-
利用基于密度泛函理论的第一性原理计算方法, 研究了Se边用H饱和、In边用各种非金属元素X(X = H, B, N, P, F和Cl)端接的锯齿型InSe纳米带(H-ZN(7)-X)的几何结构、磁电子特性及应变效应. 计算的形成能和Forcite退火模拟表明H-ZN(7)-X具有稳定的几何结构. F和Cl端接时, 纳米带具有和H端接时类似的磁金属性质. N端接时, 纳米带磁性最强. 但B和P端接使得纳米带边缘的磁性完全消失. 特别是, 我们发现外加的机械应变可以增强H-ZN(7)-N磁稳定性, 并且有效地调节费米能级处的自旋极化率(SP), 能在0—92%之间变化, 这意味着可设计机械开关来控制低偏压下的自旋输运. 应变调制机制与应变诱导的键长变化导致不成对的电子的重新分布或消失有关. N-ZN(7)-N的磁性主要来源于In, Se及N原子的p轨道, 这对于研发非过渡金属磁性材料有重要意义.Employing the first-principles calculation based on the density functional theory, the geometries, magneto-electronicproperties, and strain effects of the zigzag-edged InSe nanoribbons with the Se-edge saturated by H atoms and In-edge terminated by various non-metallic elements X (X = H, B, N, P, F and Cl) are studied. The calculated formation energy and Forcite annealing simulations show that the H-ZN(7)-X has a stable geometry. For F- and Cl- terminated ribbons, they have a magnetic metallic property similar to that in the case of H termination, and for the N termination the nanoribbon has the strongest magnetic property. However, the B and P terminations cause the magnetic properties at the ribbon edge to completely disappear, particularly when the mechanical strain is applied. The magnetic stability of H-ZN(7)-N is enhanced, and the spin polarization efficiency (SP) at the Fermi level can be effectively modulated in a range from zero to 92%, which means that it is possible to design a mechanical switch for controlling the spin transport at low bias. The strain modulating mechanism is related to the fact that the variation of strain-induced bond length leads the unpaired electrons to be redistributed or disappear. The magnetic properties of N-ZN(7)-N are mainly derived from the p orbitals of In, Se and N atoms, thus it is very important to develop non-transition metal magnetic materials.
-
Keywords:
- InSe nanoribbon /
- non-metallic atom /
- magneto-electronic property /
- strain effect
[1] Weiss N O, Zhou H L, Liao L, Liu Y, Jiang S, Huang Y, Duan X F 2012 Adv. Mater. 24 5782
 Google Scholar
Google Scholar
[2] Lee C, Wei X D, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[3] Yu W, Niu C, Zhu Z, Wang X, Zhang W B 2016 J. Mater. Chem. C 4 6581
 Google Scholar
Google Scholar
[4] Kuang W, Hu R, Fan Z, Zhang Z 2019 Nanotechnology 30 145201
 Google Scholar
Google Scholar
[5] Yu W W, Chen X A, Mei W, Chen C S, Tsang Y H 2017 Appl. Surf. Sci. 400 129
 Google Scholar
Google Scholar
[6] Hu R, Li Y H, Zhang Z H, Fan Z Q, Sun L 2019 J. Mater. Chem. C 7 7745
[7] Sheng Y, You Y, Cao Z, Liu L, Wu H 2018 Analyst 143 2411
 Google Scholar
Google Scholar
[8] Chen C S, Yu W W, Liu T G, Cao S Y, Tsang Y H 2017 Sol. Energy Mater. Sol. Cells 160 43
 Google Scholar
Google Scholar
[9] Magda G Z, Jin X, Hagy I, Vancsó P, Osváth Z, Nemes P, Hwang C P, Biró L, Tapasztó L 2014 Nature 514 608
 Google Scholar
Google Scholar
[10] Ding Y, Ni J 2009 Appl. Phys. Lett. 95 083115
 Google Scholar
Google Scholar
[11] Pan H, Zhang Y W 2012 J. Mater. Chem. 22 7280
 Google Scholar
Google Scholar
[12] Yuan P F, Hu R, Fan Z Q, Zhang Z H 2018 J. Phys. Condens. Matter 30 445802
 Google Scholar
Google Scholar
[13] Liu W, Deng X, Cai S 2016 AIP Adv. 6 075103
 Google Scholar
Google Scholar
[14] Chen Q, Tang L, Chen K, Zhao H 2013 J. Appl. Phys. 114 084301
 Google Scholar
Google Scholar
[15] Zhu Z, Zhang Z H, Wang D, Deng X Q, Fan Z Q, Tang G P 2015 J. Mater. Chem. 3 9657
[16] Zhang H, Meng S, Yang H 2015 J. Appl. Phys. 117 112108
[17] Wu M, Wu X, Zeng X C 2010 J. Phys. Chem. C 114 3937
 Google Scholar
Google Scholar
[18] Zhu Z L, Li C, Yu W, Chang D, Sun Q, Jia Y 2014 Appl. Phys. Lett. 105 113105
 Google Scholar
Google Scholar
[19] Lei S, Ge L, Najmaei S 2014 ACS Nano 8 1263
 Google Scholar
Google Scholar
[20] Ho C H 2016 2D Mater. 3 025019
 Google Scholar
Google Scholar
[21] Mudd G W, Molas M R, Chen X, Zólyomi V 2016 Sci. Rep. 6 39619
 Google Scholar
Google Scholar
[22] Bandurin D A, Tyurnina A V, Yu G L 2016 Nat. Nanotechnol. 12 223
[23] Wu M, Shi J, Zhang M, Ding Y, Wang H, Cen Y, Guo W, Pan S, Zhu Y 2018 Nanotechnology 29 205708
 Google Scholar
Google Scholar
[24] Yao A L, Wang X F, Liu Y S, Sun Y N 2018 Nanoscale Res. Lett. 13 107
 Google Scholar
Google Scholar
[25] Zhou W, Yu G, Rudenko A N, Yuan S 2018 Phys. Rev. Mater. 2 114001
 Google Scholar
Google Scholar
[26] Brandbyge M, Mozos J L, Ordejón P, Taylor J, Stokbro K. 2002 Phys. Rev. B 65 165401
 Google Scholar
Google Scholar
[27] Taylor J, Guo H, Wang J 2001 Phys. Rev. B 63 245407
 Google Scholar
Google Scholar
[28] Chen S, Zhou W, Yu J, Chen K Q 2018 Carbon 129 809
 Google Scholar
Google Scholar
[29] Deng Y, Chen S, Zeng Y, Feng Y, Zhou W, Tang L, Chen K 2018 Org. Electron. 63 310
 Google Scholar
Google Scholar
[30] Yuan J, Zhang L W, Liew K M 2016 Curr. Nanosci. 12 636
 Google Scholar
Google Scholar
[31] 张华林, 孙琳, 韩佳凝 2017 66 246101
 Google Scholar
Google Scholar
Zhang H L, Sun L, Han J N 2017 Acta Phys. Sin. 66 246101
 Google Scholar
Google Scholar
[32] 左博敏, 健美, 冯志, 毛宇亮 2019 68 113103
 Google Scholar
Google Scholar
Zuo B M, Yuan J M, Feng Z, Mao Y L 2019 Acta Phys. Sin. 68 113103
 Google Scholar
Google Scholar
[33] Kang H S, Jeong S 2004 Phys. Rev. B 70 233411
 Google Scholar
Google Scholar
-
图 1 (a) InSe单层的顶视图(上图)和侧视图(下图).沿着X方向裁剪InSe单层可以得到锯齿型InSe纳米带, 图中淡绿色填充区域表示; (b) H-ZN(7)-X的顶视图(左图)和侧视图(右图). 红色的虚线框表示计算的单胞
Fig. 1. (a) Top and side views of monolayer InSe. Tailoring monolayer InSe along X-axis direction to achieve zigzag InSe nanoribbons, denoted by a pale green filled area; (b) top and side views of H-ZN(7)-X. The red dotted box represents a unit cell.
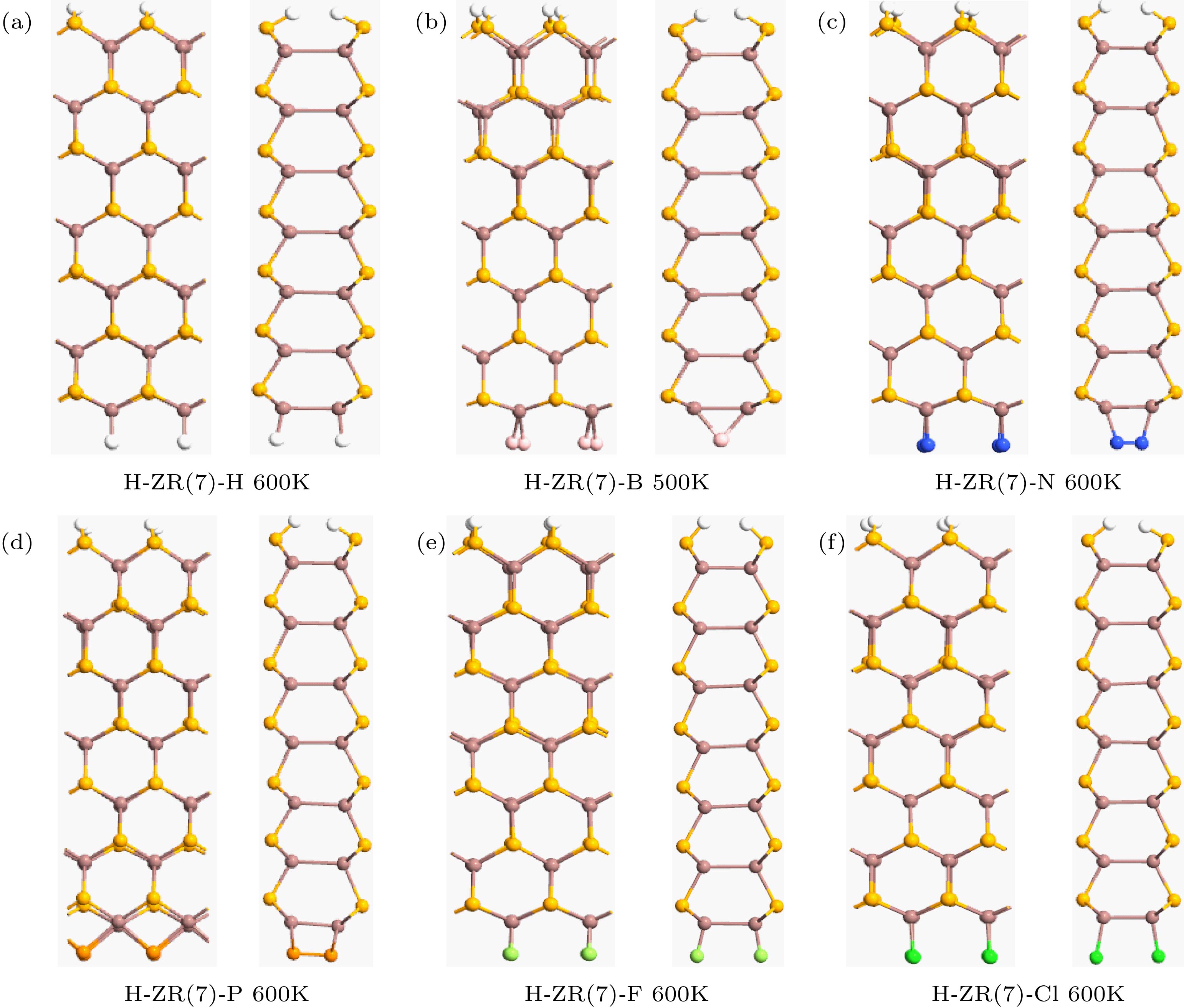
图 2 使用BOMD模拟检测H-ZN(7)-X的热稳定性. 在8 ps模拟后, 对于H-ZN(7)-H, H-ZN(7)-N, H-ZN(7)-F, H-ZN(7)-P和H-ZN(7)-Cl, 在600 K时出现小变形, 对于H-ZN(7)-B在500 K处出现小变形, 但是没有观察到边缘重构
Fig. 2. BOMD simulations for examining thermal stability of the H-ZN(7)-X. The small deformations occur at 500 K for H-ZN(7)-B and 600 K for other ribbons after 8 ps of simulation, but no edge reconstruction is observed.
图 3 (a)−(f)分别为H-ZN(7)-H, H-ZN(7)-B, H-ZN(7)-N, H-ZN(7)-P, H-ZN(7)-F, 和H-ZN(7)-Cl在NM (无磁)态下的的能带结构(BS)、态密度(DOS)和最外边缘Se (In)原子的投影态密度(PDOS); (g)费米能级附近能带a1 (a2)相对应的部分电荷密度分布. 等值面设置为0.05|e|Å–3.
Fig. 3. (a)−(f) Correspond to the band structure (BS), density of the state (DOS), and projected density of the state (PDOS) of H-ZN(7)-H, H-ZN(7)-B, H-ZN(7)-N, H-ZN(7)-P, H-ZN(7)-F, and H-ZN(7)-Cl, respectively; (g) the partial charge density distribution corresponds to subbands a1 (a2) labeled in figures (a)−(f), respectively. The isosurface value is set as 0.05|e|Å–3.
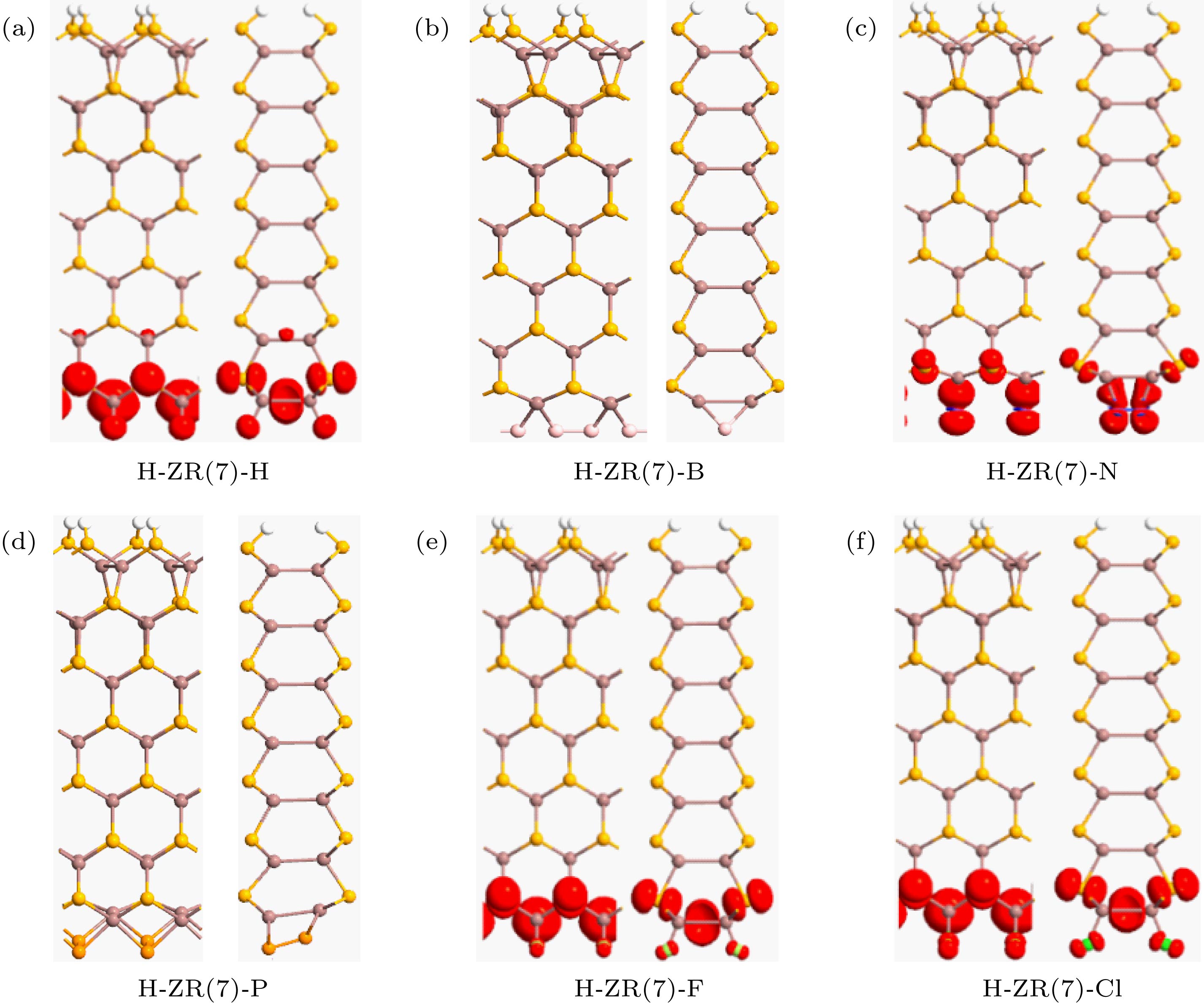
图 4 自旋极化电荷密度等值面图, 等值面取为 ± 0.005|e|/Å3 (a) H-ZN(7)-H; (b) H-ZN(7)-B; (c) H-ZN(7)-N; (d) H-ZN(7)-P; (e) H-ZN(7)-F; (f) H-ZN(7)-Cl
Fig. 4. The isosurface plots for the spin polarized density. The isosurface value is 0.005|e|/Å3: (a) H-ZN(7)-H; (b) H-ZN(7)-B; (c) H-ZN(7)-N; (d) H-ZN(7)-P; (e) H-ZN(7)-F; (f) H-ZN(7)-Cl
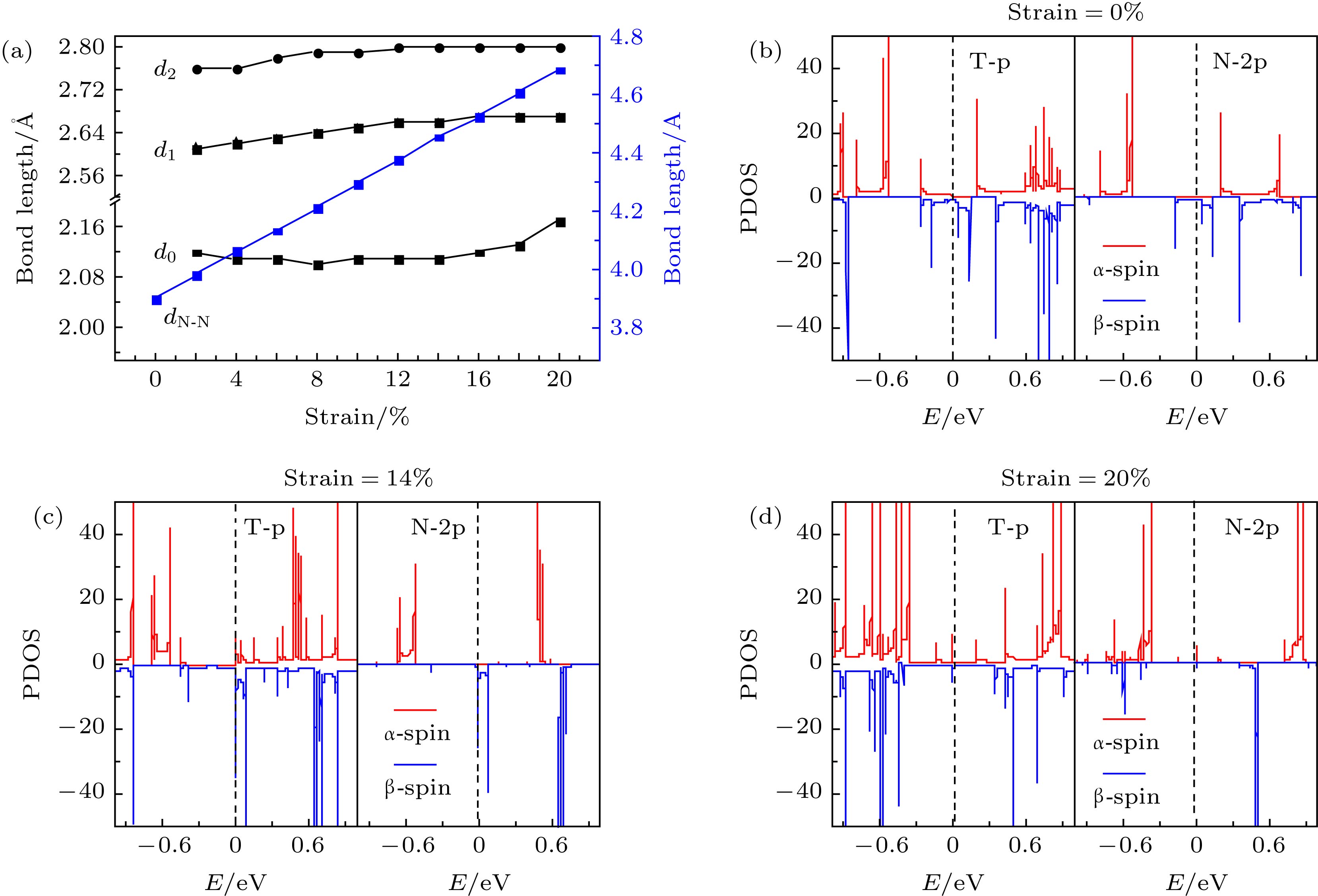
图 6 (a)拉伸总能和费米能级处自旋极化率随拉伸形变的变化; (b)磁矩及磁化能随拉伸形变的变化; (c), (d)几个典型形变0%, 14%, 20%下的自旋极化电荷密度和能带变化. 等值面被设为 ± 0.005|e|/Å3
Fig. 6. (a) The evolution of spin polarization efficiency (SP) at the Fermi level and the strain energy versus strain; (b) the magnetic moment (M) and magnetized energy(EM) in one unit cell versus strain; (c) the spin polarized density and (d) the band structure at several typical strains. The isosurface value is set as 0.005|e|Å–3.
表 2 H-ZN(7)-X在铁磁态(FM)的结构参数. M, µ(InL), µ(SeL), µ(X)分别为总磁矩和下边缘In, Se和X的磁矩(单位: μB/单胞). EM和SP分别是磁化能(单位: meV/单胞)与费米能级处的自旋极化率
Table 2. The structural parameters of H-ZN(7)-X in the FM state. M represents the net magnetic moment of unit cell, µ(InL), µ(SeL) and µ(X) represent the net magnetic moment of lower (L) edge In, Se and X atoms, respectively(unit: μB/unit cell). EM represent the magnetized energy (unit: meV/unit cell) and SP is the spin polarization efficiency at the Fermi level.
Structure EM µ(InL) µ(SeL) µ(X) M SP H-ZN(7)-H 7.95 0.15 0.21 0.07 0.47 43.0% B-ZN(7)-B 0 0 0 0 0 0% H-ZN(7)-N 78.32 0.02 0.10 0.52 0.632 55.6% H-ZN(7)-P 0 0 0 0 0 0% H-ZN(7)-F 8.82 0.14 0.25 0.03 0.44 38.8% H-ZN(7)-Cl 8.70 0.15 0.25 0.05 0.47 39.5% 表 1 H-ZN(7)-X的形成能(EFE) (单位: eV/原子)和键长或两相关原子间的空间位置(单位: Å)
Table 1. The formation energy (EFE) (unit: eV/atom) of H-ZN (7)-X and the bond length or space position between the two related atoms (unit: Å).
Structure EEF(ribbon) dX-X d0 d1 d2 d3 H-ZN(7)-H –3.12 3.93 1.72 2.83 2.60 2.57 H-ZN(7)-B –4.25 1.85 2.37 3.02 2.57 2.62 H-ZN(7)-N –5.47 3.90 2.12 2.75 2.60 2.66 H-ZN(7)-P –4.69 3.83 2.66 2.86 2.66 2.59 H-ZN(7)-F –4.86 3.93 1.72 2.82 2.60 2.57 H-ZN(7)-Cl –3.82 3.94 2.16 2.82 2.61 2.57 -
[1] Weiss N O, Zhou H L, Liao L, Liu Y, Jiang S, Huang Y, Duan X F 2012 Adv. Mater. 24 5782
 Google Scholar
Google Scholar
[2] Lee C, Wei X D, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[3] Yu W, Niu C, Zhu Z, Wang X, Zhang W B 2016 J. Mater. Chem. C 4 6581
 Google Scholar
Google Scholar
[4] Kuang W, Hu R, Fan Z, Zhang Z 2019 Nanotechnology 30 145201
 Google Scholar
Google Scholar
[5] Yu W W, Chen X A, Mei W, Chen C S, Tsang Y H 2017 Appl. Surf. Sci. 400 129
 Google Scholar
Google Scholar
[6] Hu R, Li Y H, Zhang Z H, Fan Z Q, Sun L 2019 J. Mater. Chem. C 7 7745
[7] Sheng Y, You Y, Cao Z, Liu L, Wu H 2018 Analyst 143 2411
 Google Scholar
Google Scholar
[8] Chen C S, Yu W W, Liu T G, Cao S Y, Tsang Y H 2017 Sol. Energy Mater. Sol. Cells 160 43
 Google Scholar
Google Scholar
[9] Magda G Z, Jin X, Hagy I, Vancsó P, Osváth Z, Nemes P, Hwang C P, Biró L, Tapasztó L 2014 Nature 514 608
 Google Scholar
Google Scholar
[10] Ding Y, Ni J 2009 Appl. Phys. Lett. 95 083115
 Google Scholar
Google Scholar
[11] Pan H, Zhang Y W 2012 J. Mater. Chem. 22 7280
 Google Scholar
Google Scholar
[12] Yuan P F, Hu R, Fan Z Q, Zhang Z H 2018 J. Phys. Condens. Matter 30 445802
 Google Scholar
Google Scholar
[13] Liu W, Deng X, Cai S 2016 AIP Adv. 6 075103
 Google Scholar
Google Scholar
[14] Chen Q, Tang L, Chen K, Zhao H 2013 J. Appl. Phys. 114 084301
 Google Scholar
Google Scholar
[15] Zhu Z, Zhang Z H, Wang D, Deng X Q, Fan Z Q, Tang G P 2015 J. Mater. Chem. 3 9657
[16] Zhang H, Meng S, Yang H 2015 J. Appl. Phys. 117 112108
[17] Wu M, Wu X, Zeng X C 2010 J. Phys. Chem. C 114 3937
 Google Scholar
Google Scholar
[18] Zhu Z L, Li C, Yu W, Chang D, Sun Q, Jia Y 2014 Appl. Phys. Lett. 105 113105
 Google Scholar
Google Scholar
[19] Lei S, Ge L, Najmaei S 2014 ACS Nano 8 1263
 Google Scholar
Google Scholar
[20] Ho C H 2016 2D Mater. 3 025019
 Google Scholar
Google Scholar
[21] Mudd G W, Molas M R, Chen X, Zólyomi V 2016 Sci. Rep. 6 39619
 Google Scholar
Google Scholar
[22] Bandurin D A, Tyurnina A V, Yu G L 2016 Nat. Nanotechnol. 12 223
[23] Wu M, Shi J, Zhang M, Ding Y, Wang H, Cen Y, Guo W, Pan S, Zhu Y 2018 Nanotechnology 29 205708
 Google Scholar
Google Scholar
[24] Yao A L, Wang X F, Liu Y S, Sun Y N 2018 Nanoscale Res. Lett. 13 107
 Google Scholar
Google Scholar
[25] Zhou W, Yu G, Rudenko A N, Yuan S 2018 Phys. Rev. Mater. 2 114001
 Google Scholar
Google Scholar
[26] Brandbyge M, Mozos J L, Ordejón P, Taylor J, Stokbro K. 2002 Phys. Rev. B 65 165401
 Google Scholar
Google Scholar
[27] Taylor J, Guo H, Wang J 2001 Phys. Rev. B 63 245407
 Google Scholar
Google Scholar
[28] Chen S, Zhou W, Yu J, Chen K Q 2018 Carbon 129 809
 Google Scholar
Google Scholar
[29] Deng Y, Chen S, Zeng Y, Feng Y, Zhou W, Tang L, Chen K 2018 Org. Electron. 63 310
 Google Scholar
Google Scholar
[30] Yuan J, Zhang L W, Liew K M 2016 Curr. Nanosci. 12 636
 Google Scholar
Google Scholar
[31] 张华林, 孙琳, 韩佳凝 2017 66 246101
 Google Scholar
Google Scholar
Zhang H L, Sun L, Han J N 2017 Acta Phys. Sin. 66 246101
 Google Scholar
Google Scholar
[32] 左博敏, 健美, 冯志, 毛宇亮 2019 68 113103
 Google Scholar
Google Scholar
Zuo B M, Yuan J M, Feng Z, Mao Y L 2019 Acta Phys. Sin. 68 113103
 Google Scholar
Google Scholar
[33] Kang H S, Jeong S 2004 Phys. Rev. B 70 233411
 Google Scholar
Google Scholar
计量
- 文章访问数: 13374
- PDF下载量: 88
- 被引次数: 0














 下载:
下载: