-
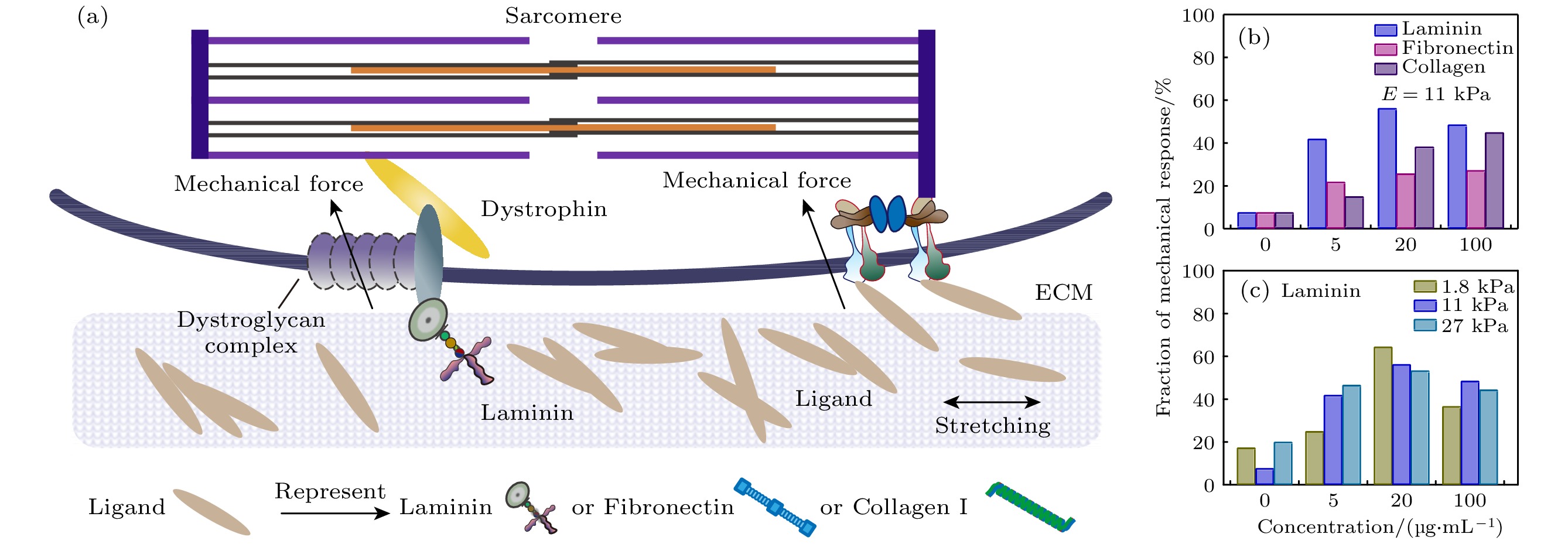
The mechanical behavior of cardiomyocytes plays an essential role in maintaining life and health. It is traditionally believed that both electrical signals and chemical signals modulate the cardiomyocytes behaviors. Recent discoveries have elucidated that the physical cues of microenvironment can regulate cell activities such as proliferation, spreading, migration, and differentiation. However, there is still limited research on regulating cardiomyocytes beating through mechanical force. Herein we prepare different polyacrylamide gels coated with different cell adhesion ligand proteins to simulate the physical microenvironment of cardiomyocytes. Then the mechanical loading forces are loaded by using a tungsten probe to stretch elastic hydrogels which can emulate the mechanical oscillations induced by the beating of adjacent cardiomyocytes. We investigate the responsive behavior of cardiomyocytes to external mechanical oscillations within various physical microenvironments. Firstly, we load 1 Hz mechanical oscillation on the matrix (E = 11 kPa) with different kinds and concentrations of ligands (0, 5, 20, 100 μg/mL) to stimulate cardiomyocytes and observe their mechanical response behavior. Our findings indicate that all kinds of ligands including Laminin, Fibronectin and Collagen I , can mediate the cardiomyocytes response to extrinsic mechanical oscillatory stimuli, which might be due to distinct mechanisms of mechanical force coupling (Fig. (b)). This suggests that mechanical force signals can regulate the beating of cardiomyocytes through matrix-ligand-cell signaling pathway, thereby inducing intercellular coupled oscillations for rhythmic control of cardiomyocytes. Cardiomyocytes cultured on the matrix coated with 20 μg/mL Laminin show the highest and most stable response fraction. We hypothesize that there exist dual force transduction pathways for Laminin binding to integrin and dystrophin glycoprotein complex (DGC) (Fig. (a)). We further analyze the cardiomyocytes behaviors under mechanical oscillation with different values of substrate stiffness (E = 1.8, 11, 27 kPa) and concentrations of Laminin (0, 5, 20, 100 μg/mL). We find that cardiomyocytes cultured on 1.8 kPa coated with 20 μg/mL Laminin show the highest response fraction (Fig. (c)). Our results demonstrate that the stiffness of substrate, the type and density of cell adhesion ligands, as well as the strength and rhythm of the mechanical signals can synergetically affect the cardiomyocytes responses to external mechanical stimulations, which provides the foundation for understanding the diseases such as cardiac arrhythmias and heart failure following myocardial infarction.
-
Keywords:
- cardiomyocyte /
- hydrogel /
- mechanical stimulation /
- intercellular interaction
[1] Dogan A, Parmaksız M, Elçin A E, Elçin Y M 2016 Stem Cell Rev. Rep. 12 202
 Google Scholar
Google Scholar
[2] World Health Organization 2023. Licence: CC BY-NC-SA 3.0 IGO
[3] Thayer J F, Yamamoto S S, Brosschot J F 2010 Int. J. Cardiol. 141 122
 Google Scholar
Google Scholar
[4] Quinn T A, Kohl P 2021 Physiol. Rev. 101 37
 Google Scholar
Google Scholar
[5] De Mello WC 1982 Circ. Res. 51 1
 Google Scholar
Google Scholar
[6] McCain M L, Lee H, Aratyn-Schaus Y, Kléber A G, Parker K K 2012 Proc. Natl. Acad. Sci. U.S.A. 109 9881
 Google Scholar
Google Scholar
[7] Kumar N M, Gilula N B 1996 Cell 84 381
 Google Scholar
Google Scholar
[8] Barr L, Dewey M M, Berger W 1965 J. Gen. Physiol. 48 797
 Google Scholar
Google Scholar
[9] Corrado D, Basso C, Thiene G, et al. 1997 J. Am. Coll. Cardiol. 30 1512
 Google Scholar
Google Scholar
[10] Pelham R J, Wang Y L 1997 Proc. Natl. Acad. Sci. U.S.A. 94 13661
 Google Scholar
Google Scholar
[11] Harris A 1973 Exp. Cell Res. 77 285
 Google Scholar
Google Scholar
[12] Folkman J, Moscona A 1978 Nature 273 345
 Google Scholar
Google Scholar
[13] Wang W Y, Davidson C D, Lin D, Baker B M 2019 Nat. Commun. 10 1186
 Google Scholar
Google Scholar
[14] Isomursu A, Park K Y, Hou J, et al. 2022 Nat. Mater. 21 1081
 Google Scholar
Google Scholar
[15] Adebowale K, Gong Z, Hou J C, Wisdom K M, Garbett D, Lee H P, Nam S, Meyer T, Odde D J, Shenoy V B, Chaudhuri O 2021 Nat. Mater. 20 1290
 Google Scholar
Google Scholar
[16] Bera K, Kiepas A, Godet I, Li Y, Mehta P, Ifemembi B, Paul C D, Sen A, Serra S A, Stoletov K, Tao J, Shatkin G, Lee S J, Zhang Y, Boen A, Mistriotis P, Gilkes D M, Lewis J D, Fan C M, Feinberg A P, Valverde M A, Sun S X, Konstantopoulos K 2022 Nature 611 365
 Google Scholar
Google Scholar
[17] 王璟, 杨根, 刘峰 2015 64 058707
 Google Scholar
Google Scholar
Wang J, Yang G, Liu F 2015 Acta Phys. Sin. 64 058707
 Google Scholar
Google Scholar
[18] Lecuit T, Le Goff L 2007 Nature 450 189
 Google Scholar
Google Scholar
[19] Assoian R K, Klein E A 2008 Trends Cell Biol. 18 347
 Google Scholar
Google Scholar
[20] Wen J H, Vincent L G, Fuhrmann A, Choi Y S, Hribar K C, Taylor-Weiner H, Chen S, Engler A J 2014 Nat. Mater. 13 979
 Google Scholar
Google Scholar
[21] Engler A J, Sen S, Sweeney H L, Discher D E 2006 Cell 126 677
 Google Scholar
Google Scholar
[22] Zhang J S, Wong S H D, Wu X, Lei H, Qin M, Shi P, Wang W, Bian L, Cao Y 2021 Adv. Mater. 33 2105765
 Google Scholar
Google Scholar
[23] Chowdhury F, Na S, Li D, Poh Y C, Tanaka T S, Wang F, Wang N 2010 Nat. Mater. 9 82
 Google Scholar
Google Scholar
[24] Ingber D E, Folkman J 1989 J. Cell Biol. 109 317
 Google Scholar
Google Scholar
[25] Reinhart-King C A, Dembo M, Hammer D A 2008 Biophys. J. 95 6044
 Google Scholar
Google Scholar
[26] Tang X, Bajaj P, Bashir R, Saif T A 2011 Soft Matter 7 6151
 Google Scholar
Google Scholar
[27] Nitsan I, Drori S, Lewis Y E, Cohen S, Tzlil S 2016 Nature Phys. 12 472
 Google Scholar
Google Scholar
[28] Cohen O, Safran S A 2018 Sci. Rep. 8 2237
 Google Scholar
Google Scholar
[29] Bick R J, Snuggs M B, Poindexter B J, Buja L M, Winkle W B V 1998 Cell Adhes. Commun. 6 301
 Google Scholar
Google Scholar
[30] Engler A J, Carag-Krieger C, Johnson C P, Raab M, Tang H Y, Speicher D W, Sanger J W, Sanger J M, Discher D E 2008 J. Cell Sci. 121 3794
 Google Scholar
Google Scholar
[31] Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher D E 2013 Curr. Biol. 23 2434
 Google Scholar
Google Scholar
[32] Jacot J G, McCulloch A D, Omens J H 2008 Biophys. J. 95 3479
 Google Scholar
Google Scholar
[33] Ehler E, Moore-Morris T, Lange S 2013 J. Vis. Exp. e50154
[34] Gittenberger-de Groot A C, Vrancken Peeters M P F M, Mentink M M T, Gourdie R G, Poelmann R E 1998 Circ. Res. 82 1043
 Google Scholar
Google Scholar
[35] Berry M F, Engler A J, Woo Y J, Pirolli T J, Bish L T, Jayasankar V, Morine K J, Gardner T J, Discher D E, Sweeney H L 2006 Am. J. Physiol. Heart Circ. Physiol. 290 H2196
 Google Scholar
Google Scholar
[36] Eisner D A, Choi H S, Díaz M E, O'Neill S C, Trafford A W 2000 Circ. Res. 87 1087
 Google Scholar
Google Scholar
[37] Bers D M 2002 Nature 415 198
 Google Scholar
Google Scholar
[38] 白永强, 唐爱辉, 王世强, 朱 星 2007 56 3607
 Google Scholar
Google Scholar
Bai Y Q, Tang A H, Wang S Q, Zhu X 2007 Acta Phys. Sin. 56 3607
 Google Scholar
Google Scholar
[39] Ross R S, Borg T K 2001 Circ. Res. 88 1112
 Google Scholar
Google Scholar
[40] Israeli-Rosenberg S, Manso A M, Okada H, Ross R S 2014 Circ. Res. 114 572
 Google Scholar
Google Scholar
[41] Tan P M, Buchholz K S, Omens J H, McCulloch A D, Saucerman J J 2017 PLOS Comput. Biol. 13 e1005854
 Google Scholar
Google Scholar
[42] Harvey P A, Leinwand L A 2011 J. of Cell Biol. 194 355
 Google Scholar
Google Scholar
[43] Chen-Izu Y, Izu L T 2017 J. Physiol. 595 3949
 Google Scholar
Google Scholar
[44] Roca-Cusachs P, Gauthier N C, del Rio A, Sheetz M P 2009 Proc. Natl. Acad. Sci. U. S. A. 106 16245
 Google Scholar
Google Scholar
[45] Kechagia J Z, Ivaska J, Roca-Cusachs P 2019 Nat. Rev. Mol. Cell Biol. 20 457
 Google Scholar
Google Scholar
[46] Sun Z, Guo S S, Fässler R 2016 J. Cell Biol. 215 445
 Google Scholar
Google Scholar
[47] Han Y L, Ronceray P, Xu G, Malandrino A, Kamm R D, Lenz M, Broedersz C P, Guo M 2018 Proc. Natl. Acad. Sci. U. S. A. 115 4075
 Google Scholar
Google Scholar
[48] Hall M S, Alisafaei F, Ban E, Feng X, Hui C Y, Shenoy V B, Wu M 2016 Proc. Natl. Acad. Sci. U. S. A. 113 14043
 Google Scholar
Google Scholar
[49] 孙波 2015 64 058201
 Google Scholar
Google Scholar
Sun B 2015 Acta Phys. Sin. 64 058201
 Google Scholar
Google Scholar
[50] Hu J Y, Li J H, Jiang J, Wang L L, Roth J, McGuinness K N, Baum J, Dai W, Sun Y, Nanda V, Xu F 2022 Nat. Commun. 13 6761
 Google Scholar
Google Scholar
[51] Discher D E, Janmey P, Wang Y L 2005 Science 310 1139
 Google Scholar
Google Scholar
[52] Hersch N, Wolters B, Dreissen G, Springer R, Kirchgeßner N, Merkel R, Hoffmann B 2013 Biol. Open 2 351
 Google Scholar
Google Scholar
-
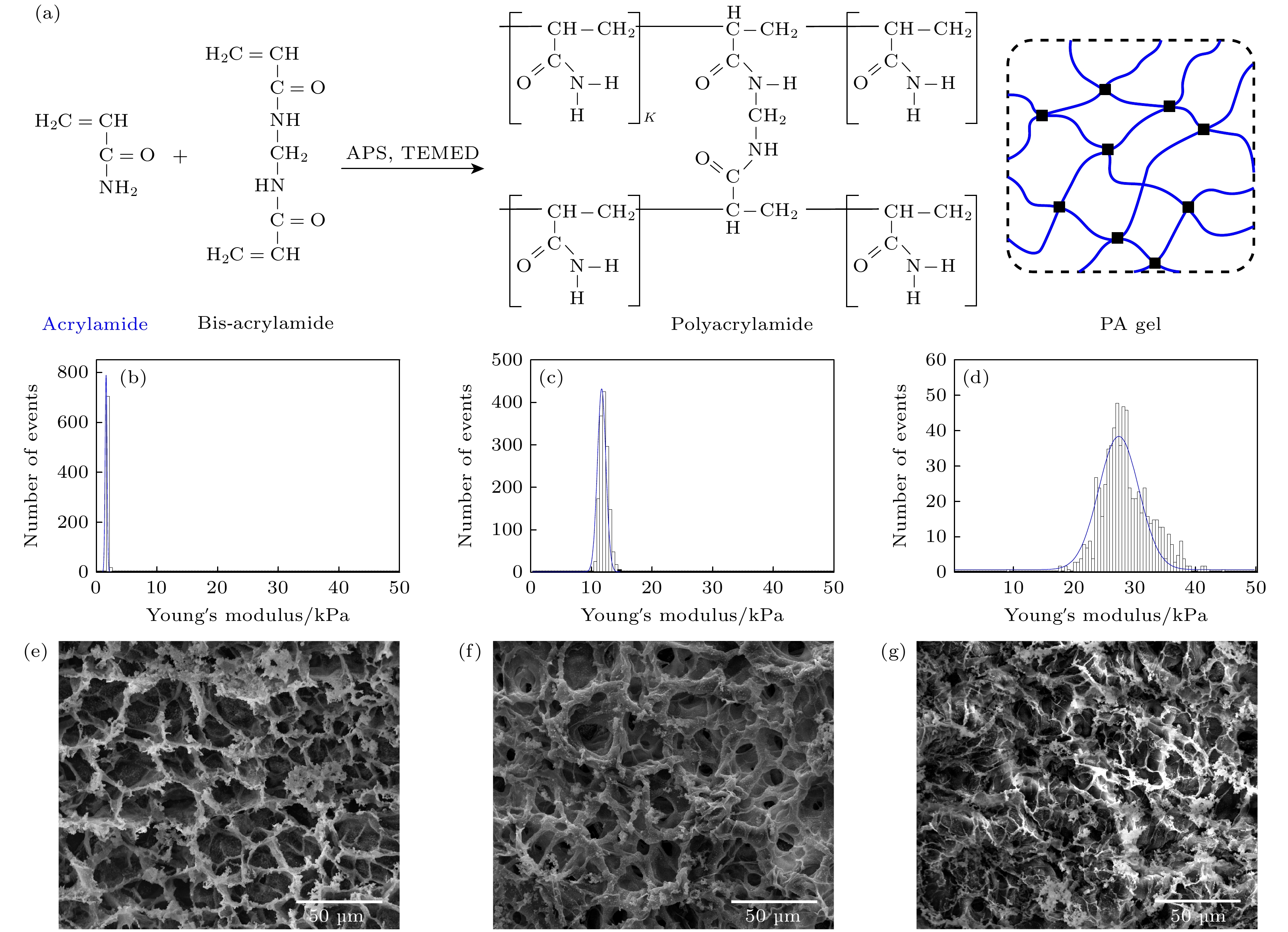
图 2 聚丙烯酰胺水凝胶的制备与表征 (a) 聚丙烯酰胺水凝胶的制备示意图; (b)—(d) 基于AFM的3种不同水凝胶的杨氏模量分布, 其杨氏模量分别为(1.824 ± 0.111) kPa, (11.688 ± 0.493) kPa和(27.358 ± 2.331) kPa; (e)—(g)对应3种水凝胶的SEM结果
Figure 2. Preparation and characterization of PA gel: (a) Schematic illustration of the preparation of PA gel; (b)–(d) the Young’ modulus distributions of three different of PA gel measured by AFM, which are (1.824 ± 0.111) kPa, (11.688 ± 0.493) kPa, and (27.358 ± 2.331) kPa, respectively; (e)–(g) SEM images of three different of PA gel.
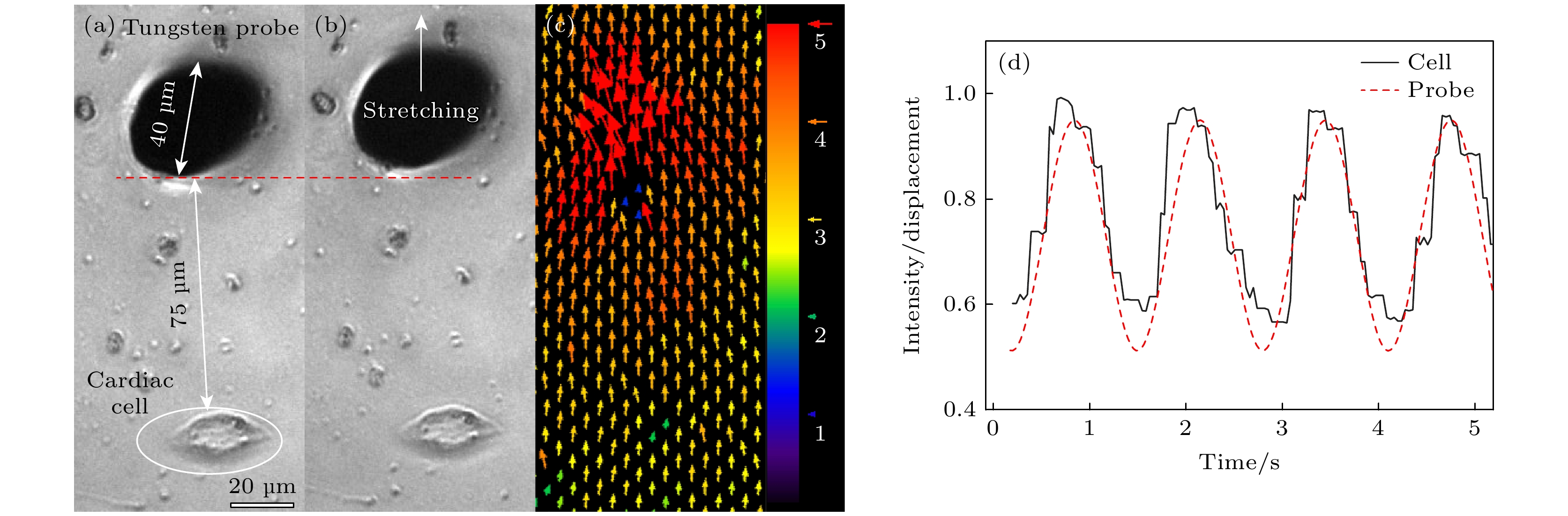
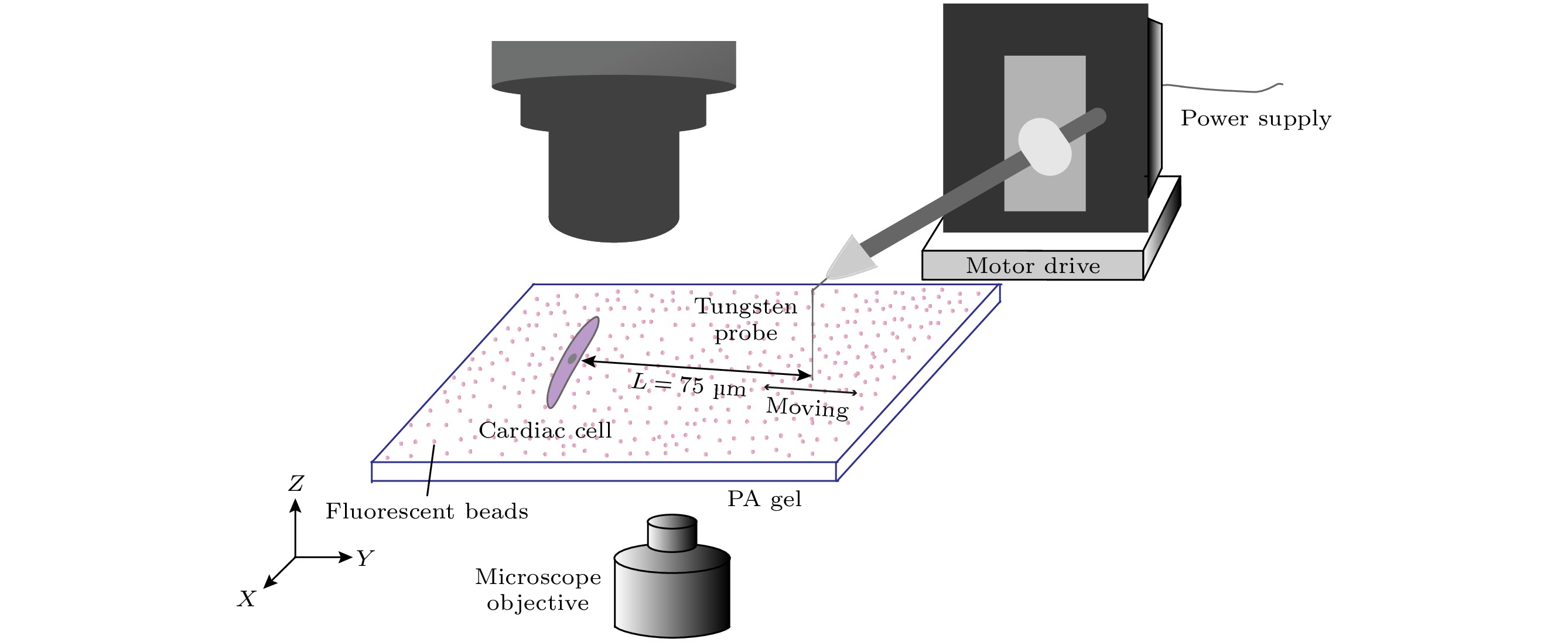
图 3 力学刺激加载及荧光表征 (a), (b)分别为对单个心肌细胞施加力学刺激前后的实物图; (c) 钨针运动到最大振幅时, PA gel上各点的位移分布图(单位为 μm); (d) 心肌细胞机械行为检测, 红色虚线为钨针输出的力振荡信号, 黑色实线是心肌细胞达到同频后Fluo4探针检测到的Ca2+ 振荡信号
Figure 3. Mechanical loading and fluorescence characterization: (a), (b) Phase-contrast images of individual cardiomyocyte before and after mechanical stimulation; (c) displacement distribution of each point on PA gel when the tungsten probe moves to the maximum amplitude (unit: μm); (d) mechanical behavior detection of cardiomyocytes, the red dotted line is the signal of mechanical oscillation output by tungsten probe, and the black solid line is the Ca2+ oscillation signal detected by Fluo4 probe after cardiomyocytes reach the same frequency with the tungsten probe.
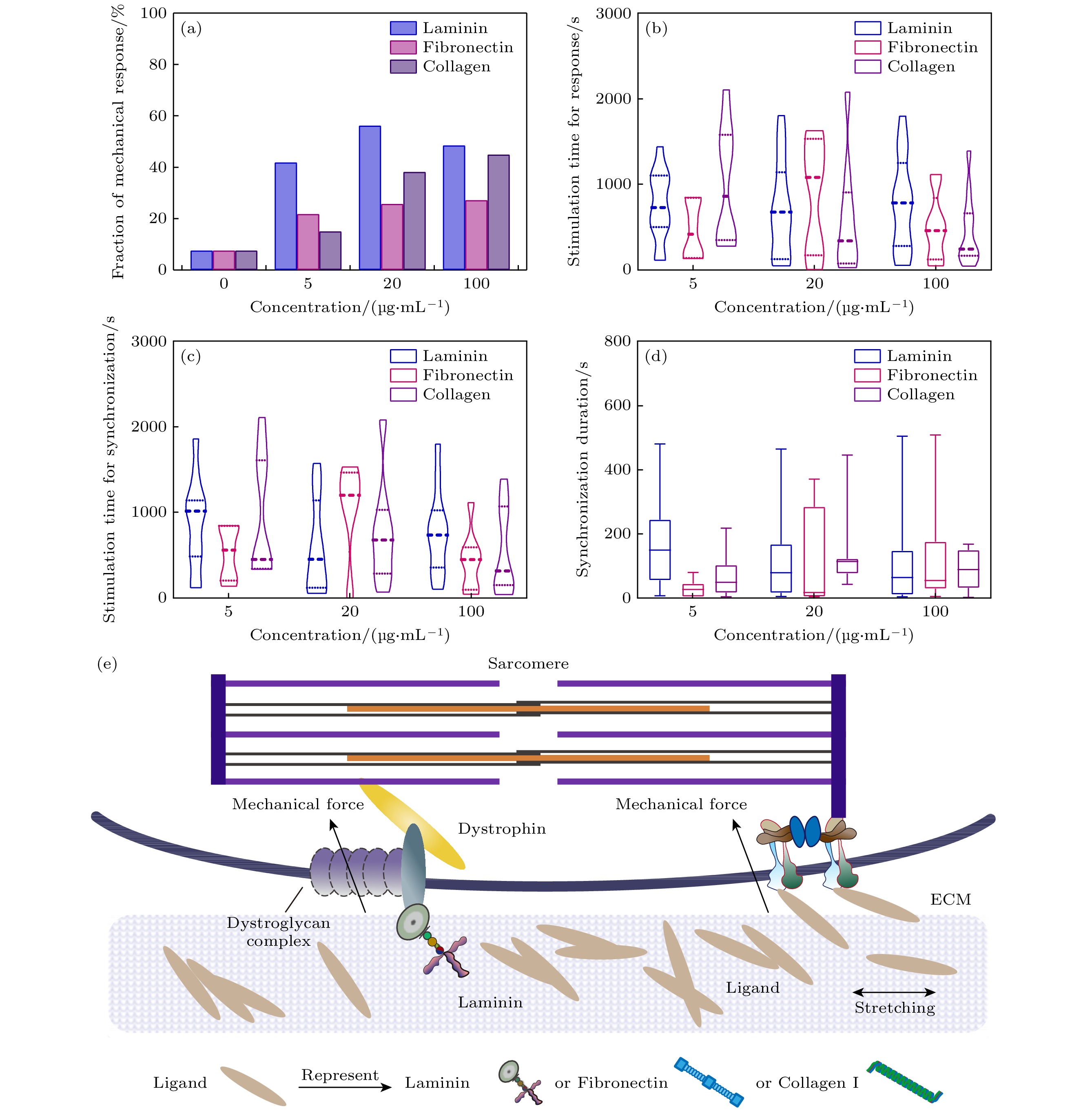
图 4 心肌细胞在不同种类及浓度配体的基质(E = 11 kPa)上发生机械响应行为 (a) 心肌细胞机械响应百分比(n > 31); (b) 心肌细胞产生响应所需的机械刺激时间 (n > 7); (c)心肌细胞与钨针同频所需的机械刺激时间 (n > 5); (d) 心肌细胞同频持续时长(n > 5); (e) 3种不同配体( Laminin / Fibronectin / Collagen I ) 分别与心肌细胞膜受体结合示意图
Figure 4. Mechanical response behavior of cardiomyocytes on the matrix (E = 11 kPa) with different kinds and concentrations of ligands: (a) The fraction of mechanical response of cardiomyocytes (n > 31); (b) the mechanical stimulation time required for cardiomyocytes to respond the stimulation (n > 7); (c) the mechanical stimulation time required for cardiomyocytes to couple with the tungsten probe (n > 5); (d) duration of cardiomyocyte coupling with tungsten probe (n > 5); (e) schematics of three different ligands (Laminin/Fibronectin/Collagen I) binding to myocardial cell membrane receptors.
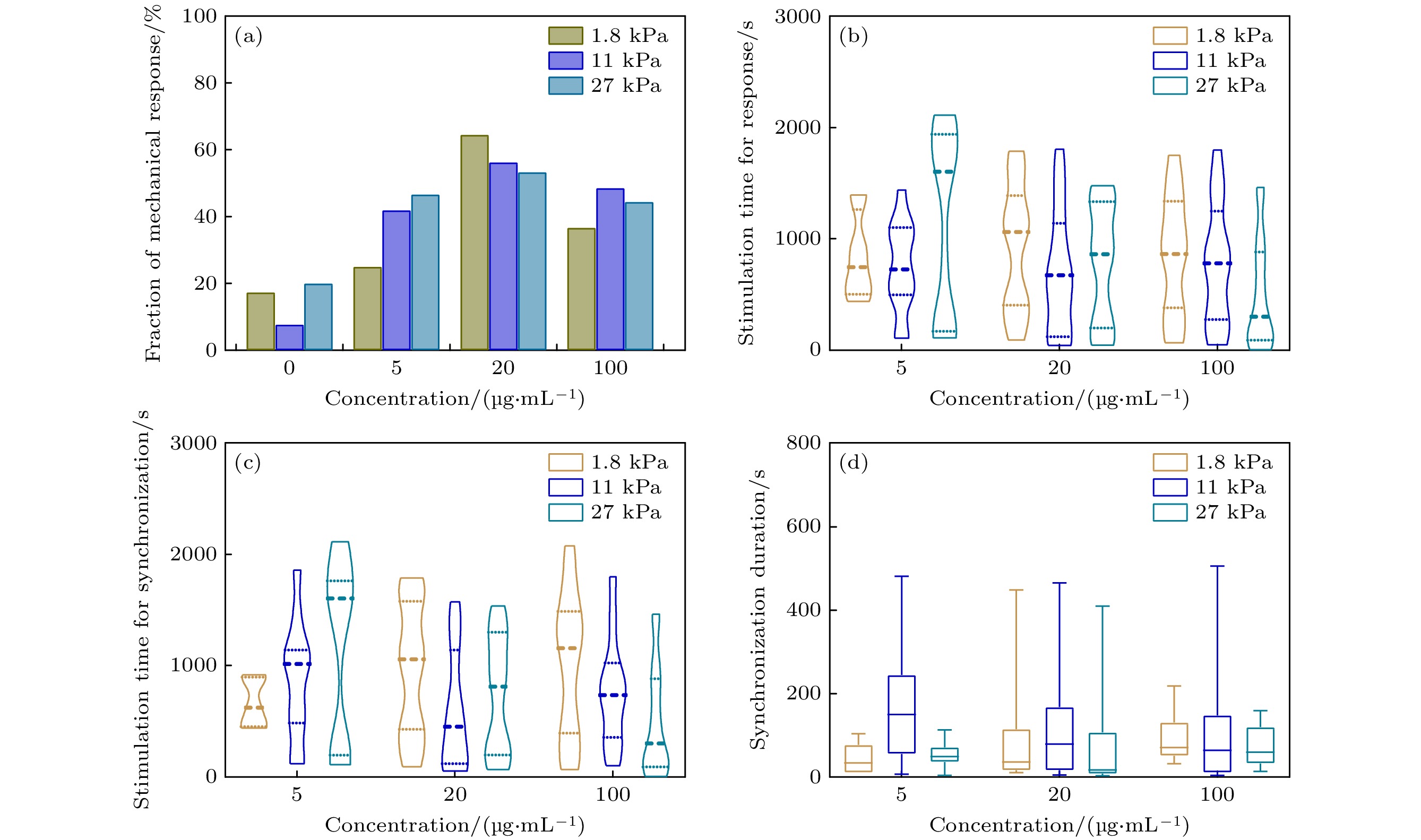
图 5 心肌细胞在不同Laminin浓度和基质硬度上的机械响应行为 (a) 心肌细胞机械响应百分比 (n > 23); (b) 心肌细胞产生响应所需的机械刺激时间 (n > 8); (c) 心肌细胞与钨针同频所需的机械刺激时间 (n > 5); (d)心肌细胞同频持续时长 (n > 5)
Figure 5. Mechanical response behavior of cardiomyocytes at different Laminin concentrations and matrix stiffness: (a) The fraction of mechanical response of cardiomyocytes (n > 23); (b) The mechanical stimulation time required for cardiomyocytes to respond the stimulation (n > 8); (c)The mechanical stimulation time required for cardiomyocytes to couple with the tungsten probe (n > 5); (d) Duration of cardiomyocytes coupling with tungsten probe (n > 5).
-
[1] Dogan A, Parmaksız M, Elçin A E, Elçin Y M 2016 Stem Cell Rev. Rep. 12 202
 Google Scholar
Google Scholar
[2] World Health Organization 2023. Licence: CC BY-NC-SA 3.0 IGO
[3] Thayer J F, Yamamoto S S, Brosschot J F 2010 Int. J. Cardiol. 141 122
 Google Scholar
Google Scholar
[4] Quinn T A, Kohl P 2021 Physiol. Rev. 101 37
 Google Scholar
Google Scholar
[5] De Mello WC 1982 Circ. Res. 51 1
 Google Scholar
Google Scholar
[6] McCain M L, Lee H, Aratyn-Schaus Y, Kléber A G, Parker K K 2012 Proc. Natl. Acad. Sci. U.S.A. 109 9881
 Google Scholar
Google Scholar
[7] Kumar N M, Gilula N B 1996 Cell 84 381
 Google Scholar
Google Scholar
[8] Barr L, Dewey M M, Berger W 1965 J. Gen. Physiol. 48 797
 Google Scholar
Google Scholar
[9] Corrado D, Basso C, Thiene G, et al. 1997 J. Am. Coll. Cardiol. 30 1512
 Google Scholar
Google Scholar
[10] Pelham R J, Wang Y L 1997 Proc. Natl. Acad. Sci. U.S.A. 94 13661
 Google Scholar
Google Scholar
[11] Harris A 1973 Exp. Cell Res. 77 285
 Google Scholar
Google Scholar
[12] Folkman J, Moscona A 1978 Nature 273 345
 Google Scholar
Google Scholar
[13] Wang W Y, Davidson C D, Lin D, Baker B M 2019 Nat. Commun. 10 1186
 Google Scholar
Google Scholar
[14] Isomursu A, Park K Y, Hou J, et al. 2022 Nat. Mater. 21 1081
 Google Scholar
Google Scholar
[15] Adebowale K, Gong Z, Hou J C, Wisdom K M, Garbett D, Lee H P, Nam S, Meyer T, Odde D J, Shenoy V B, Chaudhuri O 2021 Nat. Mater. 20 1290
 Google Scholar
Google Scholar
[16] Bera K, Kiepas A, Godet I, Li Y, Mehta P, Ifemembi B, Paul C D, Sen A, Serra S A, Stoletov K, Tao J, Shatkin G, Lee S J, Zhang Y, Boen A, Mistriotis P, Gilkes D M, Lewis J D, Fan C M, Feinberg A P, Valverde M A, Sun S X, Konstantopoulos K 2022 Nature 611 365
 Google Scholar
Google Scholar
[17] 王璟, 杨根, 刘峰 2015 64 058707
 Google Scholar
Google Scholar
Wang J, Yang G, Liu F 2015 Acta Phys. Sin. 64 058707
 Google Scholar
Google Scholar
[18] Lecuit T, Le Goff L 2007 Nature 450 189
 Google Scholar
Google Scholar
[19] Assoian R K, Klein E A 2008 Trends Cell Biol. 18 347
 Google Scholar
Google Scholar
[20] Wen J H, Vincent L G, Fuhrmann A, Choi Y S, Hribar K C, Taylor-Weiner H, Chen S, Engler A J 2014 Nat. Mater. 13 979
 Google Scholar
Google Scholar
[21] Engler A J, Sen S, Sweeney H L, Discher D E 2006 Cell 126 677
 Google Scholar
Google Scholar
[22] Zhang J S, Wong S H D, Wu X, Lei H, Qin M, Shi P, Wang W, Bian L, Cao Y 2021 Adv. Mater. 33 2105765
 Google Scholar
Google Scholar
[23] Chowdhury F, Na S, Li D, Poh Y C, Tanaka T S, Wang F, Wang N 2010 Nat. Mater. 9 82
 Google Scholar
Google Scholar
[24] Ingber D E, Folkman J 1989 J. Cell Biol. 109 317
 Google Scholar
Google Scholar
[25] Reinhart-King C A, Dembo M, Hammer D A 2008 Biophys. J. 95 6044
 Google Scholar
Google Scholar
[26] Tang X, Bajaj P, Bashir R, Saif T A 2011 Soft Matter 7 6151
 Google Scholar
Google Scholar
[27] Nitsan I, Drori S, Lewis Y E, Cohen S, Tzlil S 2016 Nature Phys. 12 472
 Google Scholar
Google Scholar
[28] Cohen O, Safran S A 2018 Sci. Rep. 8 2237
 Google Scholar
Google Scholar
[29] Bick R J, Snuggs M B, Poindexter B J, Buja L M, Winkle W B V 1998 Cell Adhes. Commun. 6 301
 Google Scholar
Google Scholar
[30] Engler A J, Carag-Krieger C, Johnson C P, Raab M, Tang H Y, Speicher D W, Sanger J W, Sanger J M, Discher D E 2008 J. Cell Sci. 121 3794
 Google Scholar
Google Scholar
[31] Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher D E 2013 Curr. Biol. 23 2434
 Google Scholar
Google Scholar
[32] Jacot J G, McCulloch A D, Omens J H 2008 Biophys. J. 95 3479
 Google Scholar
Google Scholar
[33] Ehler E, Moore-Morris T, Lange S 2013 J. Vis. Exp. e50154
[34] Gittenberger-de Groot A C, Vrancken Peeters M P F M, Mentink M M T, Gourdie R G, Poelmann R E 1998 Circ. Res. 82 1043
 Google Scholar
Google Scholar
[35] Berry M F, Engler A J, Woo Y J, Pirolli T J, Bish L T, Jayasankar V, Morine K J, Gardner T J, Discher D E, Sweeney H L 2006 Am. J. Physiol. Heart Circ. Physiol. 290 H2196
 Google Scholar
Google Scholar
[36] Eisner D A, Choi H S, Díaz M E, O'Neill S C, Trafford A W 2000 Circ. Res. 87 1087
 Google Scholar
Google Scholar
[37] Bers D M 2002 Nature 415 198
 Google Scholar
Google Scholar
[38] 白永强, 唐爱辉, 王世强, 朱 星 2007 56 3607
 Google Scholar
Google Scholar
Bai Y Q, Tang A H, Wang S Q, Zhu X 2007 Acta Phys. Sin. 56 3607
 Google Scholar
Google Scholar
[39] Ross R S, Borg T K 2001 Circ. Res. 88 1112
 Google Scholar
Google Scholar
[40] Israeli-Rosenberg S, Manso A M, Okada H, Ross R S 2014 Circ. Res. 114 572
 Google Scholar
Google Scholar
[41] Tan P M, Buchholz K S, Omens J H, McCulloch A D, Saucerman J J 2017 PLOS Comput. Biol. 13 e1005854
 Google Scholar
Google Scholar
[42] Harvey P A, Leinwand L A 2011 J. of Cell Biol. 194 355
 Google Scholar
Google Scholar
[43] Chen-Izu Y, Izu L T 2017 J. Physiol. 595 3949
 Google Scholar
Google Scholar
[44] Roca-Cusachs P, Gauthier N C, del Rio A, Sheetz M P 2009 Proc. Natl. Acad. Sci. U. S. A. 106 16245
 Google Scholar
Google Scholar
[45] Kechagia J Z, Ivaska J, Roca-Cusachs P 2019 Nat. Rev. Mol. Cell Biol. 20 457
 Google Scholar
Google Scholar
[46] Sun Z, Guo S S, Fässler R 2016 J. Cell Biol. 215 445
 Google Scholar
Google Scholar
[47] Han Y L, Ronceray P, Xu G, Malandrino A, Kamm R D, Lenz M, Broedersz C P, Guo M 2018 Proc. Natl. Acad. Sci. U. S. A. 115 4075
 Google Scholar
Google Scholar
[48] Hall M S, Alisafaei F, Ban E, Feng X, Hui C Y, Shenoy V B, Wu M 2016 Proc. Natl. Acad. Sci. U. S. A. 113 14043
 Google Scholar
Google Scholar
[49] 孙波 2015 64 058201
 Google Scholar
Google Scholar
Sun B 2015 Acta Phys. Sin. 64 058201
 Google Scholar
Google Scholar
[50] Hu J Y, Li J H, Jiang J, Wang L L, Roth J, McGuinness K N, Baum J, Dai W, Sun Y, Nanda V, Xu F 2022 Nat. Commun. 13 6761
 Google Scholar
Google Scholar
[51] Discher D E, Janmey P, Wang Y L 2005 Science 310 1139
 Google Scholar
Google Scholar
[52] Hersch N, Wolters B, Dreissen G, Springer R, Kirchgeßner N, Merkel R, Hoffmann B 2013 Biol. Open 2 351
 Google Scholar
Google Scholar
-
 8-20240095Suppl.pdf
8-20240095Suppl.pdf
Catalog
Metrics
- Abstract views: 7520
- PDF Downloads: 223
- Cited By: 0
















 DownLoad:
DownLoad: