-
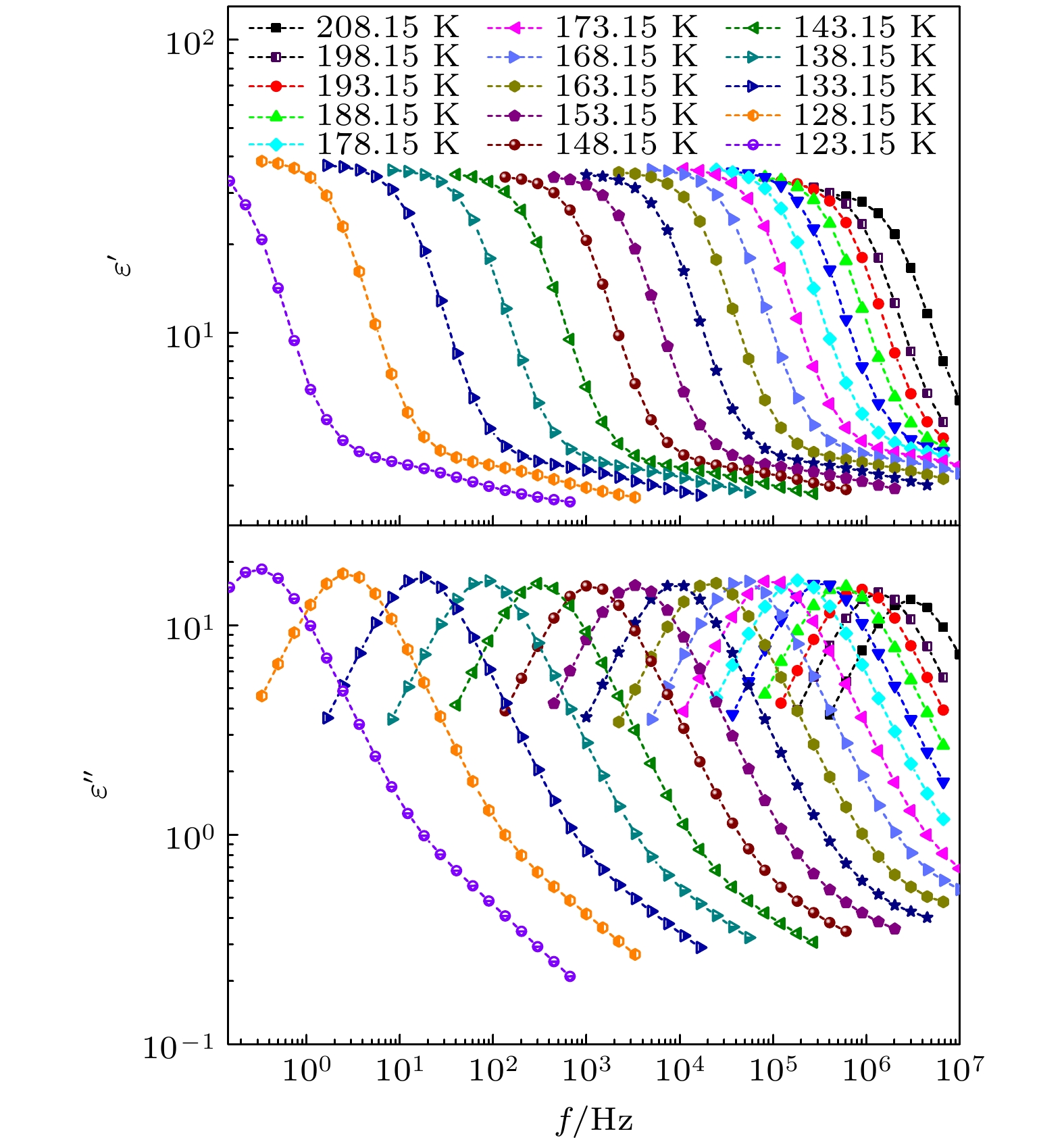
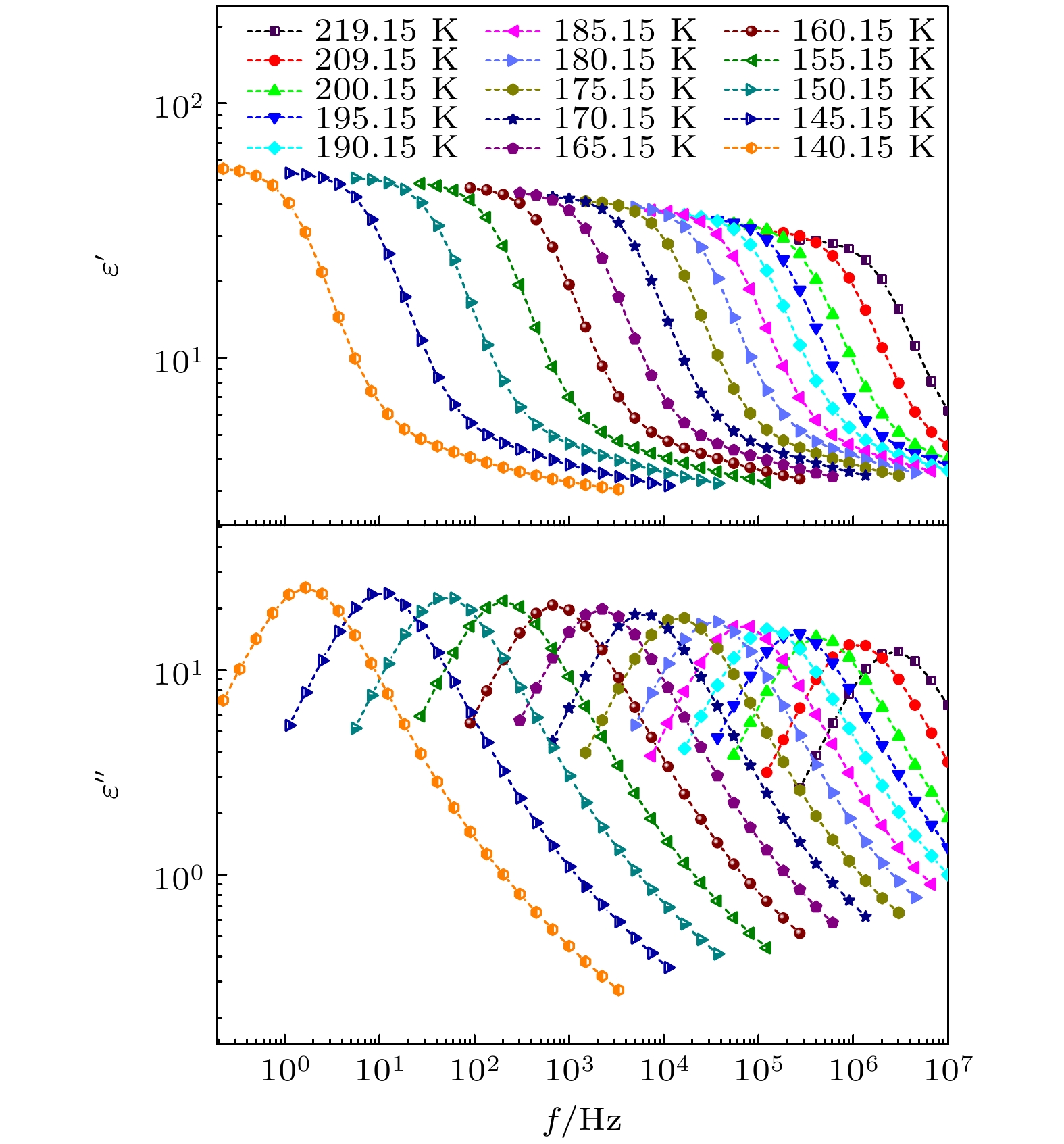
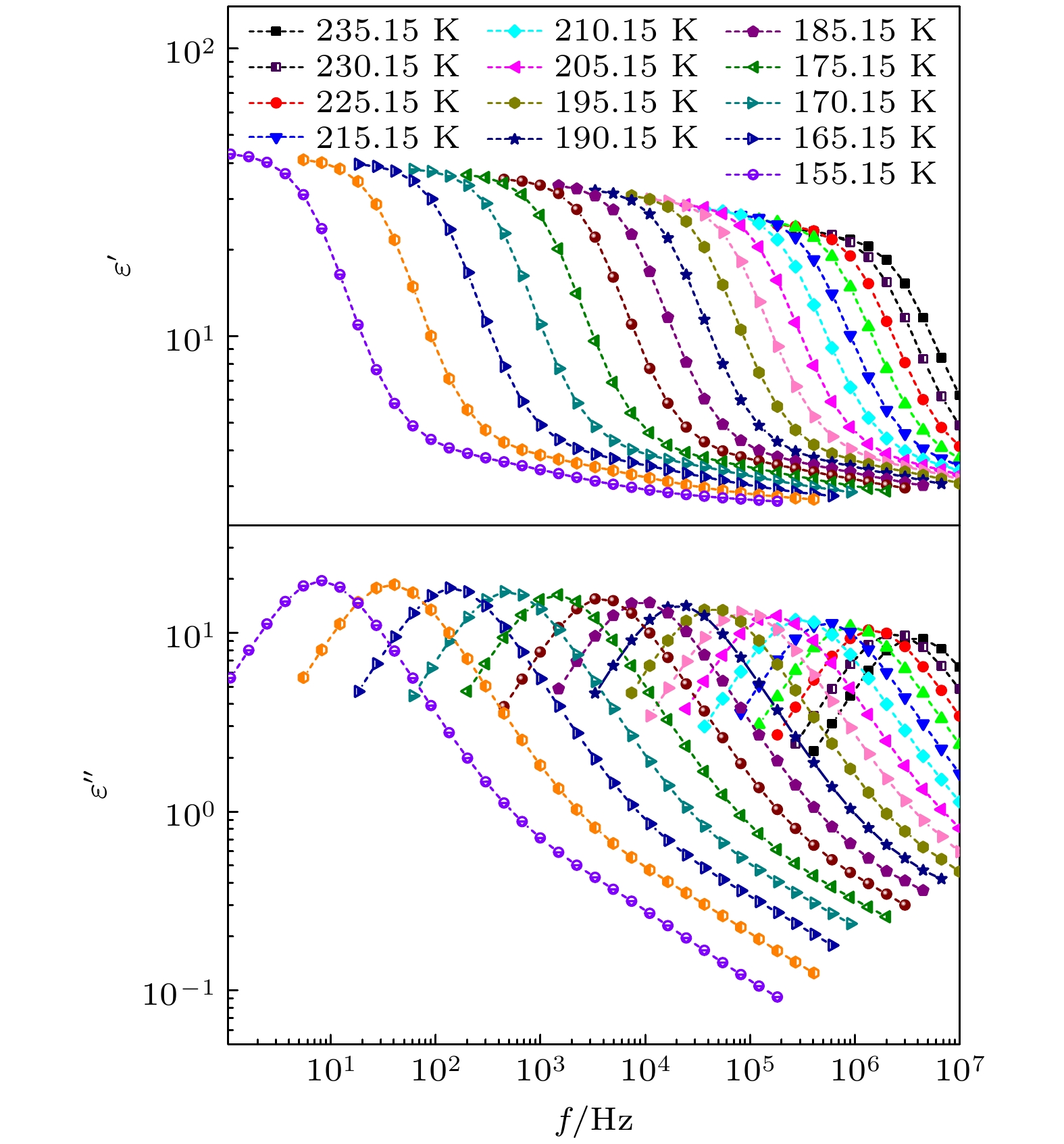
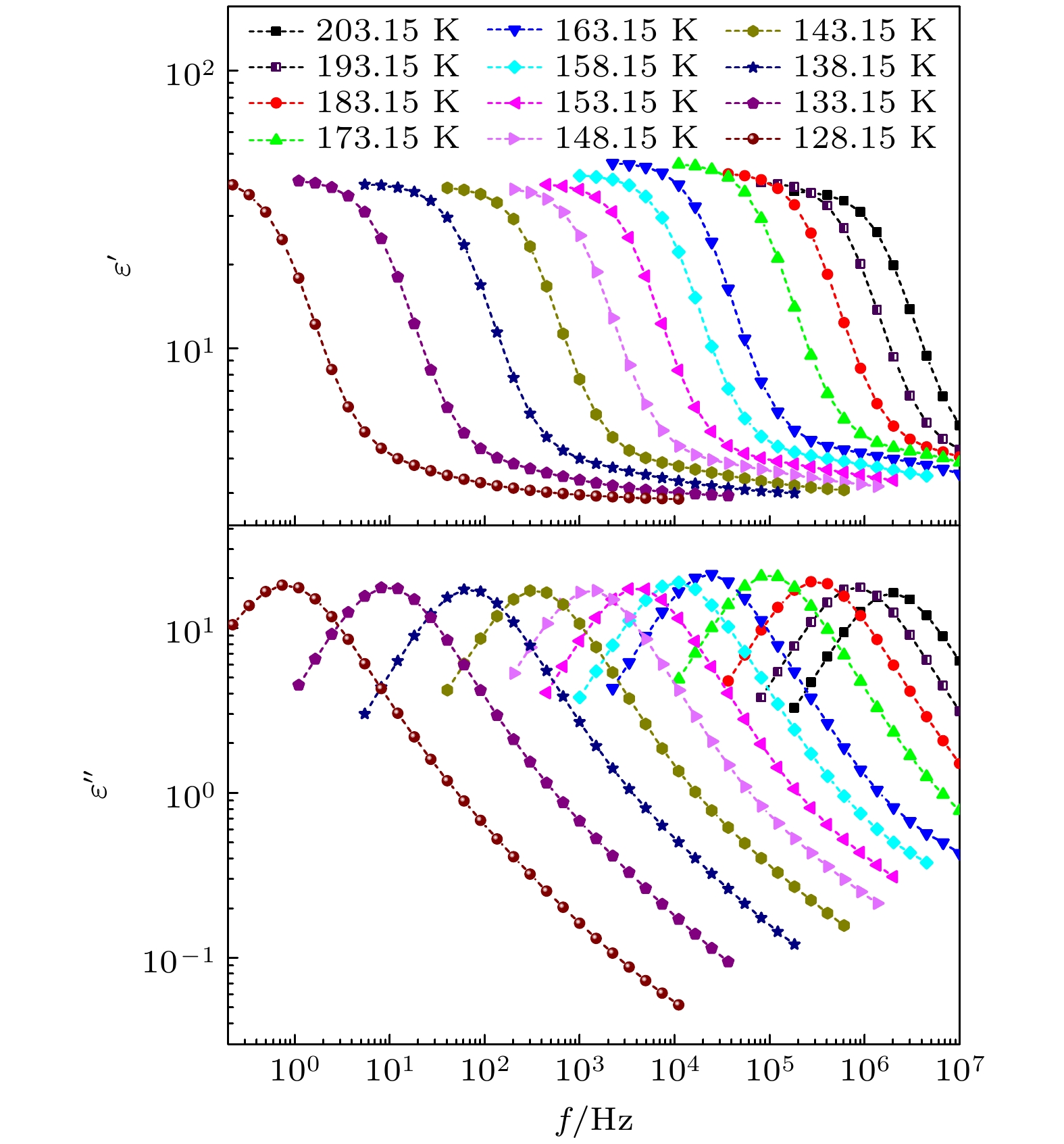
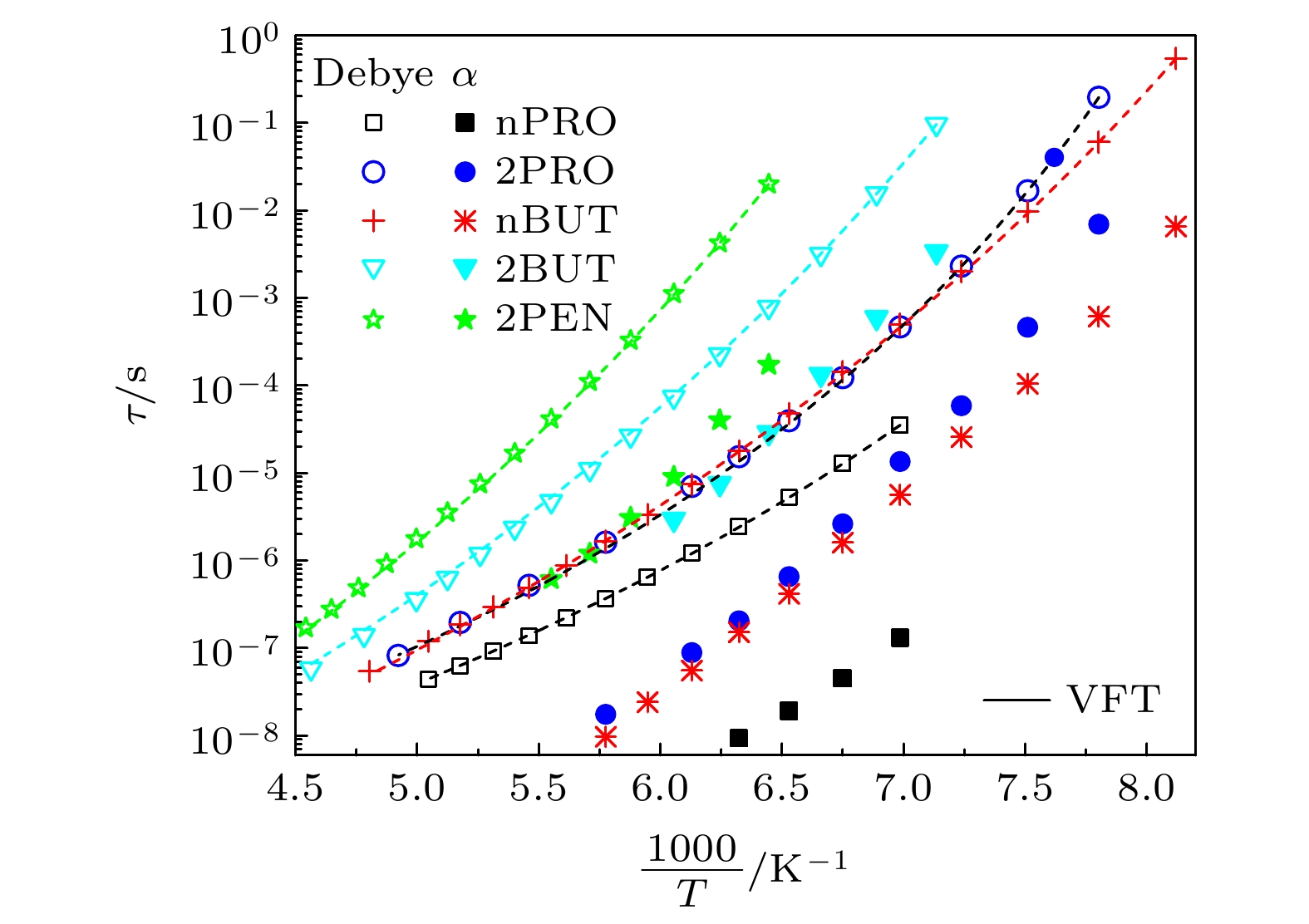
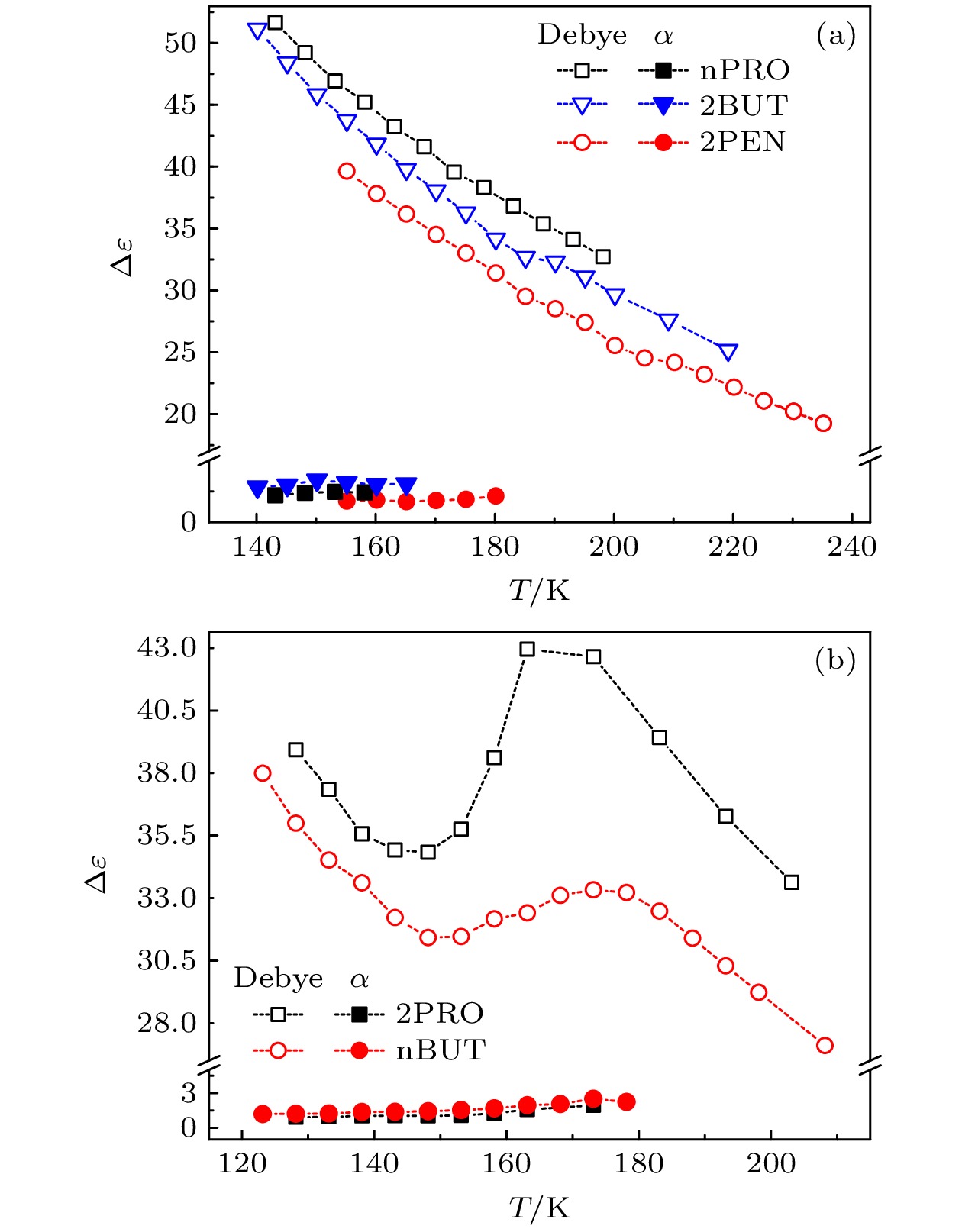
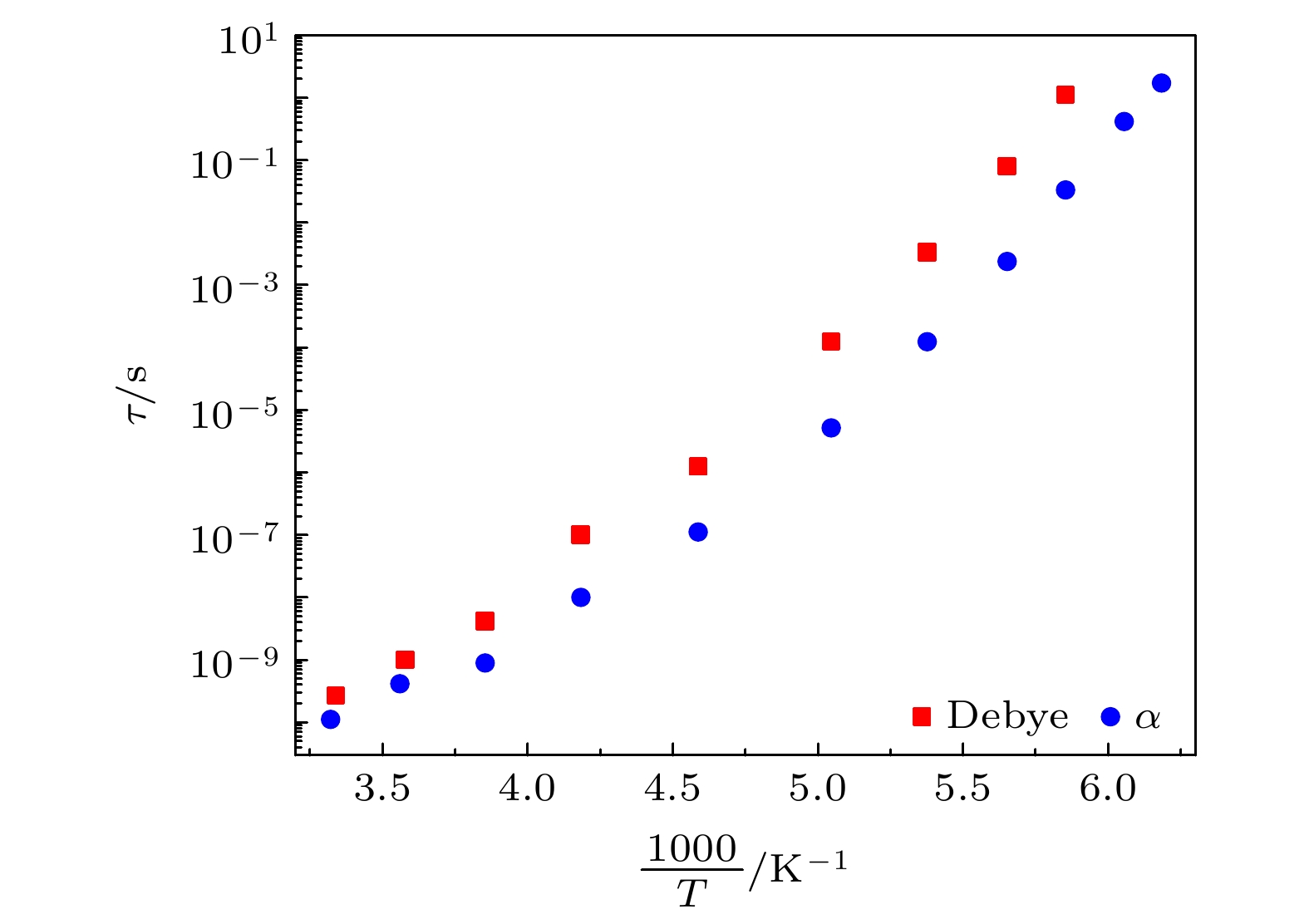
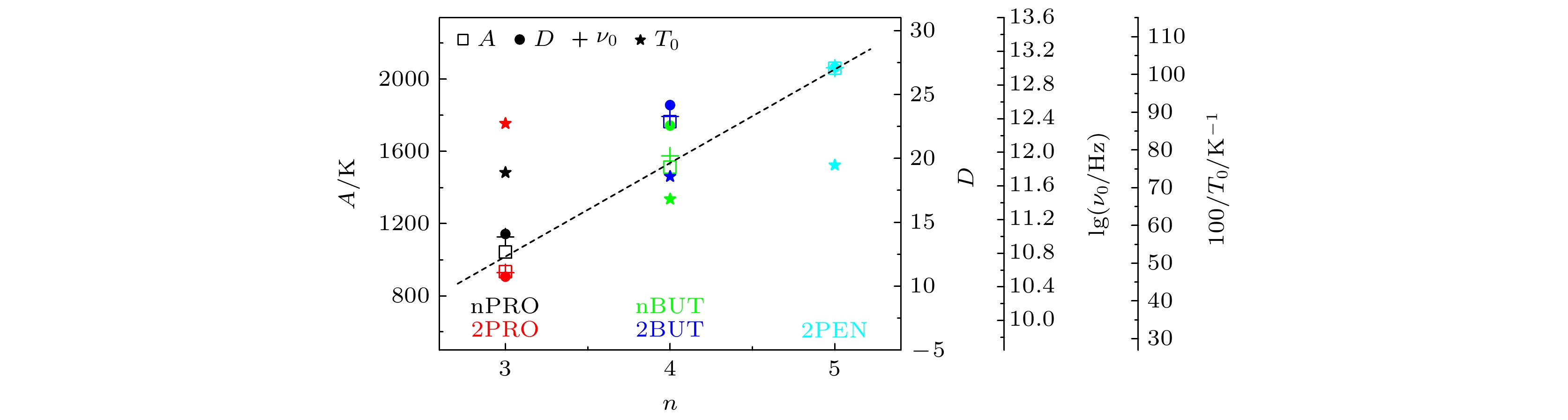
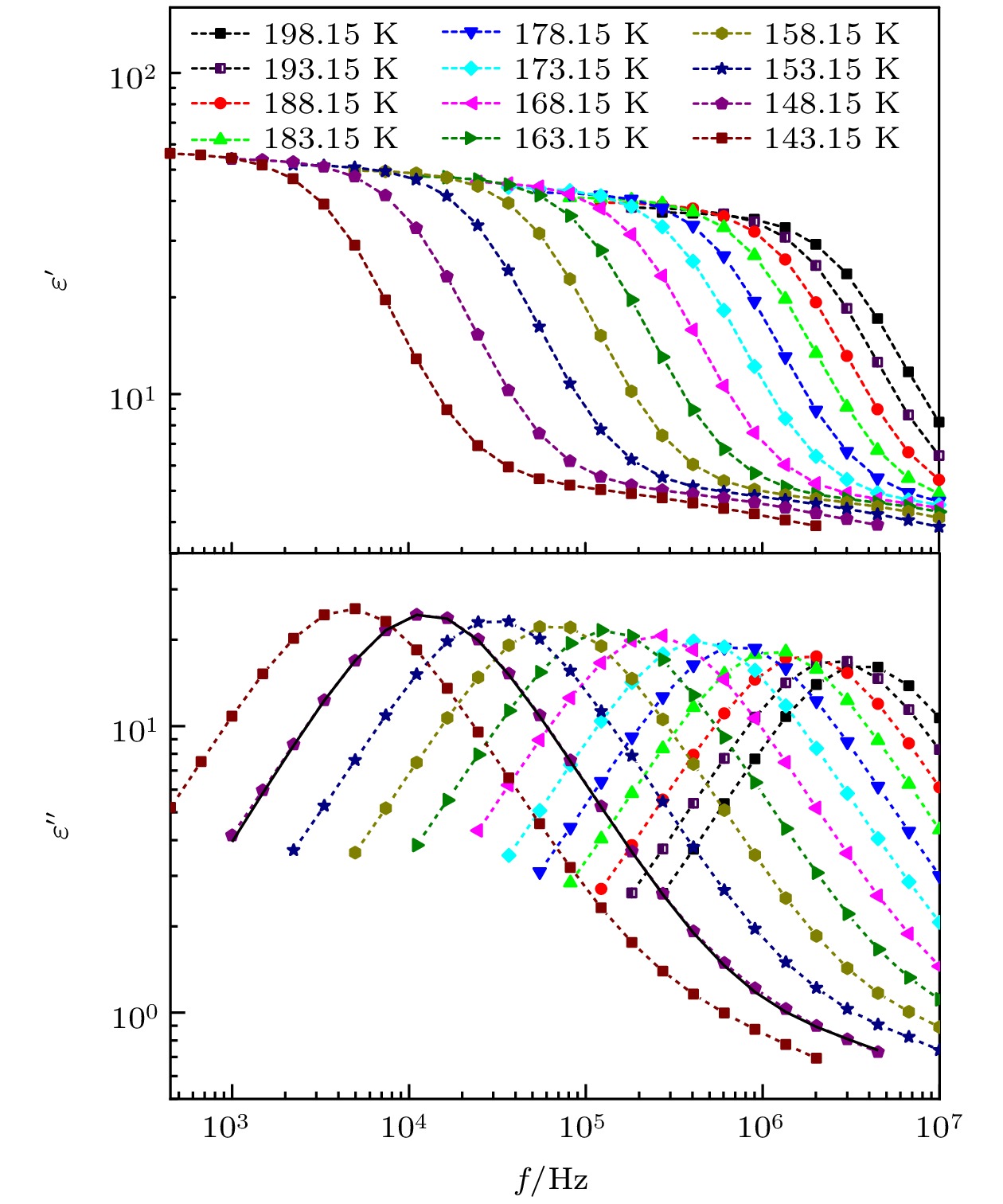
The five linear primary and secondary alcohols, i.e. n-propanol, isopropanol, n-butanol, 2-butanol and 2-pentanol, have similar chain lengths and slightly different structures. In this work, dielectric spectroscopy is used to investigate the properties of monohydroxy alcohols. The dielectric spectra of isopropanol and n-butanol show an abnormal change. i.e. the relaxation peaks with the highest strength gradually increases with temperature rising in a range of about 145–175 K. The analyses indicate that the abnormal variation originates from that of the Debye dielectric relaxation strength (DDRS) in the monohydroxy alcohols at above temperatures. According to the theoretical model of the DDRS for the monohydroxy alcohol, the abnormal variation is believed to be the result of the combined effects of decrease and increase of the DDRS caused by temperature, and the transformation of the structure of the hydrogen bonding molecular chain caused by the variation of the mobility of molecules. By comparing the relaxation times of the five monohydroxy alcohols, it is found that the conditions should be more stringent to cause the above-mentioned abnormal variation. In addition, the results also show that strength parameter of Debye processes, intrinsic vibration frequency of the relaxation units and their activation energy in the high-temperature limit in secondary alcohols also rise with the increase of the number of carbon atoms, similar to the scenario in the case of primary alcohols. These results can not only provide a new breakthrough point for the investigation of exotic properties in monohydroxy alcohols but also give a reference to explore the effect of molecular chain length on their dynamics.
-
Keywords:
- Debye relaxation /
- monohydroxy alcohol /
- dielectric relaxation /
- abnormal variation
[1] Kennedy D, Norman C 2005 Science 309 75
 Google Scholar
Google Scholar
[2] Böhmer R, Gainaru C, Richert R 2014 Phys. Rep. 545 125
 Google Scholar
Google Scholar
[3] Tsai S D, Yao H Y, Chang T H 2024 J. Mol. Liq. 405 125043
 Google Scholar
Google Scholar
[4] Arrese-Igor S, Alegría A, Colmenero J 2018 Phys. Chem. Chem. Phys. 20 27758
 Google Scholar
Google Scholar
[5] Fragiadakis D, Roland C M, Casalini R 2010 J. Chem. Phys. 132 144505
 Google Scholar
Google Scholar
[6] Bergman R, Jansson H, Swenson J 2010 J. Chem. Phys. 132 044504
 Google Scholar
Google Scholar
[7] Huth H, Wang L M, Schick C, Richert R 2007 J. Chem. Phys. 126 104503
 Google Scholar
Google Scholar
[8] Mandanici A, Huang W, Cutroni M, Richert R 2008 J. Chem. Phys. 128 124505
 Google Scholar
Google Scholar
[9] Wang L M, Tian Y, Liu R, Richert R 2008 J. Chem. Phys. 128 084503
 Google Scholar
Google Scholar
[10] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 457
 Google Scholar
Google Scholar
[11] Gainaru C, Kastner S, Mayr F, et al. 2011 Phys. Rev. Lett. 107 118304
 Google Scholar
Google Scholar
[12] Wikarek M, Pawlus S, Tripathy S N, Szulc A, Paluch M 2016 J. Phys. Chem. B 120 5744
 Google Scholar
Google Scholar
[13] Gainaru C, Meier R, Schildmann S, Lederle C, Hiller W, Rössler E A, Böhmer R 2010 Phys. Rev. Lett. 105 258303
 Google Scholar
Google Scholar
[14] Ananiadou A, Papamokos G, Steinhart M, Floudas G 2021 J. Chem. Phys. 155 184504
 Google Scholar
Google Scholar
[15] Sillrén P, Matic A, Karlsson M, et al. 2014 J. Chem. Phys. 140 124501
 Google Scholar
Google Scholar
[16] Bauer S, Burlafinger K, Gainaru C, Lunkenheimer P, Hiller W, Loidl A, Böhmer R 2013 J. Chem. Phys. 138 094505
 Google Scholar
Google Scholar
[17] Wang L N, Zhao X Y, Huang Y N 2019 Int. J. Mod. Phys. B 33 1950313
 Google Scholar
Google Scholar
[18] Wang L N, Zhao X Y, Huang Y N 2019 Chin. Phys. Lett. 36 097701
 Google Scholar
Google Scholar
[19] Wang L N, Zhao X Y, Shang J Y, Zhou H W 2023 Acta Phys. Sin. 72 037701
 Google Scholar
Google Scholar
[20] Zhao X Y, Wang L N, He Y F, Zhou H W, Huang Y N 2020 Chem. Phys. 528 110473
 Google Scholar
Google Scholar
[21] 赵兴宇, 王丽娜, 韩宏博, 尚洁莹 2024 73 147701
 Google Scholar
Google Scholar
Zhao X Y, Wang L N, Han H B, Shang J Y 2024 Acta Phys. Sin. 73 147701
 Google Scholar
Google Scholar
[22] Havriliak S, Negami S 1966 J. Polym. Sci. 14 99
 Google Scholar
Google Scholar
[23] Fulcher G S 1925 J. Am. Ceram. Soc. 8 339
 Google Scholar
Google Scholar
[24] Tammann G, Hesse W 1926 Z. Anorg. Allg. Chem. 156 245
 Google Scholar
Google Scholar
[25] Wang L M, Richert R 2005 J. Chem. Phys. 123 054516
 Google Scholar
Google Scholar
[26] Xu D, Feng S, Wang J Q, Wang L M, Richert R 2020 J. Phys. Chem. Lett. 11 5792
 Google Scholar
Google Scholar
[27] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 115
 Google Scholar
Google Scholar
-
表 1 单羟基醇的分子结构
Table 1. Molecular structures of monohydroxy alcohols.
Monohydroxy alcohols Molecular structures n-propanol (nPRO) 
Isopropanol (2PRO) 
n-butanol (nBUT) 
2-Butanol (2BUT) 
2-Pentanol (2PEN) 
-
[1] Kennedy D, Norman C 2005 Science 309 75
 Google Scholar
Google Scholar
[2] Böhmer R, Gainaru C, Richert R 2014 Phys. Rep. 545 125
 Google Scholar
Google Scholar
[3] Tsai S D, Yao H Y, Chang T H 2024 J. Mol. Liq. 405 125043
 Google Scholar
Google Scholar
[4] Arrese-Igor S, Alegría A, Colmenero J 2018 Phys. Chem. Chem. Phys. 20 27758
 Google Scholar
Google Scholar
[5] Fragiadakis D, Roland C M, Casalini R 2010 J. Chem. Phys. 132 144505
 Google Scholar
Google Scholar
[6] Bergman R, Jansson H, Swenson J 2010 J. Chem. Phys. 132 044504
 Google Scholar
Google Scholar
[7] Huth H, Wang L M, Schick C, Richert R 2007 J. Chem. Phys. 126 104503
 Google Scholar
Google Scholar
[8] Mandanici A, Huang W, Cutroni M, Richert R 2008 J. Chem. Phys. 128 124505
 Google Scholar
Google Scholar
[9] Wang L M, Tian Y, Liu R, Richert R 2008 J. Chem. Phys. 128 084503
 Google Scholar
Google Scholar
[10] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 457
 Google Scholar
Google Scholar
[11] Gainaru C, Kastner S, Mayr F, et al. 2011 Phys. Rev. Lett. 107 118304
 Google Scholar
Google Scholar
[12] Wikarek M, Pawlus S, Tripathy S N, Szulc A, Paluch M 2016 J. Phys. Chem. B 120 5744
 Google Scholar
Google Scholar
[13] Gainaru C, Meier R, Schildmann S, Lederle C, Hiller W, Rössler E A, Böhmer R 2010 Phys. Rev. Lett. 105 258303
 Google Scholar
Google Scholar
[14] Ananiadou A, Papamokos G, Steinhart M, Floudas G 2021 J. Chem. Phys. 155 184504
 Google Scholar
Google Scholar
[15] Sillrén P, Matic A, Karlsson M, et al. 2014 J. Chem. Phys. 140 124501
 Google Scholar
Google Scholar
[16] Bauer S, Burlafinger K, Gainaru C, Lunkenheimer P, Hiller W, Loidl A, Böhmer R 2013 J. Chem. Phys. 138 094505
 Google Scholar
Google Scholar
[17] Wang L N, Zhao X Y, Huang Y N 2019 Int. J. Mod. Phys. B 33 1950313
 Google Scholar
Google Scholar
[18] Wang L N, Zhao X Y, Huang Y N 2019 Chin. Phys. Lett. 36 097701
 Google Scholar
Google Scholar
[19] Wang L N, Zhao X Y, Shang J Y, Zhou H W 2023 Acta Phys. Sin. 72 037701
 Google Scholar
Google Scholar
[20] Zhao X Y, Wang L N, He Y F, Zhou H W, Huang Y N 2020 Chem. Phys. 528 110473
 Google Scholar
Google Scholar
[21] 赵兴宇, 王丽娜, 韩宏博, 尚洁莹 2024 73 147701
 Google Scholar
Google Scholar
Zhao X Y, Wang L N, Han H B, Shang J Y 2024 Acta Phys. Sin. 73 147701
 Google Scholar
Google Scholar
[22] Havriliak S, Negami S 1966 J. Polym. Sci. 14 99
 Google Scholar
Google Scholar
[23] Fulcher G S 1925 J. Am. Ceram. Soc. 8 339
 Google Scholar
Google Scholar
[24] Tammann G, Hesse W 1926 Z. Anorg. Allg. Chem. 156 245
 Google Scholar
Google Scholar
[25] Wang L M, Richert R 2005 J. Chem. Phys. 123 054516
 Google Scholar
Google Scholar
[26] Xu D, Feng S, Wang J Q, Wang L M, Richert R 2020 J. Phys. Chem. Lett. 11 5792
 Google Scholar
Google Scholar
[27] Hu L N, Zhang C Z, Yue Y Z, Bian X F 2010 Chin. Sci. Bull. 55 115
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 858
- PDF Downloads: 32
- Cited By: 0














 DownLoad:
DownLoad: