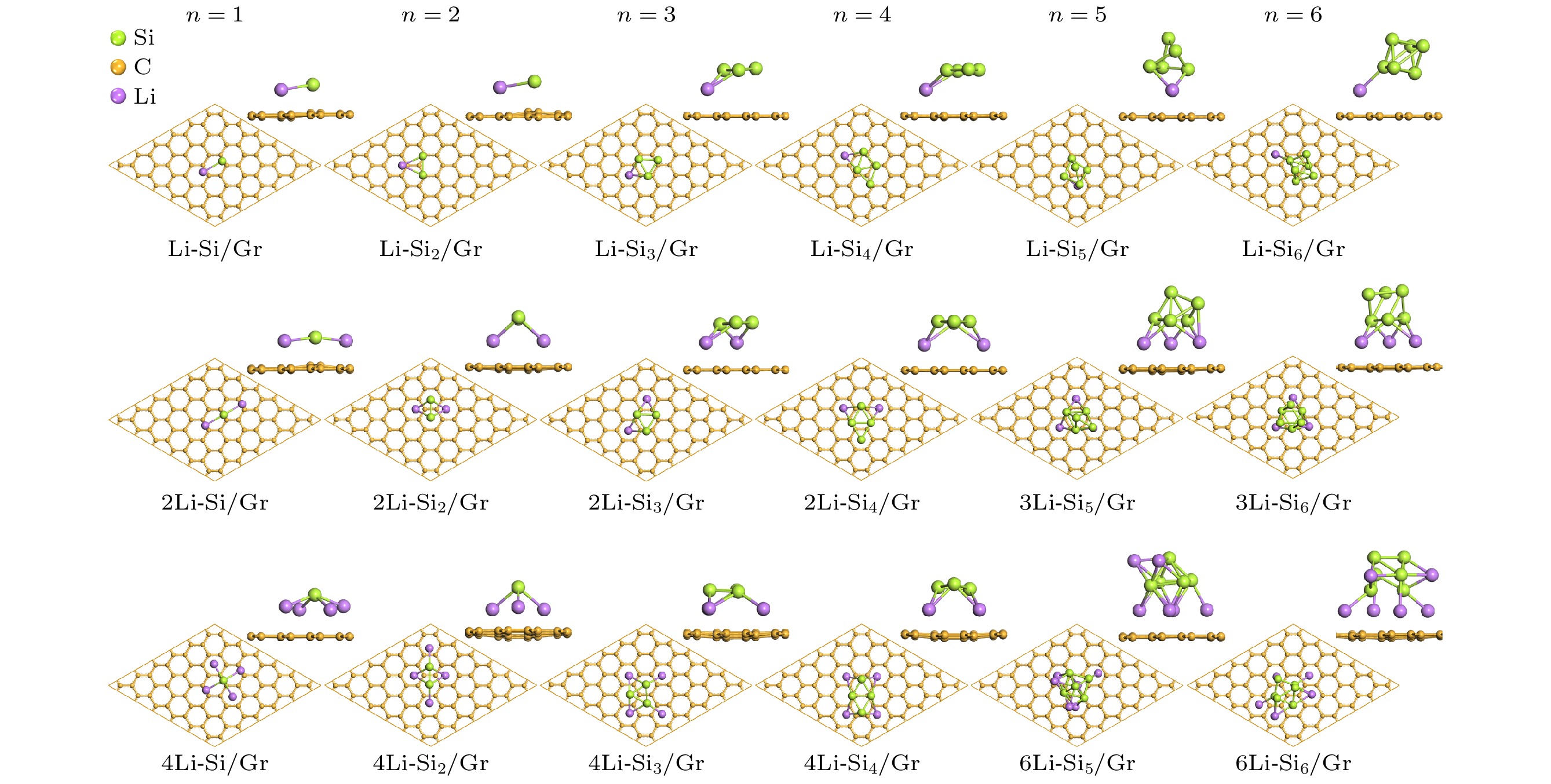
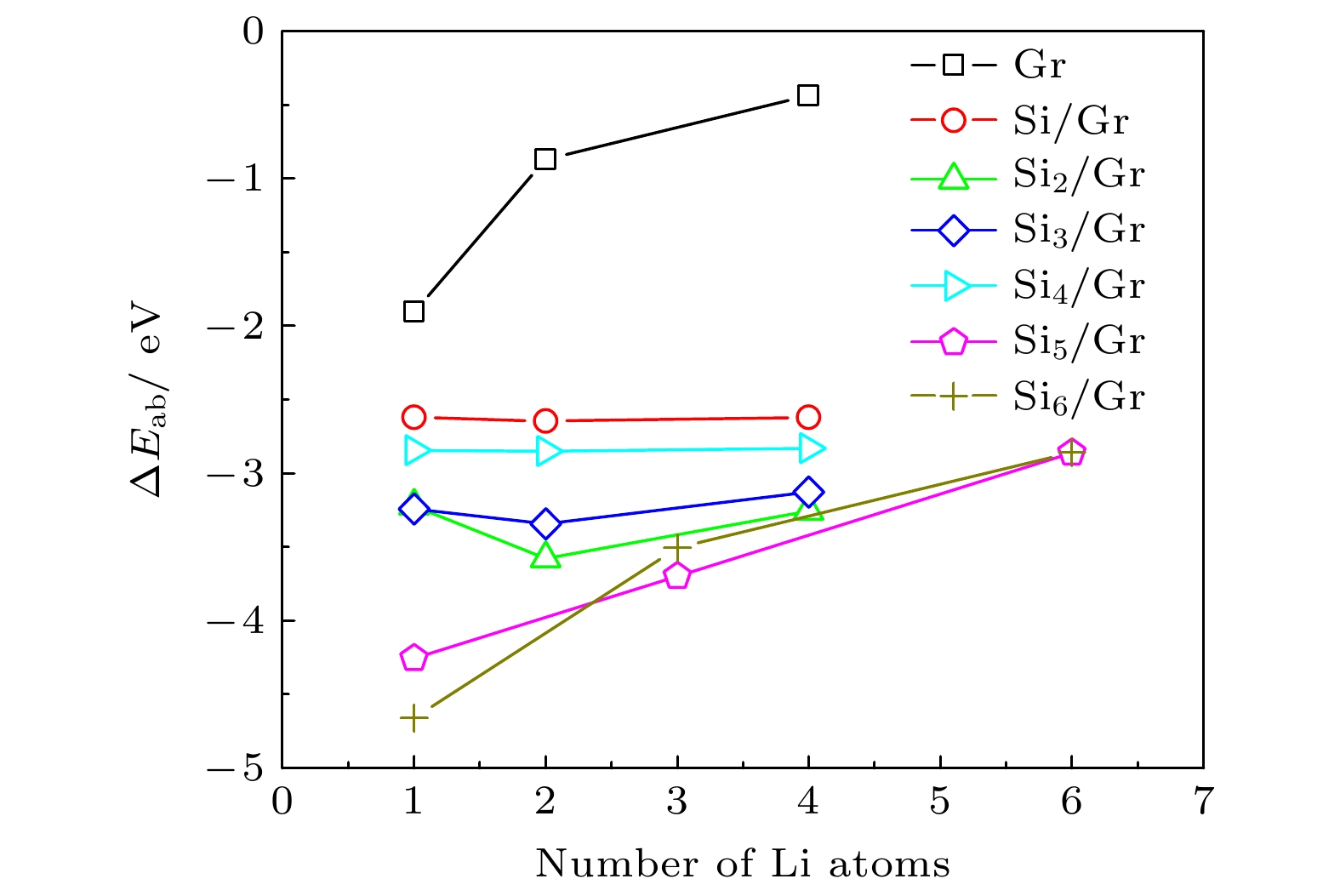
目前, 硅/碳复合材料是锂离子电池最有潜在应用前景的高容量负极材料之一, 硅与碳材料的界面状态是影响其电化学性能的重要因素. 本文在作为碳材料结构单元的石墨烯表面构建了Sin(n ≤ 6)团簇, 采用基于密度泛函理论(DFT)的第一性原理方法研究了Sin团簇/石墨烯(Sin/Gr)的几何构型、结构稳定性和电子性质. 结果表明, 当Si原子数n ≤ 4时, Sin团簇优先以平行于石墨烯的二维构型沉积在石墨烯表面, 当n ≥ 5时, Sin团簇优先以三维立体构型沉积在石墨烯表面. 随着n的增大, Sin团簇在石墨烯表面的热力学稳定性显著降低, 两者之间的界面结合减弱, 并且伴随着Sin团簇与石墨烯之间的电荷转移也越来越少. 同时还研究了Sin/Gr复合构型的储锂能力, Li原子主要存储在Sin团簇临近的石墨烯表面和Sin团簇周围, Sin团簇与石墨烯复合形成的协同作用增强了Li原子吸附的热力学稳定性. 当n ≤ 4时, 存储2个Li原子有利于提高xLi-Sin/Gr体系的热力学稳定性, 继续增加Li原子数x会导致稳定性降低; 当n ≥ 5时, 稳定性随着Li原子数x的增多而逐渐降低.Silicon/carbon composite is one of the most potential high-capacity anode materials for lithium-ion batteries. The interface state between silicon and carbon of silicon/carbon composite is an important factor affecting its electrochemical performance. In this paper, Sin (n ≤ 6) clusters with different numbers of Si atoms are constructed on graphene as a structural unit of carbon material. The geometric configuration, structure stability and electronic property of Sin clusters adsorbed on graphene (Sin/Gr) are studied by the first-principles method based on density functional theory (DFT). The results show that when the number of Si atoms n ≤ 4, the Sin clusters are preferentially adsorbed on graphene in a two-dimensional configuration parallel to graphene. When n ≥ 5, the Sin clusters are preferentially adsorbed on graphene in a three-dimensional configuration. With the increase of the number of Si atoms n, the thermodynamic stability of Sin clusters on graphene decreases significantly, the interface binding strength between Sin clusters and graphene decreases, and the charge transfer between Sin clusters and graphene becomes less. At the same time, the storage capacity of Li atoms in Sin/Gr complex is also studied. Li atoms are mainly stored on the graphene surface near Sin clusters and around Sin clusters. The complex synergistic effect of Sin clusters and graphene enhances the thermodynamic stability of Li adsorption. When n ≤ 4, storing two Li atoms is beneficial to improving the thermodynamic stability of xLi-Sin/Gr system, and the thermodynamic stability decreases with the increase of Li atom number. When n ≥ 5, the thermodynamic stability of xLi-Sin/Gr system decreases with the increase of Li atom number. In the xLi-Si5/Gr system, the C-C bond and Si-Si bond are mainly covalent bonds, while the Li-C bond and Li-Si bond are mainly ionic bonds with certain covalent properties.
-
Keywords:
- graphene /
- Sin clusters /
- structural stability /
- lithium storage property
[1] 王晓钰, 张渝, 马磊, 魏良明 2019 化学学报 77 24
 Google Scholar
Google Scholar
Wang X Y, Zhang Y, Ma L, Wei L M 2019 Acta Chim. Sin. 77 24
 Google Scholar
Google Scholar
[2] Feng K, Li M, Liu W, Kashkooli A G, Chen Z 2018 Small 14 1702737
 Google Scholar
Google Scholar
[3] 魏剑, 秦葱敏, 苏欢, 王佳敏, 李雪婷 2020 新型炭材料 35 97
Wei J, Qin C M, Su H, Wang J M, Li X T 2020 New Carbon Materials 35 97
[4] Zhao X Y, Lehto V P 2021 Nanotechnology 32 042002
 Google Scholar
Google Scholar
[5] Gao H, Xiao L, Plümel I, Xu G L, Ren Y, Zuo X, Liu Y, Schulz C, Wiggers H, Amine K 2017 Nano Lett. 17 1512
 Google Scholar
Google Scholar
[6] Liu J, Kopold P, Aken P A, Maier J, Yu Y 2015 Angew. Chem. Int. Ed. 54 9632
 Google Scholar
Google Scholar
[7] Li X, Gu M, Hu S, Kennard R, Yan P, Chen X, Wang C, Sailor M J, Zhang J G, Liu J 2014 Nat. Commun. 5 4105
 Google Scholar
Google Scholar
[8] Song H C, Wang H X, Lin Z X, et al. 2016 Adv. Funct. Mater. 26 524
 Google Scholar
Google Scholar
[9] Su J M, Zhang C C, Chen X Liu S Y, Huang T, Yu A S 2018 J. Power Sources 381 66
 Google Scholar
Google Scholar
[10] Zuo X X, Wang X Y, Xia Y G, Yin S S Ji Q, Yang Z H, Wang M M, Zheng X F, Qiu B, Liu Z P, Zhu J, Müller P, Cheng Y J 2019 J. Power Sources 412 93
 Google Scholar
Google Scholar
[11] Shi J, Jiang X S, Sun J F, Ban B Y, Li J W, Chen J 2021 J. Colloid Interface Sci. 588 737
 Google Scholar
Google Scholar
[12] Ko M, Chae S, Ma J, Kim N, Lee H W, Cui Y Cho J 2016 Nat. Energy 1 16113
 Google Scholar
Google Scholar
[13] 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安 2021 无机材料学报 3 454
Li K R, Hu X H, Zhang Z F, Guo Y Z, Huang R A 2021 Journal of Inorganic Materials 3 454
[14] 刘振源, 刘烈凯, 金鑫, 汤昊, 孙润光 2019 复合材料学报 36 1568
Liu Z Y, Liu L K, Jin X, Tang H, Sun R G 2019 Acta Mater. Compos. Sin. 36 1568
[15] Luo W, Wang Y X, Chou S L, Xu Y F, Li W, Kong B, Dou S X, Liu H K, Yang J P 2016 Nano Energy 27 255
 Google Scholar
Google Scholar
[16] Cai W, Liu X, Zhu Y, Lan Y, Ma K, Qian Y 2016 Dalton Trans. 45 13667
 Google Scholar
Google Scholar
[17] 林伟国, 孙伟航, 曲宗凯, 冯晓磊, 荣峻峰, 陈旭, 杨文胜 2019 高等学校化学学报 40 1216
 Google Scholar
Google Scholar
Lin W G, Sun W H, Qu Z K, Feng X L, Rong J F, Chen X, Yang W S 2019 Chem. J. Chin. Univ. 40 1216
 Google Scholar
Google Scholar
[18] Zhu X, Choi S H, Tao R, Jia X L, Lu Y F 2019 J. Alloys Compd. 791 1105
 Google Scholar
Google Scholar
[19] Yu Y, Li G, Zhou S, Chen X, Yang W 2017 Carbon 120 397
 Google Scholar
Google Scholar
[20] Deiss E, Wokaun A, Barras J L, Daul C, Dufek P 1997 J. Electrochem. Soc. 144 3877
 Google Scholar
Google Scholar
[21] Ullah A, Majid A, Rani N 2018 J. Energy Chem. 27 219
[22] 闫小童, 侯育花, 郑寿红, 黄有林, 陶小马 2019 68 187101
 Google Scholar
Google Scholar
Yan X Tong, Hou Y H, Zheng S H, Huang Y L, Tao X M 2019 Acta Phys. Sin. 68 187101
 Google Scholar
Google Scholar
[23] 张伟, 齐小鹏, 方升, 张健华, 史碧梦, 杨娟玉 2020 化学进展 32 454
Zhang W, Qing X P, Fang S, Zhang J H, Shi B M, Yang J Y 2020 Prog. Chem. 32 454
[24] Li G C, Yang Z W, Yin Z L, Guo H J, Wang Z X, Yan G C, Liu Y, Li L J, Wang J X 2019 J. Mater. Chem. A 26 15541
[25] Clark S J, Segall M D, Pickard C J, Hasnip P J, Probert M J, Refson K, Payne M C 2005 Z. Kristallogr. 220 567
[26] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[27] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[28] Vanderbilt D 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[29] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[30] Gao H, Jian Z, Lu M, Wei F, Chen Y 2010 J. Appl. Phys. 107 666
[31] Aktuerk E, Ataca C, Ciraci S 2010 Appl. Phys. Lett. 96 123112
 Google Scholar
Google Scholar
[32] Stockmeier M, Müller R, Sakwe S A, Wellmann P J, Magerl A 2009 J. Appl. Phys. 105 033511
 Google Scholar
Google Scholar
[33] Tsirelson V, Stash A 2002 Chem. Phys. Lett. 351 142
 Google Scholar
Google Scholar
[34] Zhang S, Wang Q, Kawazoe Y, Jena P 2013 J. Am. Chem. Soc. 135 18216
 Google Scholar
Google Scholar
-
图 3 孤立Sin团簇和Sin/Gr复合材料的态密度图 (a) 孤立Sin团簇的分波态密度; (b), (c) Sin/Gr复合材料的分波态密度; (d) Sin/Gr复合材料的总态密度
Fig. 3. Density of states for isolated Sin clusters and Sin/Gr composites: (a) Partial density of states for isolated Sin clusters; (b), (c) partial density of states for Sin/Gr composites; (d) total density of states for Sin/Gr composites.
表 1 石墨烯表面Sin团簇的平均吸附能、结构参数和Mulliken布局
Table 1. Average adsorption energy, structural parameters and Mulliken population for Sin clusters on graphene.
n ΔEab/eV h/Å Δh/Å dSi—C/Å dSi—Si/Å Mulliken
population/e1 –1.216 2.168 0.078 2.093 — 0.50 2 –0.312 2.532 0.167 2.223 2.262 0.30 3 –0.116 3.365 0.007 3.456 2.187 –0.02 4 –0.152 3.235 0.010 3.246 2.335 –0.02 5 –0.088 3.314 0.005 3.403 2.316 0.02 6 –0.083 3.425 0.008 3.512 2.371 0.04 -
[1] 王晓钰, 张渝, 马磊, 魏良明 2019 化学学报 77 24
 Google Scholar
Google Scholar
Wang X Y, Zhang Y, Ma L, Wei L M 2019 Acta Chim. Sin. 77 24
 Google Scholar
Google Scholar
[2] Feng K, Li M, Liu W, Kashkooli A G, Chen Z 2018 Small 14 1702737
 Google Scholar
Google Scholar
[3] 魏剑, 秦葱敏, 苏欢, 王佳敏, 李雪婷 2020 新型炭材料 35 97
Wei J, Qin C M, Su H, Wang J M, Li X T 2020 New Carbon Materials 35 97
[4] Zhao X Y, Lehto V P 2021 Nanotechnology 32 042002
 Google Scholar
Google Scholar
[5] Gao H, Xiao L, Plümel I, Xu G L, Ren Y, Zuo X, Liu Y, Schulz C, Wiggers H, Amine K 2017 Nano Lett. 17 1512
 Google Scholar
Google Scholar
[6] Liu J, Kopold P, Aken P A, Maier J, Yu Y 2015 Angew. Chem. Int. Ed. 54 9632
 Google Scholar
Google Scholar
[7] Li X, Gu M, Hu S, Kennard R, Yan P, Chen X, Wang C, Sailor M J, Zhang J G, Liu J 2014 Nat. Commun. 5 4105
 Google Scholar
Google Scholar
[8] Song H C, Wang H X, Lin Z X, et al. 2016 Adv. Funct. Mater. 26 524
 Google Scholar
Google Scholar
[9] Su J M, Zhang C C, Chen X Liu S Y, Huang T, Yu A S 2018 J. Power Sources 381 66
 Google Scholar
Google Scholar
[10] Zuo X X, Wang X Y, Xia Y G, Yin S S Ji Q, Yang Z H, Wang M M, Zheng X F, Qiu B, Liu Z P, Zhu J, Müller P, Cheng Y J 2019 J. Power Sources 412 93
 Google Scholar
Google Scholar
[11] Shi J, Jiang X S, Sun J F, Ban B Y, Li J W, Chen J 2021 J. Colloid Interface Sci. 588 737
 Google Scholar
Google Scholar
[12] Ko M, Chae S, Ma J, Kim N, Lee H W, Cui Y Cho J 2016 Nat. Energy 1 16113
 Google Scholar
Google Scholar
[13] 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安 2021 无机材料学报 3 454
Li K R, Hu X H, Zhang Z F, Guo Y Z, Huang R A 2021 Journal of Inorganic Materials 3 454
[14] 刘振源, 刘烈凯, 金鑫, 汤昊, 孙润光 2019 复合材料学报 36 1568
Liu Z Y, Liu L K, Jin X, Tang H, Sun R G 2019 Acta Mater. Compos. Sin. 36 1568
[15] Luo W, Wang Y X, Chou S L, Xu Y F, Li W, Kong B, Dou S X, Liu H K, Yang J P 2016 Nano Energy 27 255
 Google Scholar
Google Scholar
[16] Cai W, Liu X, Zhu Y, Lan Y, Ma K, Qian Y 2016 Dalton Trans. 45 13667
 Google Scholar
Google Scholar
[17] 林伟国, 孙伟航, 曲宗凯, 冯晓磊, 荣峻峰, 陈旭, 杨文胜 2019 高等学校化学学报 40 1216
 Google Scholar
Google Scholar
Lin W G, Sun W H, Qu Z K, Feng X L, Rong J F, Chen X, Yang W S 2019 Chem. J. Chin. Univ. 40 1216
 Google Scholar
Google Scholar
[18] Zhu X, Choi S H, Tao R, Jia X L, Lu Y F 2019 J. Alloys Compd. 791 1105
 Google Scholar
Google Scholar
[19] Yu Y, Li G, Zhou S, Chen X, Yang W 2017 Carbon 120 397
 Google Scholar
Google Scholar
[20] Deiss E, Wokaun A, Barras J L, Daul C, Dufek P 1997 J. Electrochem. Soc. 144 3877
 Google Scholar
Google Scholar
[21] Ullah A, Majid A, Rani N 2018 J. Energy Chem. 27 219
[22] 闫小童, 侯育花, 郑寿红, 黄有林, 陶小马 2019 68 187101
 Google Scholar
Google Scholar
Yan X Tong, Hou Y H, Zheng S H, Huang Y L, Tao X M 2019 Acta Phys. Sin. 68 187101
 Google Scholar
Google Scholar
[23] 张伟, 齐小鹏, 方升, 张健华, 史碧梦, 杨娟玉 2020 化学进展 32 454
Zhang W, Qing X P, Fang S, Zhang J H, Shi B M, Yang J Y 2020 Prog. Chem. 32 454
[24] Li G C, Yang Z W, Yin Z L, Guo H J, Wang Z X, Yan G C, Liu Y, Li L J, Wang J X 2019 J. Mater. Chem. A 26 15541
[25] Clark S J, Segall M D, Pickard C J, Hasnip P J, Probert M J, Refson K, Payne M C 2005 Z. Kristallogr. 220 567
[26] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[27] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[28] Vanderbilt D 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[29] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[30] Gao H, Jian Z, Lu M, Wei F, Chen Y 2010 J. Appl. Phys. 107 666
[31] Aktuerk E, Ataca C, Ciraci S 2010 Appl. Phys. Lett. 96 123112
 Google Scholar
Google Scholar
[32] Stockmeier M, Müller R, Sakwe S A, Wellmann P J, Magerl A 2009 J. Appl. Phys. 105 033511
 Google Scholar
Google Scholar
[33] Tsirelson V, Stash A 2002 Chem. Phys. Lett. 351 142
 Google Scholar
Google Scholar
[34] Zhang S, Wang Q, Kawazoe Y, Jena P 2013 J. Am. Chem. Soc. 135 18216
 Google Scholar
Google Scholar
计量
- 文章访问数: 8184
- PDF下载量: 117
- 被引次数: 0














 下载:
下载: