-
多室神经元的精细结构能够同时捕捉时空特性, 具有丰富的响应和内在机理. 本研究基于Pinsky-Rinzel两室神经元模型, 提出多室神经元通道阻塞与噪声对神经元响应状态影响的分析方法. 首先, 钙离子(Ca2+)浓度影响神经递质释放的概率, 对多室神经元的节律性放电具有关键作用, 因此特别引入Ca2+通道阻塞, 构建带离子通道阻塞的多室神经元模型. 其次推导跃迁矩阵等核心参数构建Pinsky-Rinzel神经元Conductance噪声模型, 并与Subunit噪声模型对比. 最终, 通过单参数Hopf分岔解释各个离子通道阻塞下的动力学过程; 双参数分岔显示钾离子(K+)的Hopf节点随输入电流呈近似线性递增关系, 而钠离子(Na+)则近似为线性下降和指数上升两阶段; 通过变异系数等指标发现K+适度阻塞促进放电, Na+阻塞抑制放电, Ca2+阻塞总体上促进放电的特性. 另外, 在低于阈值信号刺激时, 两种噪声模型均产生随机共振, Conductance模型表现出更强的编码能力. 本研究揭示了离子通道阻塞与噪声在神经信号传递中的复杂机制, 为研究神经信息编码提供新的视角和工具.
-
关键词:
- Pinsky-Rinzel神经元 /
- 通道噪声 /
- 通道阻塞 /
- Hopf分岔分析
The fine structure of multi-compartment neurons can simultaneously capture both temporal and spatial characteristics, offering rich responses and intrinsic mechanisms. However, current studies of the effects of channel blockage and noise on neuronal response states are mainly limited to single-compartment neurons. This study introduces an analytical method to explore theintrinsic mechanism of channel blockage and noise effects on the response states of multi-compartment neurons, by using the smooth Pinsky-Rinzel two-compartment neuron model as a case study. Potassium, sodium, and calcium ion channel blockage coefficient are separately introduced to develop a smooth Pinsky-Rinzel neuron model with ion channel blockage. Methods such as single-parameter bifurcation analysis, double-parameter bifurcation analysis, coefficient of variation, and frequency characteristics analysis are utilized to examine the effects of various ion channel blockages on neuronal response states. Additionally, smooth Pinsky-Rinzel neuron Subunit noise model and conductance noise model are constructed to investigate their response characteristics by using interspike interval analysis and coefficient of variation indicators. Subthreshold stimulation is used to explore the presence of stochastic resonance phenomena. Single-parameter bifurcation analysis of the ion channel blockage model elucidates the dynamic processes of two torus bifurcations and limit point bifurcations in Pinsky-Rinzel neuron firing under potassium ion blocking. Double-parameter bifurcation analysis reveals a nearly linear increase in the Hopf bifurcation node of potassium ions with input current, whereas sodium ions exhibit a two-stage pattern of linear decline followed by exponential rise. The analysis of average firing frequency and coefficient of variation indicates that the moderate potassium channel blockage promotes firing, sodium channel blockage inhibits firing, and calcium channel blockage shows the complex characteristics but mainly promotes firing. Subthreshold stimulation of the channel noise model demonstrates the stochastic resonance phenomena in both models, accompanied by more intense chaotic firing, highlighting the positive role of noise in neural signal transmission. The interspike interval and coefficient of variation indicators show consistent variation levels for both noise models, with the conductance model displaying greater sensitivity to membrane area and stronger encoding capabilities. This study analyzes the general frequency characteristics of potassium and sodium ions in a multi-compartment neuron model through ion channel blocking model, providing special insights into the unique role of calcium ions. Further, the study explores stochastic resonance by using ion channel noise model, supporting the theory of noise-enhanced signal processing and offering new perspectives and tools for future studying complex information encoding in neural systems. By constructing an ion channel blockage model, the effects of potassium and sodium ions on the frequency characteristics of multi-compartment neurons are analyzed and the special influences of calcium ions are revealed. Using the ion channel noise model, the stochastic resonance is investigated, supporting the theory that the noise enhances signal processing. This research offers a new perspective and tool for studying the complex information encoding in neural systems.-
Keywords:
- Pinsky-Rinzel neuron /
- channel noise /
- channel blocking /
- Hopf bifurcation analysis
[1] Xu Y, Jia Y, Ge M Y, Lu L L, Yang L J, Zhan X 2018 Neurocomputing 283 196
 Google Scholar
Google Scholar
[2] Zhou X Y, Xu Y, Wang G W, Jia Y 2020 Cogn. Neurodyn. 14 569
 Google Scholar
Google Scholar
[3] Zhu J L, Qiu H, Guo W L 2023 Biophys. J. 122 496
 Google Scholar
Google Scholar
[4] Yan H R, Yan J Q, Yu L C, Shao Y F 2024 Chin. Phys. B 33 058801
 Google Scholar
Google Scholar
[5] 吴静, 潘春宇 2022 71 048701
 Google Scholar
Google Scholar
Wu J, Pan C Y 2022 Acta Phys. Sin. 71 048701
 Google Scholar
Google Scholar
[6] Narahashi T, Moore J W 1968 J. Gen. Physiol. 51 93
 Google Scholar
Google Scholar
[7] 王荣, 吴莹, 刘少宝 2013 62 220504
 Google Scholar
Google Scholar
Wang R, Wu Y, Liu S B 2013 Acta Phys. Sin. 62 220504
 Google Scholar
Google Scholar
[8] 刘少宝, 吴莹, 郝忠文, 李银军, 贾宁 2012 61 020503
 Google Scholar
Google Scholar
Liu S B, Wu Y, Hao Z W, Li Y J, Jia N 2012 Acta Phys. Sin. 61 020503
 Google Scholar
Google Scholar
[9] Adair R K 2003 Proc. Natl. Acad. Sci. USA 100 12099
 Google Scholar
Google Scholar
[10] Xiao F L, Fu Z Y, Jia Y, Yang L J 2023 Chaos Soliton. Fract. 166 112969
 Google Scholar
Google Scholar
[11] 梁艳美, 陆博, 古华光 2022 71 230502
 Google Scholar
Google Scholar
Liang Y M, Lu B, Gu H G 2022 Acta Phys. Sin. 71 230502
 Google Scholar
Google Scholar
[12] Gong Y B, Hao Y H, Lin X, Wang L, Ma X G 2011 BioSystems 106 76
 Google Scholar
Google Scholar
[13] Longtin A 1993 J. Stat. Phys. 70 309
 Google Scholar
Google Scholar
[14] Faisal A A, Selen L P J, Wolpert D M 2008 Nat. Rev. Neurosci. 9 292
 Google Scholar
Google Scholar
[15] Ermentrout G B, Galán R F, Urban N N 2008 Trends Neurosci. 31 428
 Google Scholar
Google Scholar
[16] Chow C C, White J A 1996 Biophys. J. 71 3013
 Google Scholar
Google Scholar
[17] Mahapatra C, Samuilik I 2024 Mathematics 12 1149
 Google Scholar
Google Scholar
[18] van Rossum M C W, O’Brien B J, Smith R G 2003 J. Neurophysiol. 89 2406.
 Google Scholar
Google Scholar
[19] Chen Y, Yu L C, Qin S M 2008 Phys. Rev. E 78 051909
 Google Scholar
Google Scholar
[20] Stacey W C, Durand D M 2001 J. Neurophysiol. 86 1104
 Google Scholar
Google Scholar
[21] Lu L, Jia Y, Kirunda J B, Xu Y, Ge M Y, Pei Q M, Yang L J 2019 Nonlinear Dyn. 95 1673
 Google Scholar
Google Scholar
[22] Sengupta B, Laughlin S B, Niven J E 2010 Phys. Rev. E 81 011918
 Google Scholar
Google Scholar
[23] Maisel B, Lindenberg K 2017 Phys. Rev. E 95 022414
 Google Scholar
Google Scholar
[24] Anderson D F, Ermentrout B, Thomas P J 2015 J. Comput. Neurosci. 38 67
 Google Scholar
Google Scholar
[25] Kilinc D, Demir A 2017 IEEE Trans. Biomed. Circuits Syst. 11 958
 Google Scholar
Google Scholar
[26] Fox R F, Lu Y 1994 Phys. Rev. E 49 3421
 Google Scholar
Google Scholar
[27] Goldwyn J H, Shea-Brown E 2011 PloS Comput. Biol. 7 e1002247
 Google Scholar
Google Scholar
[28] Goldwyn J H, Imennov N S, Famulare M, Shea-Brown E 2011 Phys. Rev. E 83 041908
 Google Scholar
Google Scholar
[29] Huang Y D, Rüdiger S, Shuai J W 2015 Phys. Biol. 12 061001
 Google Scholar
Google Scholar
[30] Cox D R 2017 The Theory of Stochastic Processes (New York: Routledge) pp1–408
[31] Linaro D, Storace M, Giugliano M 2011 PloS Comput. Biol. 7 e1001102
 Google Scholar
Google Scholar
[32] Tuckerman L S, Barkley D 2000 Bifurcation Analysis for Timesteppers (New York: Springer) pp453–466
[33] Guckenheimer J, Labouriau J S 1993 Bull. Math. Biol. 55 937
 Google Scholar
Google Scholar
[34] 黎丽, 赵志国, 古华光 2022 71 050504
 Google Scholar
Google Scholar
Li L, Zhao Z G, Gu H G 2022 Acta Phys. Sin. 71 050504
 Google Scholar
Google Scholar
[35] Guo Z H, Li Z J, Wang M J, Ma M L 2023 Chin. Phys. B 32 038701
 Google Scholar
Google Scholar
[36] Xie Y, Chen L N, Kang Y M, Aihara K 2008 Phys. Rev. E 77 061921
 Google Scholar
Google Scholar
[37] Erhardt A H, Mardal K A, Schreiner J E 2020 J. Comput. Neurosci. 48 229
 Google Scholar
Google Scholar
[38] Hu B, Xu M B, Zhu L Y, Lin J H, Wang Z Z, Wang D J, Zhang D M 2022 J. Theor. Biol. 536 110979
 Google Scholar
Google Scholar
[39] Wang Z Z, Hu B, Zhu L Y, Lin J H, Xu M B, Wang D J 2022 Commun. Nonlinear Sci. Numer. Simul. 114 106614
 Google Scholar
Google Scholar
[40] Ward M, Rhodes O 2022 Front. Neurosci. 16 881598
 Google Scholar
Google Scholar
[41] Stöckel A, Eliasmith C 2022 Neuromorph. Comput. Eng. 2 024011
 Google Scholar
Google Scholar
[42] Nomura M, Chen T Q, Tang C, Todo Y, Sun R, Li B, Tang Z 2024 Electronics 13 1367
 Google Scholar
Google Scholar
[43] Kühn S, Gallinat J 2014 Hum. Brain Mapp. 35 1129
 Google Scholar
Google Scholar
[44] Biagini G, D’Arcangelo G, Baldelli E, D’Antuono M, Tancredi V, Avoli M 2005 Neuromol. Med. 7 325
 Google Scholar
Google Scholar
[45] Sendrowski K, Sobaniec W 2013 Pharmacol. Rep. 65 555
 Google Scholar
Google Scholar
[46] Pinsky P F, Rinzel J 1994 J. Comput. Neurosci. 1 39
 Google Scholar
Google Scholar
[47] Taxidis J, Coombes S, Mason R, Owen M R 2012 Hippocampus 22 995
 Google Scholar
Google Scholar
[48] Kamondi A, Acsády L, Wang X J, Buzsáki G 1998 Hippocampus 8 244
 Google Scholar
Google Scholar
[49] Booth V, Bose A 2001 J. Neurophysiol. 85 2432
 Google Scholar
Google Scholar
[50] Mainen Z F, Sejnowski T J 1996 Nature 382 363
 Google Scholar
Google Scholar
[51] Zhang S M, Yang Q, Ma C X, Wu J B, Li H Z, Tan K C 2024 Proceedings of the AAAI Conference on Artificial Intelligence Vancouver, Canada, February 20–27, 2024 p16838
[52] Hahn P J, Durand D M 2001 J. Comput. Neurosci. 11 5
 Google Scholar
Google Scholar
[53] Atherton L A, Prince L Y, Tsaneva A K 2016 J. Comput. Neurosci. 41 91
 Google Scholar
Google Scholar
[54] Harnett M T, Makara J K, Spruston N, Kath W L, Magee J C 2012 Nature 491 599
 Google Scholar
Google Scholar
[55] Clarke S G, Scarnati M S, Paradiso K G 2016 J. Neurosci. 36 11559
 Google Scholar
Google Scholar
[56] Koudriavtsev A B, Jameson R F, Linert W 2001 The Law of Mass Action (Berlin: Springer Science & Business Media) pp1–441
[57] Johnston D, Wu S M S 1994 Foundations of Cellular Neurophysiology (Cambridge, MA: MIT Press) pp1–710
-
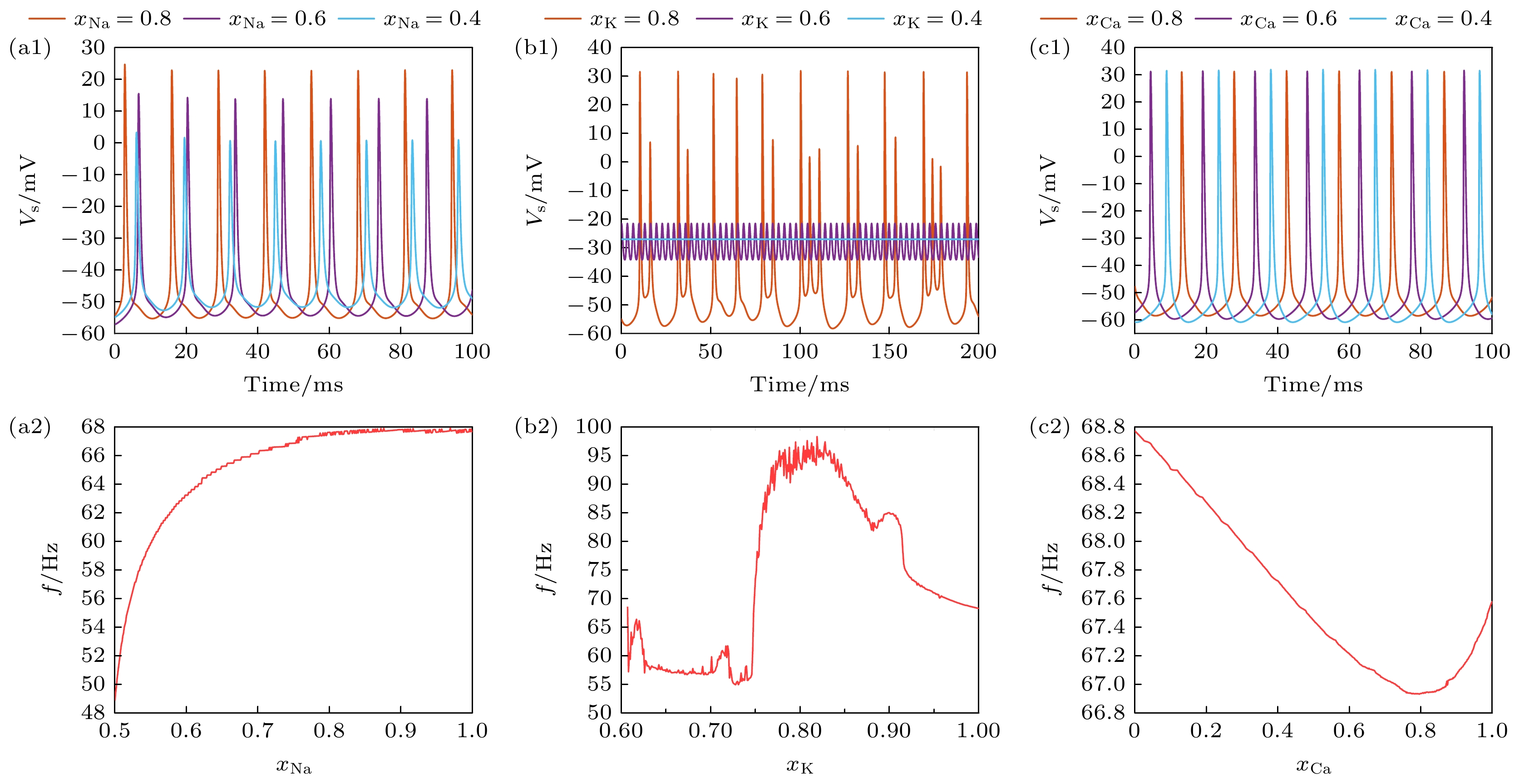
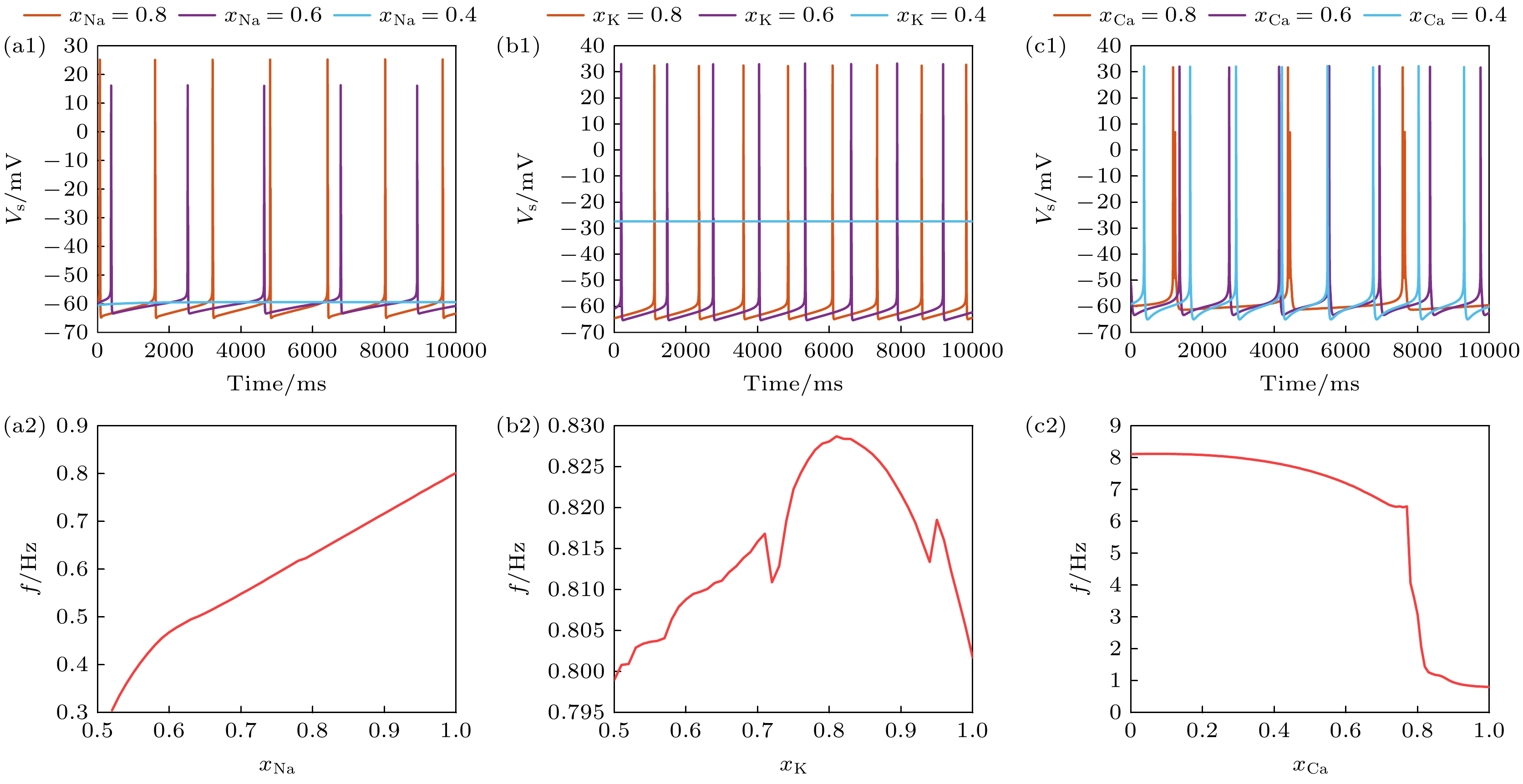
图 1 在$ {I_{{\text{Sapp}}}} = 0.3 {\text{ mA}} $时, 不同离子通道阻塞程度下PR神经元动作电位序列与放电频率 (a1) Na+通道阻塞下的动作电位序列; (a2)频率随Na+通道阻塞的变化; (b1) K+通道阻塞下PR神经元动作电位序列; (b2) 频率随K+通道阻塞的变化; (c1) Ca2+通道阻塞下PR神经元动作电位序列; (c2) 频率随Ca2+通道阻塞的变化, (a1), (b1), (c1)中红色实线代表阻塞系数为0.8, 绿色实线代表阻塞系数为0.6, 蓝色实线代表阻塞系数为0.4
Fig. 1. Action potential sequence and discharge frequency of PR neurons under different degree of ion channel obstruction, when $ {I_{{\text{Sapp}}}} = 0.3 {\text{ mA}} $: (a1) Sequence of action potentials induced by obstruction of Na+ ion channels; (a2) variation in frequency with obstruction of Na+ ion channels; (b1) action potential sequence of PR neurons blocked by K+ ion channels; (b2) variation in frequency with obstruction of K+ ion channels; Action potential sequence of PR neurons under obstruction of (c1) Ca2+ ion channels; (c2) variation in frequency with obstruction of Ca2+ ion channels. In (a1), (b1) and (c1), the red solid line represents the blocking coefficient of 0.8, the green solid line represents the blocking coefficient of 0.6, and the blue solid line represents the blocking coefficient of 0.4.
图 2 $ {I_{{\text{Sapp}}}} = 5 {\text{ mA}} $时, 不同离子通道阻塞程度下PR神经元动作电位序列与放电频率 (a1) Na+通道阻塞下的动作电位序列; (a2)频率随Na+通道阻塞的变化; (b1) K+通道阻塞下PR神经元动作电位序列; (b2) 频率随K+通道阻塞的变化; (c1) Ca2+通道阻塞下PR神经元动作电位序列; (c2) 频率随Ca2+通道阻塞的变化
Fig. 2. Action potential sequence and discharge frequency of PR neurons under different degree of ion channel obstruction, when $ {I_{{\text{Sapp}}}} = 5 {\text{ mA}} $: (a1) Sequence of action potentials induced by obstruction of Na+ ion channels; (a2) variation in frequency with obstruction of Na+ ion channels; (b1) action potential sequence of PR neurons blocked by K+ ion channels; (b2) variation in frequency with obstruction of K+ ion channels; (c1) action potential sequence of PR neurons under obstruction of Ca2+ ion channels; (c2) variation in frequency with obstruction of Ca2+ ion channels.
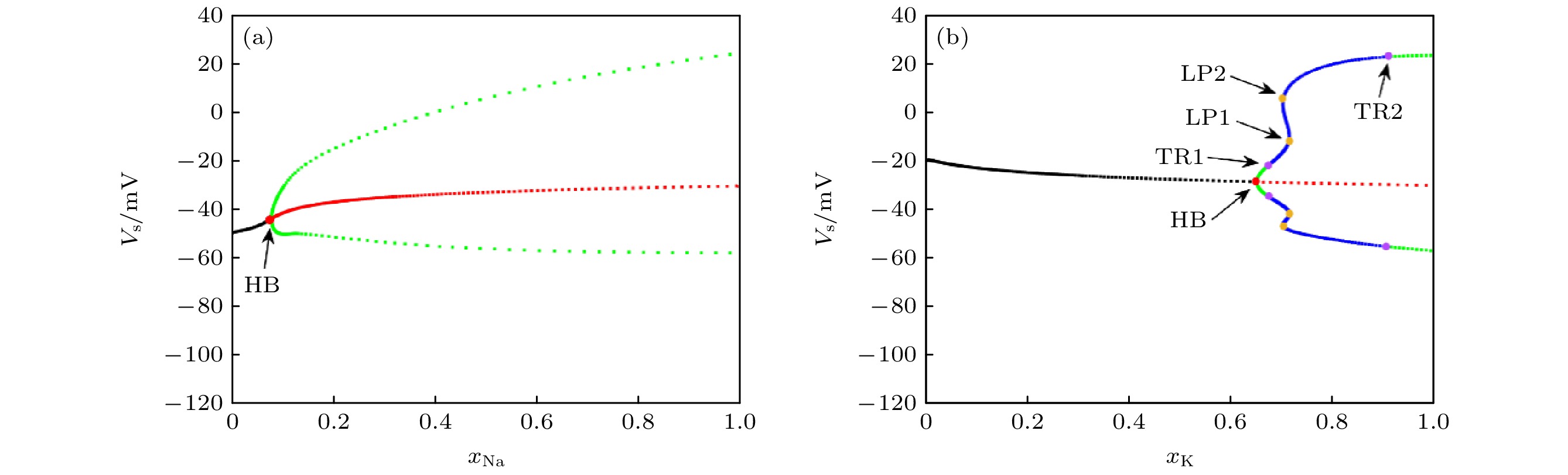
图 3 $ {I_{{\text{Sapp}}}} = 3 {\text{ mA}} $时, K+, Na+通道阻塞的分岔分析 (a) K+通道阻塞下的分岔分析结果; (b) Na+通道阻塞下的分岔分析结果, 图中HB表示Hopf分岔(Hopf bifurcation)节点, 表示系统中一个稳定的固定点变为不稳定的固定点, 同时在该点附近产生一个稳定的极限环; TR表示环面分岔(torus bifurcation), 系统中一个稳定的极限环进一步发生变化, 形成一个准周期轨道; LP表示极限点分岔(limit point bifurcation), 也称为鞍结分岔(fold bifurcation), 表示一个稳定和一个不稳定的点, 随着参数变化而碰撞并消失; 图中黑色表示稳定状态, 红色表示不稳定状态, 绿色代表周期状态, 蓝色代表非周期状态
Fig. 3. Bifurcation analysis of K+, Na+ ion channel obstruction when $ {I_{{\text{Sapp}}}} = 3 {\text{ mA}} $: (a) Results of a bifurcation analysis based on K+ ion channel obstruction; (b) results of a bifurcation analysis in the sense of Na+ ion channel obstruction. In the diagram, HB represents a Hopf bifurcation node, which indicates that a stable fixed point in the system becomes an unstable fixed point, while a stable limit cycle emerges near this point; TR represents a Torus bifurcation, where a stable limit cycle in the system undergoes further changes, leading to the formation of a quasi-periodic orbit; LP represents a limit point bifurcation, also known as a fold bifurcation, indicating a collision and disappearance of a stable and an unstable point as the parameter changes. In the diagram, black represents stable states, red represents unstable states, green represents periodic states, and blue represents non-periodic states.
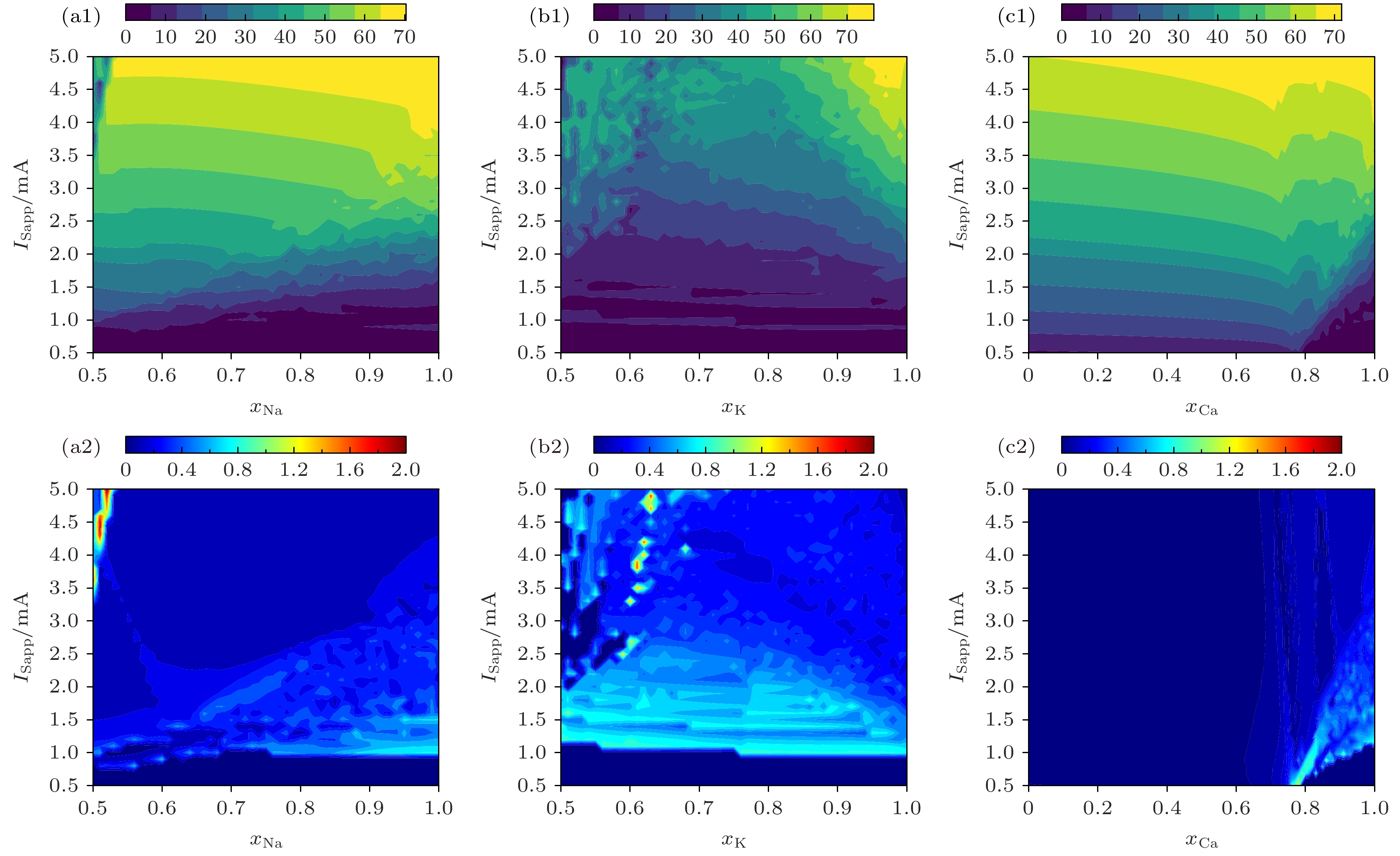
图 4 PR神经元频率特性与输入电流、离子通道阻塞的关系 (a1) 频率与Na+通道阻塞、输入电流的关系; (a2) CV与Na+通道阻塞的关系; (b1)频率与K+通道阻塞、输入电流的关系; (b2) CV与K+通道阻塞的关系; (c1)频率与Ca2+通道阻塞、输入电流的关系; (c2) CV与Ca2+通道阻塞的关系
Fig. 4. Relationship between frequency characteristics of PR neurons and input current and ion channel blocking: (a1) Relationship of frequency with Na+ ion channel obstruction and input current; (a2) relationship between CV and obstruction of Na+ ion channels; (b1) relationship of frequency with K+ ion channel obstruction and input current; (b2) relationship between CV and obstruction of K+ ion channels; (c1) relationship between frequency and Ca2+ ion channel obstruction and input current; (c2) relationship between CV and obstruction of Ca2+ ion channels.
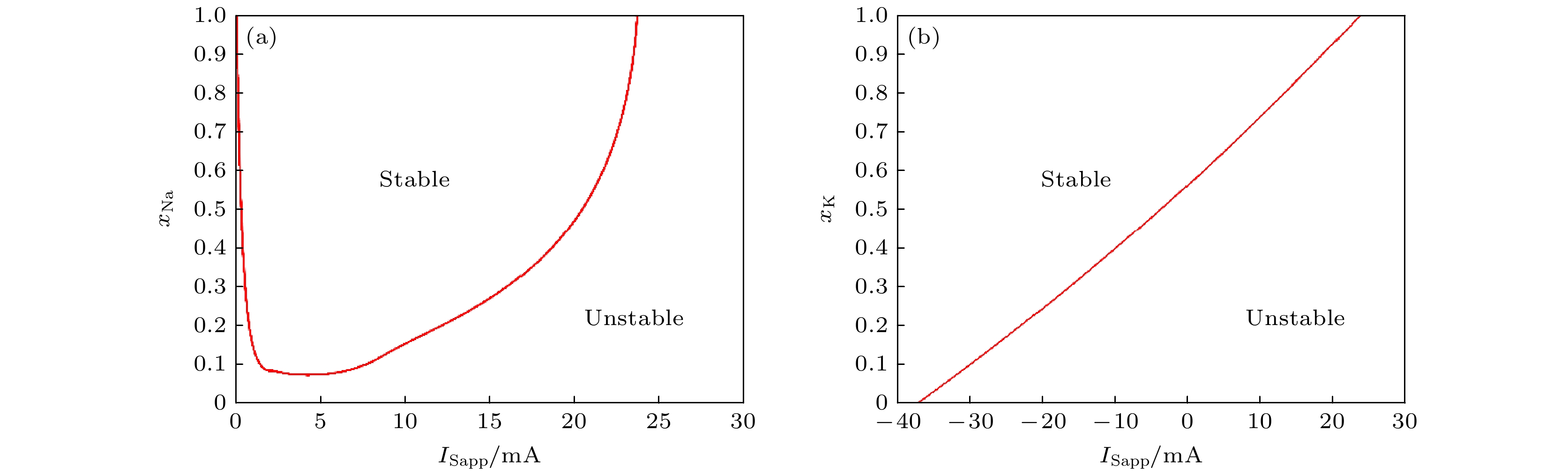
图 5 PR神经元双参数分岔分析结果 (a) PR神经元与Na+通道阻塞、输入电流的双参数分岔分析; (b) PR神经元与K+通道阻塞、输入电流的双参数分岔分析
Fig. 5. Results of two-parameter bifurcation analysis of neurons: (a) Two-parameter bifurcation analysis of PR neuron and Na+ ion channel obstruction and input current; (b) two-parameter bifurcation analysis of PR neuron and K+ ion channel obstruction and input current
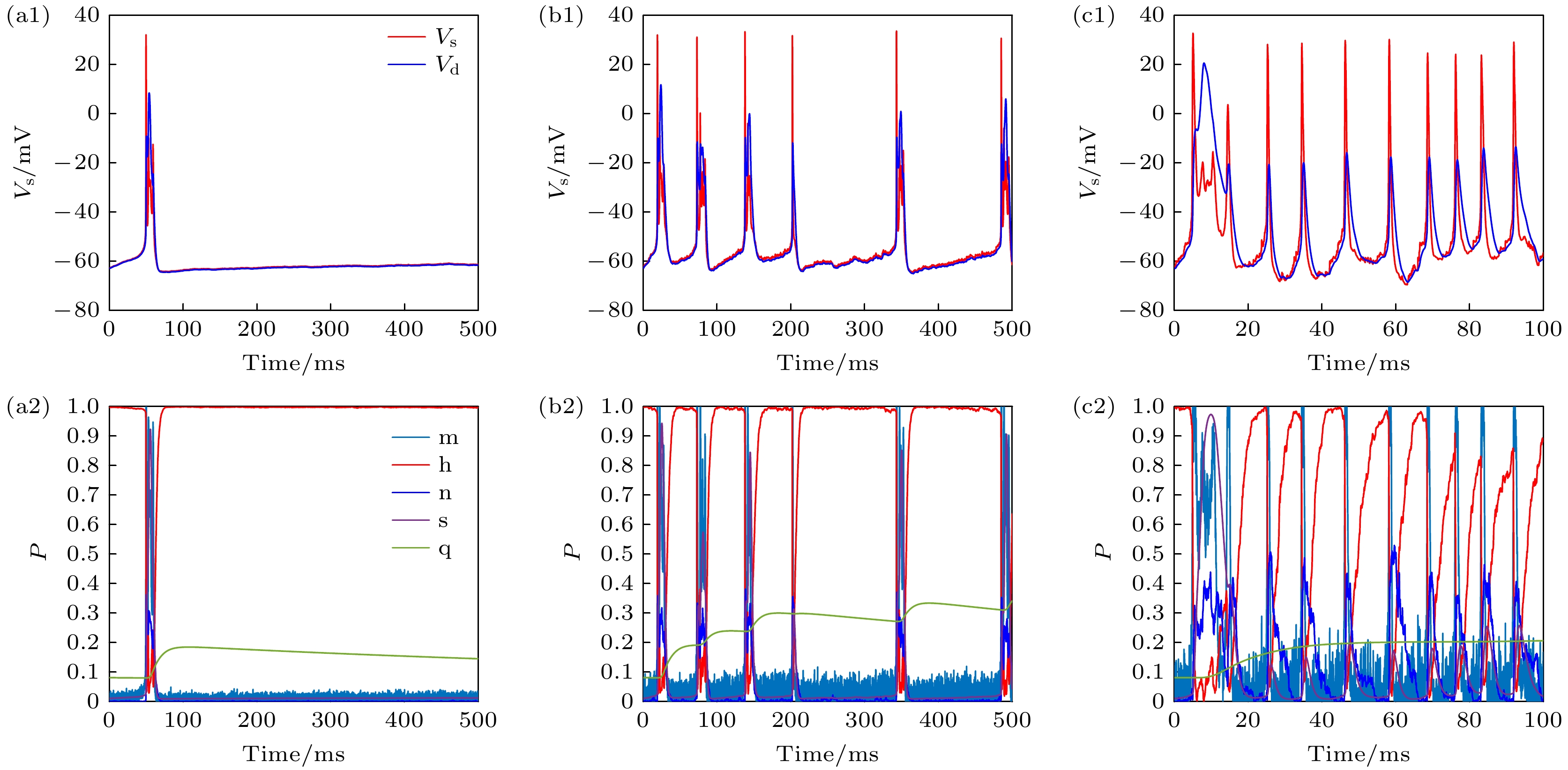
图 6 PR神经元Subunit噪声模型不同膜面积下的动作电位序列和离子通道参数变化 (a1) Area = 100, 低离子通道噪声水平下的动作电位; (a2) Area = 100, 低离子通道噪声水平下各个离子通道参数的变化; (b1) Area = 10, 中离子通道噪声水平下的动作电位; (b2) Area = 10, 中离子通道噪声水平下各个离子通道参数的变化; (c1) Area = 1, 高离子通道噪声水平下的动作电位; (c2) Area = 1, 高离子通道噪声水平下各个离子通道参数的变化
Fig. 6. Changes of action potential sequence and ion channel parameters under different membrane area of neuron Subunit noise model: (a1) Area = 100, action potential at low ion channel noise level; (a2) Area = 100, change of parameters of each ion channel at low ion channel noise level; (b1) Area = 10, action potential at medium ion channel noise level; (b2) Area = 10, change of parameters of each ion channel at medium ion channel noise level; (c1) Area = 1, action potential at high ion channel noise level; (c2) Area = 1, change of parameters of each ion channel at high ion channel noise level.
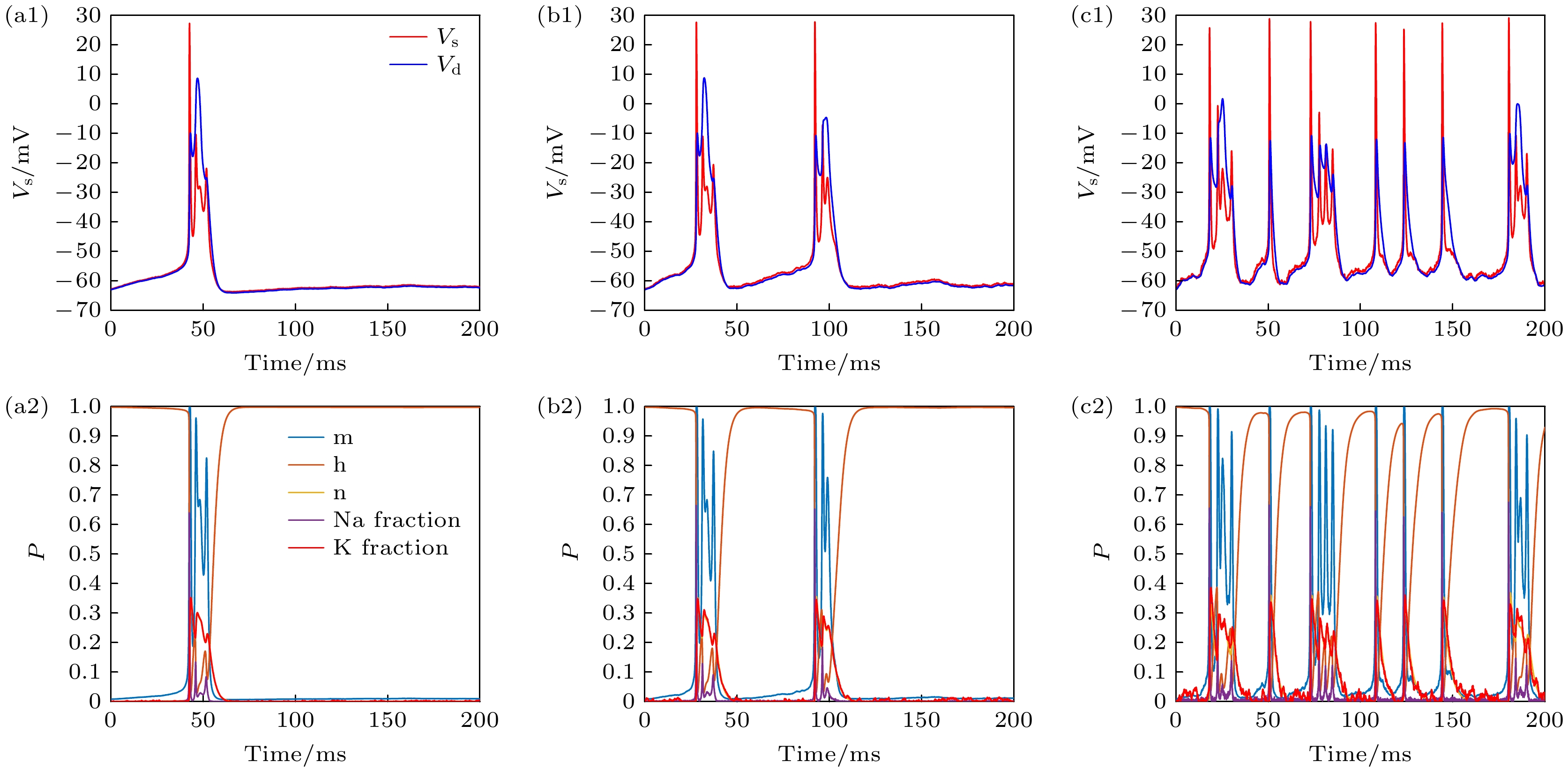
图 8 PR神经元Conductance噪声模型不同膜面积下的动作电位序列和离子通道参数变化 (a1) Area = 10, 低离子通道噪声水平下的动作电位; (a2) Area = 10, 低离子通道噪声水平下各个离子通道参数的变化; (b1) Area = 1, 中离子通道噪声水平下的动作电位; (b2) Area = 1, 中离子通道噪声水平下各个离子通道参数的变化; (c1) Area = 0.1, 高离子通道噪声水平下的动作电位; (c2) Area = 0.1, 高离子通道噪声水平下各个离子通道参数的变化
Fig. 8. Changes of action potential sequence and ion channel parameters under different membrane area of neuron Conductance noise model: (a1) Area = 10, action potential at low ion channel noise level; (a2) Area = 10, parameters of each ion channel at low ion channel noise level; (b1) Area = 1, action potential at medium ion channel noise level; (b2) Area = 1, parameters of each ion channel at medium ion channel noise level; (c1) Area = 0.1, action potential at high ion channel noise level; (c2) Area = 0.1, parameters of each ion channel at high ion channel noise level.
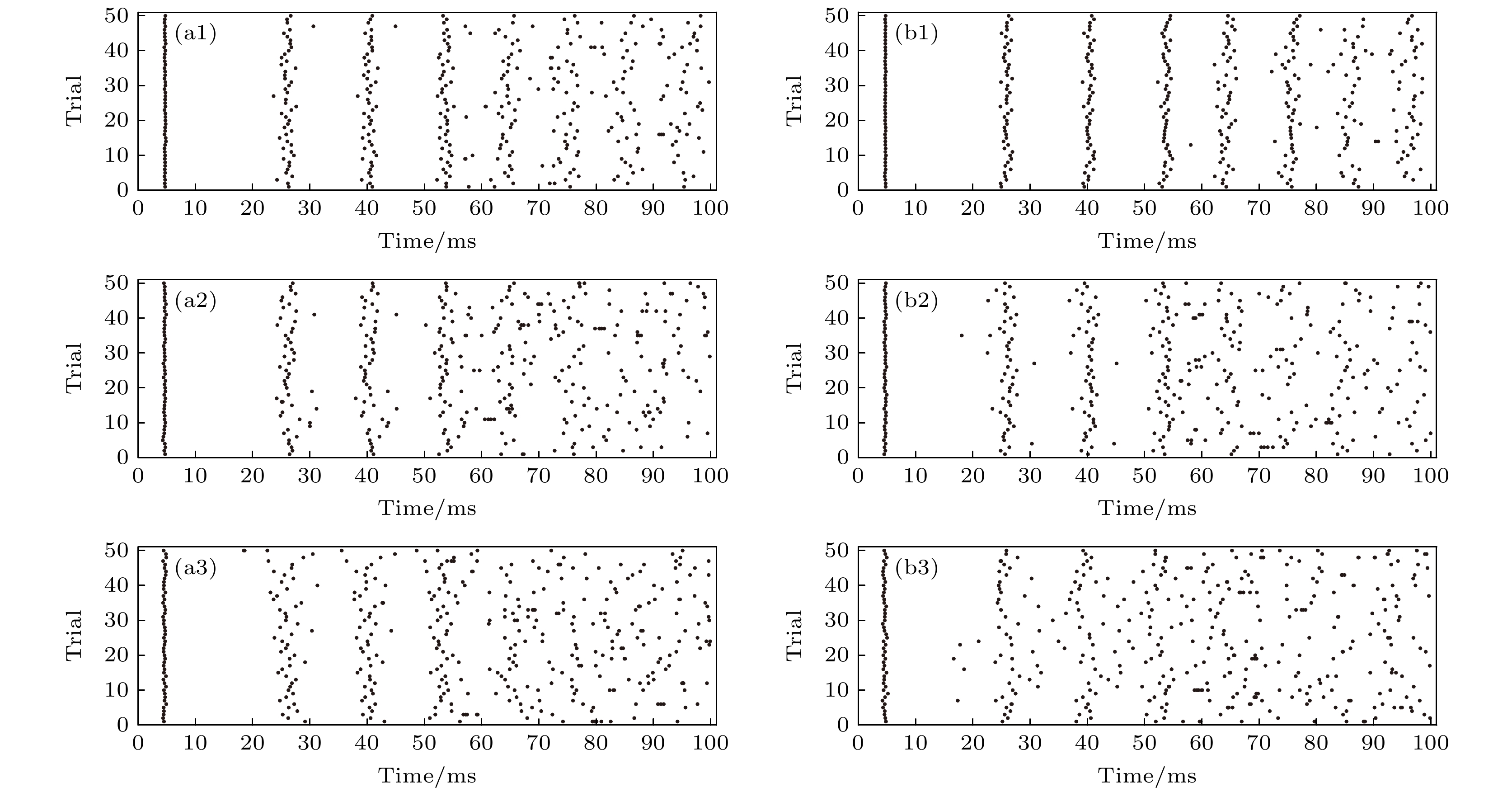
图 10 PR神经元Subunit和Conductance噪声模型在不同膜面积下的动作电位序列栅格图 (a1) Area = 100, 低噪声水平下Subunit噪声引导的栅格图; (a2) Area = 10, 中噪声水平下Subunit噪声的栅格图; (a3) Area = 1, 高噪声水平下Subunit噪声引导的栅格图; (b1) Area = 100, 低噪声水平下Conductance噪声引导的栅格图; (b2) Area = 10, 中噪声水平下Conductance噪声引导的栅格图; (b3) Area = 1, 高噪声水平下Conductance噪声引导的栅格图; 图中横坐标代表时间, 纵坐标代表实验次数, 在本实验中我们进行了50次放电
Fig. 10. Raster plots of action potential sequences for PR neuron Subunits and Conductance noise model at different membrane areas: (a1) Raster plot driven by Subunit noise with Area = 100, under low noise level; (a2) raster plot driven by Subunit noise with Area = 10, under medium noise level; (a3) raster plot driven by Subunit noise with Area = 1, under high noise level; (b1) raster plot driven by Conductance noise with Area = 100, under low noise level; (b2) raster plot driven by Conductance noise with Area = 10, under medium noise level; (b3) raster plot driven by Conductance noise with Area = 1, under high noise level. In the figure, the horizontal coordinate represents the time, and the vertical coordinate represents the number of experiments. In this experiment, we carried out 50 discharges.
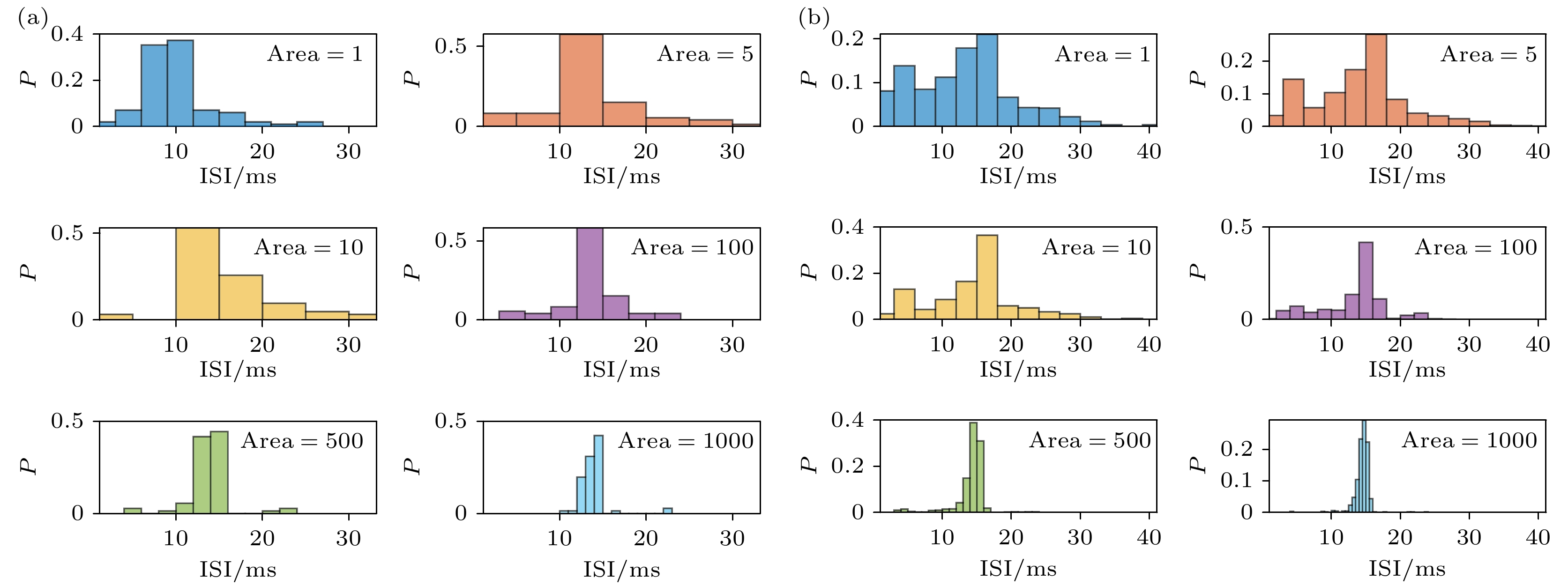
图 11 PR神经元不同噪声模型和不同噪声程度下的ISI分布, 横坐标代表相邻脉冲之间的间隔(ISI), 纵坐标P表示ISI值落在横坐标对应值上的概率 (a) Subunit噪声模型; (b) Conductance噪声模型
Fig. 11. The ISI distribution of PR neurons under different noise models and different noise levels, the horizontal axis represents the interval between adjacent spikes (ISI), and the vertical axis represents the probability of the ISI values falling within the corresponding bins on the horizontal axis, denoted as P: (a) Subunit noise model; (b) Conductance noise model.
表 1 PR神经元模型中参数取值
Table 1. Parameter value in PR neuron model.
参数 取值 参数 取值 ${C_{\text{m}}}$/(μF·cm–2) 3 $ {g_{\text{L}}} $/(μF·cm–2) 0.1 $ {g_{{\text{Na}}}} $/(μF·cm–2) 30 $ {g_{\text{C}}} $/(μF·cm–2) 2.1 $ {g_{{\text{KDR}}}} $/(μF·cm–2) 15 $ {V_{{\text{Na}}}} $/mV 60 $ {g_{{\text{KCa}}}} $/(μF·cm–2) 15 $ {V_{\text{K}}} $/mV –75 $ {g_{{\text{KAHP}}}} $/(μF·cm–2) 0.8 $ {V_{{\text{Ca}}}} $/mV 80 $ {g_{{\text{Ca}}}} $/(μF·cm–2) 10 $ {V_{\text{L}}} $/mV –60 表 2 PR神经元本征噪声模型中系数取值
Table 2. Coefficient value of PR neuron intrinsic noise model.
系数 钠 钾 钙 $\sigma _1^2$ $\dfrac{1}{N}{m^4}h(1 - h)$ $\dfrac{1}{N}n(1 - n)$ $\dfrac{2}{N}{s^3}(1 - s)$ $\sigma _2^2$ $\dfrac{2}{N}{m^3}{h^2}(1 - m)$ — $\dfrac{1}{N}{s^2}{(1 - s)^2}$ $\sigma _3^2$ $\dfrac{1}{N}{m^2}{h^2}{(1 - m)^2}$ — — $\sigma _4^2$ $\dfrac{2}{N}{m^3}h(1 - m)(1 - h)$ — — $\sigma _5^2$ $\dfrac{1}{N}{m^2}h{(1 - m)^2}(1 - h)$ — — 表 3 PR神经元本征噪声模型中时间常数取值
Table 3. Value of time constant in the intrinsic noise model of PR neuron.
系数 钠 钾 钙 系数 钠 钾 钙 ${\tau _1}$ ${\tau _h}$ ${\tau _n}$ ${\tau _s}$ ${\tau _4}$ $\dfrac{{{\tau _m}{\tau _h}}}{{{\tau _m} + {\tau _h}}}$ — — ${\tau _2}$ ${\tau _m}$ — $ {{{\tau _s}}}/{2}$ ${\tau _5}$ $\dfrac{{{\tau _m}{\tau _h}}}{{{\tau _m} + 2{\tau _h}}}$ — — ${\tau _3}$ ${{{\tau _m}}}/{2}$ — — — — — — 表 4 钾离子通道分岔分析的节点
Table 4. Nodes of bifurcation analysis of potassium ion channels.
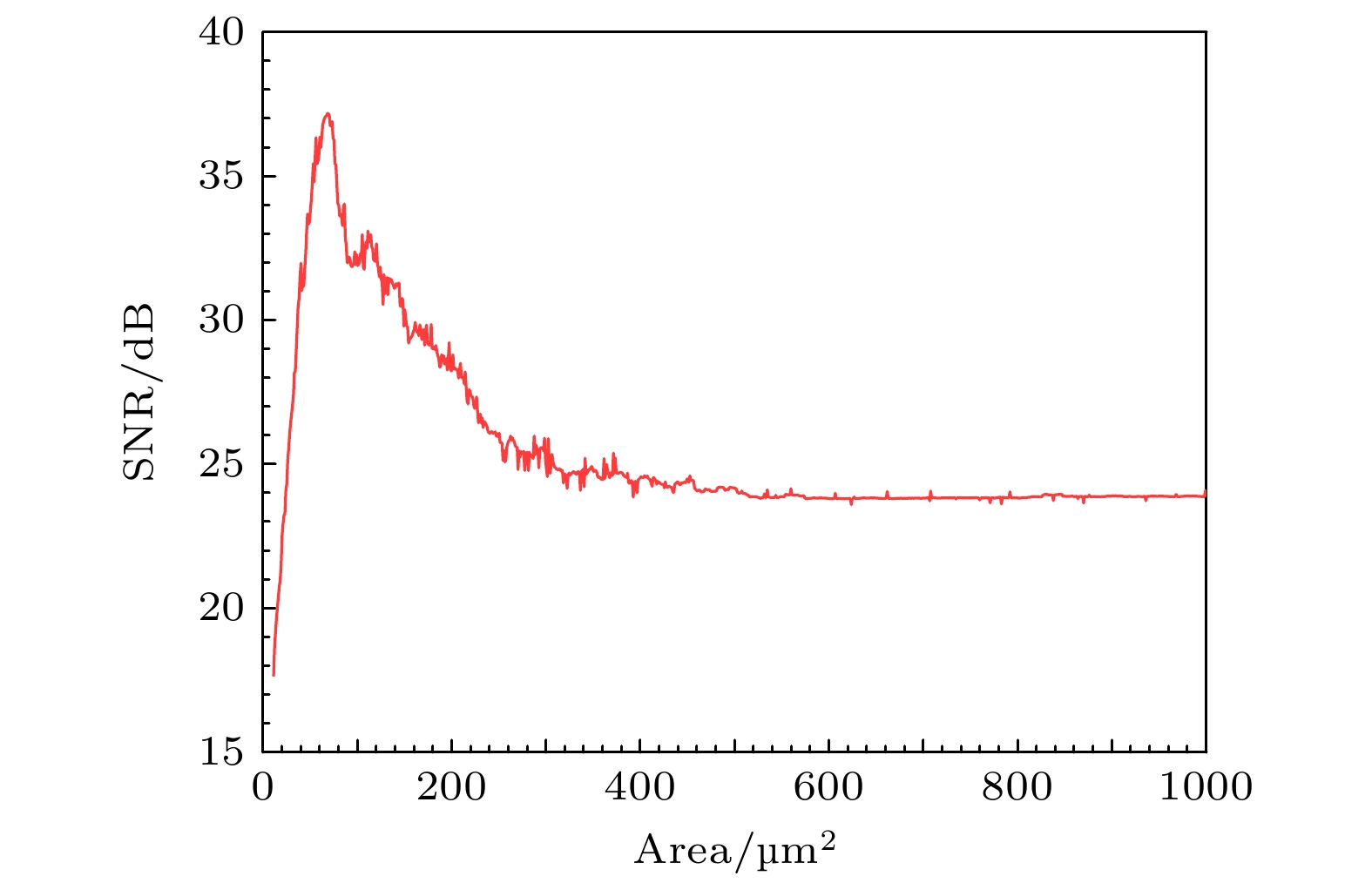
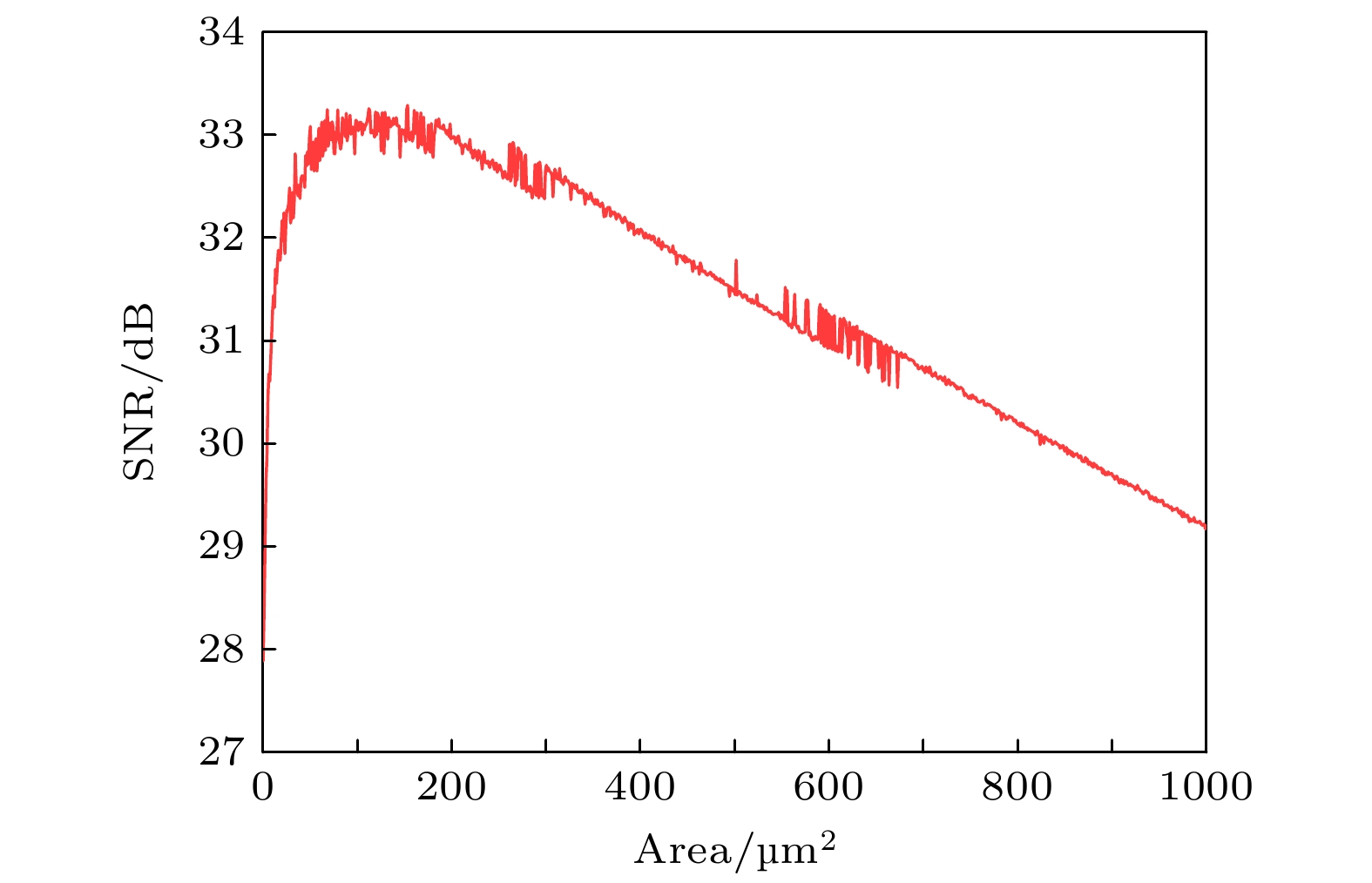
节点名称 HB TR1 LP1 LP2 TR2 $ {x_{\text{K}}} $的值 0.6507 0.6745 0.7146 0.7021 0.9104 表 5 PR神经元两种噪声模型下的信息熵
Table 5. Information entropy of PR neurons under two different noise models.
噪声模型 膜面积/μm2 单位面积 1 10 100 500 1000 Subunit(信息熵)/bits 2.39 2.17 2.06 1.81 1.66 1.87 Conductance(信息熵)/bits 3.31 3.18 3.16 3.12 2.96 3.13 -
[1] Xu Y, Jia Y, Ge M Y, Lu L L, Yang L J, Zhan X 2018 Neurocomputing 283 196
 Google Scholar
Google Scholar
[2] Zhou X Y, Xu Y, Wang G W, Jia Y 2020 Cogn. Neurodyn. 14 569
 Google Scholar
Google Scholar
[3] Zhu J L, Qiu H, Guo W L 2023 Biophys. J. 122 496
 Google Scholar
Google Scholar
[4] Yan H R, Yan J Q, Yu L C, Shao Y F 2024 Chin. Phys. B 33 058801
 Google Scholar
Google Scholar
[5] 吴静, 潘春宇 2022 71 048701
 Google Scholar
Google Scholar
Wu J, Pan C Y 2022 Acta Phys. Sin. 71 048701
 Google Scholar
Google Scholar
[6] Narahashi T, Moore J W 1968 J. Gen. Physiol. 51 93
 Google Scholar
Google Scholar
[7] 王荣, 吴莹, 刘少宝 2013 62 220504
 Google Scholar
Google Scholar
Wang R, Wu Y, Liu S B 2013 Acta Phys. Sin. 62 220504
 Google Scholar
Google Scholar
[8] 刘少宝, 吴莹, 郝忠文, 李银军, 贾宁 2012 61 020503
 Google Scholar
Google Scholar
Liu S B, Wu Y, Hao Z W, Li Y J, Jia N 2012 Acta Phys. Sin. 61 020503
 Google Scholar
Google Scholar
[9] Adair R K 2003 Proc. Natl. Acad. Sci. USA 100 12099
 Google Scholar
Google Scholar
[10] Xiao F L, Fu Z Y, Jia Y, Yang L J 2023 Chaos Soliton. Fract. 166 112969
 Google Scholar
Google Scholar
[11] 梁艳美, 陆博, 古华光 2022 71 230502
 Google Scholar
Google Scholar
Liang Y M, Lu B, Gu H G 2022 Acta Phys. Sin. 71 230502
 Google Scholar
Google Scholar
[12] Gong Y B, Hao Y H, Lin X, Wang L, Ma X G 2011 BioSystems 106 76
 Google Scholar
Google Scholar
[13] Longtin A 1993 J. Stat. Phys. 70 309
 Google Scholar
Google Scholar
[14] Faisal A A, Selen L P J, Wolpert D M 2008 Nat. Rev. Neurosci. 9 292
 Google Scholar
Google Scholar
[15] Ermentrout G B, Galán R F, Urban N N 2008 Trends Neurosci. 31 428
 Google Scholar
Google Scholar
[16] Chow C C, White J A 1996 Biophys. J. 71 3013
 Google Scholar
Google Scholar
[17] Mahapatra C, Samuilik I 2024 Mathematics 12 1149
 Google Scholar
Google Scholar
[18] van Rossum M C W, O’Brien B J, Smith R G 2003 J. Neurophysiol. 89 2406.
 Google Scholar
Google Scholar
[19] Chen Y, Yu L C, Qin S M 2008 Phys. Rev. E 78 051909
 Google Scholar
Google Scholar
[20] Stacey W C, Durand D M 2001 J. Neurophysiol. 86 1104
 Google Scholar
Google Scholar
[21] Lu L, Jia Y, Kirunda J B, Xu Y, Ge M Y, Pei Q M, Yang L J 2019 Nonlinear Dyn. 95 1673
 Google Scholar
Google Scholar
[22] Sengupta B, Laughlin S B, Niven J E 2010 Phys. Rev. E 81 011918
 Google Scholar
Google Scholar
[23] Maisel B, Lindenberg K 2017 Phys. Rev. E 95 022414
 Google Scholar
Google Scholar
[24] Anderson D F, Ermentrout B, Thomas P J 2015 J. Comput. Neurosci. 38 67
 Google Scholar
Google Scholar
[25] Kilinc D, Demir A 2017 IEEE Trans. Biomed. Circuits Syst. 11 958
 Google Scholar
Google Scholar
[26] Fox R F, Lu Y 1994 Phys. Rev. E 49 3421
 Google Scholar
Google Scholar
[27] Goldwyn J H, Shea-Brown E 2011 PloS Comput. Biol. 7 e1002247
 Google Scholar
Google Scholar
[28] Goldwyn J H, Imennov N S, Famulare M, Shea-Brown E 2011 Phys. Rev. E 83 041908
 Google Scholar
Google Scholar
[29] Huang Y D, Rüdiger S, Shuai J W 2015 Phys. Biol. 12 061001
 Google Scholar
Google Scholar
[30] Cox D R 2017 The Theory of Stochastic Processes (New York: Routledge) pp1–408
[31] Linaro D, Storace M, Giugliano M 2011 PloS Comput. Biol. 7 e1001102
 Google Scholar
Google Scholar
[32] Tuckerman L S, Barkley D 2000 Bifurcation Analysis for Timesteppers (New York: Springer) pp453–466
[33] Guckenheimer J, Labouriau J S 1993 Bull. Math. Biol. 55 937
 Google Scholar
Google Scholar
[34] 黎丽, 赵志国, 古华光 2022 71 050504
 Google Scholar
Google Scholar
Li L, Zhao Z G, Gu H G 2022 Acta Phys. Sin. 71 050504
 Google Scholar
Google Scholar
[35] Guo Z H, Li Z J, Wang M J, Ma M L 2023 Chin. Phys. B 32 038701
 Google Scholar
Google Scholar
[36] Xie Y, Chen L N, Kang Y M, Aihara K 2008 Phys. Rev. E 77 061921
 Google Scholar
Google Scholar
[37] Erhardt A H, Mardal K A, Schreiner J E 2020 J. Comput. Neurosci. 48 229
 Google Scholar
Google Scholar
[38] Hu B, Xu M B, Zhu L Y, Lin J H, Wang Z Z, Wang D J, Zhang D M 2022 J. Theor. Biol. 536 110979
 Google Scholar
Google Scholar
[39] Wang Z Z, Hu B, Zhu L Y, Lin J H, Xu M B, Wang D J 2022 Commun. Nonlinear Sci. Numer. Simul. 114 106614
 Google Scholar
Google Scholar
[40] Ward M, Rhodes O 2022 Front. Neurosci. 16 881598
 Google Scholar
Google Scholar
[41] Stöckel A, Eliasmith C 2022 Neuromorph. Comput. Eng. 2 024011
 Google Scholar
Google Scholar
[42] Nomura M, Chen T Q, Tang C, Todo Y, Sun R, Li B, Tang Z 2024 Electronics 13 1367
 Google Scholar
Google Scholar
[43] Kühn S, Gallinat J 2014 Hum. Brain Mapp. 35 1129
 Google Scholar
Google Scholar
[44] Biagini G, D’Arcangelo G, Baldelli E, D’Antuono M, Tancredi V, Avoli M 2005 Neuromol. Med. 7 325
 Google Scholar
Google Scholar
[45] Sendrowski K, Sobaniec W 2013 Pharmacol. Rep. 65 555
 Google Scholar
Google Scholar
[46] Pinsky P F, Rinzel J 1994 J. Comput. Neurosci. 1 39
 Google Scholar
Google Scholar
[47] Taxidis J, Coombes S, Mason R, Owen M R 2012 Hippocampus 22 995
 Google Scholar
Google Scholar
[48] Kamondi A, Acsády L, Wang X J, Buzsáki G 1998 Hippocampus 8 244
 Google Scholar
Google Scholar
[49] Booth V, Bose A 2001 J. Neurophysiol. 85 2432
 Google Scholar
Google Scholar
[50] Mainen Z F, Sejnowski T J 1996 Nature 382 363
 Google Scholar
Google Scholar
[51] Zhang S M, Yang Q, Ma C X, Wu J B, Li H Z, Tan K C 2024 Proceedings of the AAAI Conference on Artificial Intelligence Vancouver, Canada, February 20–27, 2024 p16838
[52] Hahn P J, Durand D M 2001 J. Comput. Neurosci. 11 5
 Google Scholar
Google Scholar
[53] Atherton L A, Prince L Y, Tsaneva A K 2016 J. Comput. Neurosci. 41 91
 Google Scholar
Google Scholar
[54] Harnett M T, Makara J K, Spruston N, Kath W L, Magee J C 2012 Nature 491 599
 Google Scholar
Google Scholar
[55] Clarke S G, Scarnati M S, Paradiso K G 2016 J. Neurosci. 36 11559
 Google Scholar
Google Scholar
[56] Koudriavtsev A B, Jameson R F, Linert W 2001 The Law of Mass Action (Berlin: Springer Science & Business Media) pp1–441
[57] Johnston D, Wu S M S 1994 Foundations of Cellular Neurophysiology (Cambridge, MA: MIT Press) pp1–710
计量
- 文章访问数: 2387
- PDF下载量: 91
- 被引次数: 0













 下载:
下载: