-
采用基于密度泛函理论的第一性原理方法, 计算了
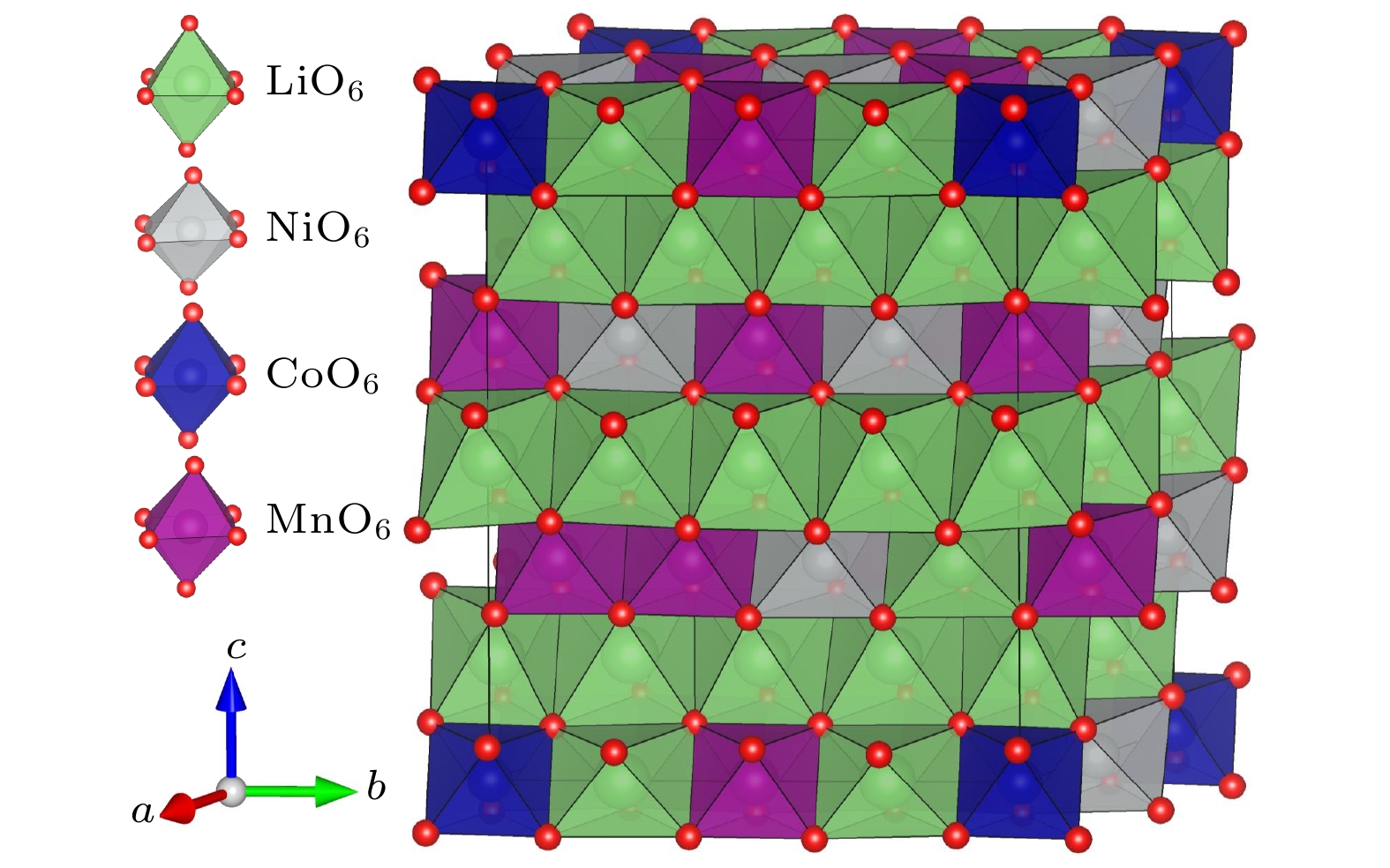
$ R\bar{3}m $ 相的富锂锰基三元正极材料Li1.208Ni0.333Co0.042Mn0.417O2的晶体结构、电子结构以及缺陷性质. 结果表明, Li1.208Ni0.333Co0.042Mn0.417O2是一种具有直接带隙的磁性半导体材料, 其价带顶是O的px, py, pz轨道与Ni的dxy, dyz, dxz原子轨道之间的杂化, 导带底除了有价带顶的特征外, 还有部分的Ni-$ {3\mathrm{d}}_{{x}^{2}-{y}^{2}} $ 和Mn-$ {3\mathrm{d}}_{{x}^{2}-{y}^{2}} $ , Mn-$ {3\mathrm{d}}_{yz} $ 轨道参与杂化. 差分电荷密度图显示, 金属原子与O原子之间的成键方式是共价键和离子键的混合. 本文还计算了脱去单个过渡金属原子的空位形成能. 脱去Mn原子时的缺陷体系的体积变化最大, 而脱去Co原子的缺陷体系的体积则几乎不变. 得到的金属空位形成能的大小依次为Ef (Mn-空位) > Ef (Co-空位) > Ef (Ni-空位). 差分电荷密度图还显示, 空位的产生仅强烈影响了空位附近的部分O原子, 体现了金属空位影响的局域性特征.Lithium-rich manganese-based ternary cathode material for lithium-ion batteries, Li1.208Ni0.333Co0.042Mn0.417O2, has excellent structural stability and electrochemical stability due to its high Ni content. In order to understand the physical properties of this lithium-rich material, its crystal structure, electronic structure and defect properties are calculated by employing the first-principles method based on the density functional theory. The obtained electronic structure shows that Li1.208Ni0.333Co0.042Mn0.417O2 is a magnetic semiconductor with a direct band gap of 0.47 eV. The analysis of the electronic state suggests that the electronic state at the valence band maximum (VBM) is the hybridization of px, py, pz orbitals of oxygen and the dxy, dyz, dxz orbitals of Ni-atom. The electronic state at the conduction band minimum has similar characteristics to those at the VBM, however, part of Ni-${3{\rm{d}}}_{{x}^{2}-{y}^{2}}$ and Mn-${3{\rm{d}}}_{{x}^{2}-{y}^{2}}$ , and Mn-${3{\rm{d}}}_{yz}$ also contribute to the electronic hybridizations. The charge density difference calculations indicate that the bonding between O and transition metal atoms are through the mixture of covalent bond with ionic bond. The vacancy formation of a single metal atom is also calculated. The results show that the volumes of the defect systems containing metal vacancies are all reduced in comparison with the volume of perfect lattice. The volume change is the largest for the formation of Mn-vacancy, while the volume is almost unchanged with Co atoms extracted. The vacancy formation energies of the metals are Ef (Mn) > Ef (Co) > Ef (Ni), and the vacancy formation energy of Mn is significantly higher than those of Ni and Co, indicating that the presence of Mn provides a major structural stability for the material. The calculated charge density differences also show that the formation of metal vacancies influences only the charge distribution of the oxygen atoms around the vacancy, showing the local character of the vacancy effect. Since the formation of metal vacancy breaks the bonding between the metal and the surrounding oxygen atoms, the O-2p states near the Fermi surface are significantly increased as shown in the calculated electronic density of states. Such a picture suggests that the electrons on oxygen atoms in vicinity of the metal vacancies become freer.-
Keywords:
- Li-rich Mn-based ternary material /
- electronic structures /
- defect properties /
- first principles calculations
[1] Whittingham M S 2004 Chem. Rev. 104 4271
 Google Scholar
Google Scholar
[2] Hong J, Gwon H, Jung S K, Ku K, Kang K 2015 J. Electrochem. Soc. 162 A2447
 Google Scholar
Google Scholar
[3] He P, Yu H J, Li D, Zhou H S 2012 J. Mater. Chem. 22 3680
 Google Scholar
Google Scholar
[4] Lu J, Chen Z H, Ma Z F, Pan F, Curtiss L A, Amine K 2016 Nat. Nanotechnol. 11 1031
 Google Scholar
Google Scholar
[5] Deng Y W, Feng C L, Jiaqiang E, Wei K X, Zhang B, Zhang Z Q, Han D D, Zhao X H, Xu W W 2019 Energy 183 869
 Google Scholar
Google Scholar
[6] Cano Z P, Banham D, Ye S Y, Hintennach A, Lu J, Fowler M, Chen Z W 2018 Nat. Energy 3 279
 Google Scholar
Google Scholar
[7] Zeng X Q, Li M, Abd El-Hady D, Alshitari W, Al-Bogami A S, Lu J, Amine K 2019 Adv. Energy Mater. 9 1900161
 Google Scholar
Google Scholar
[8] Goodenough J B, Park K S 2013 J. Am. Chem. Soc. 135 1167
 Google Scholar
Google Scholar
[9] Hou P Y, Zhang H Z, Zi Z Y, Zhang L Q, Xu X J 2017 J. Mater. Chem. A 5 4254
 Google Scholar
Google Scholar
[10] Hou P Y, Yin J M, Ding M, Huang J Z, Xu X J 2017 Small 13 1701802
 Google Scholar
Google Scholar
[11] Kim H, Lee E J, Sun Y K 2014 Mater. Today 17 285
 Google Scholar
Google Scholar
[12] Lee J K, Oh C, Kim N, Hwang J Y, Sun Y K 2016 J. Mater. Chem. A 4 5366
 Google Scholar
Google Scholar
[13] Lee J H, Yoon C S, Hwang J Y, Kim S J, Maglia F, Lamp P, Myung S T, Sun Y K 2016 Energy Environ. Sci. 9 2152
 Google Scholar
Google Scholar
[14] Lu Z H, MacNeil D D, Dahn J R 2001 Electrochem. Solid-State Lett. 4 A191
 Google Scholar
Google Scholar
[15] Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D 2011 Energy Environ. Sci. 4 3243
 Google Scholar
Google Scholar
[16] Lu Z H, MacNeil D D, Dahn J R 2001 Electrochem. Solid-State Lett. 4 A200
 Google Scholar
Google Scholar
[17] Thackeray M M, David W I F, Bruce P G, Goodenough J B 1983 Mater. Res. Bull. 18 461
 Google Scholar
Google Scholar
[18] Manthiram A, Chemelewski K, Lee E S 2014 Energy Environ. Sci. 7 1339
 Google Scholar
Google Scholar
[19] Padhi A K, Nanjundaswamy K S, Goodenough J B 1997 J. Electrochem. Soc. 144 1188
 Google Scholar
Google Scholar
[20] Yuan L X, Wang Z H, Zhang W X, Hu X L, Chen J T, Huang Y H, Goodenough J B 2011 Energy Environ. Sci. 4 269
 Google Scholar
Google Scholar
[21] Nyten A, Kamali S, Haggstrom L, Gustafsson T, Thomas J O 2006 J. Mater. Chem. 16 2266
 Google Scholar
Google Scholar
[22] Ohzuku T, Nagayama M, Tsuji K, Ariyoshi K 2011 J. Mater. Chem. 21 10179
 Google Scholar
Google Scholar
[23] Lee J, Zhang Q, Kim J, Dupre N, Avdeev M, Jeong M, Yoon W S, Gu L, Kang B 2020 Adv. Energy Mater. 10 1902231
 Google Scholar
Google Scholar
[24] Wang J, He X, Paillard E, Laszczynski N, Li J, Passerini S 2016 Adv. Energy Mater. 6 1600906
 Google Scholar
Google Scholar
[25] Zheng H, Hu Z, Liu P, Xu W, Xie Q, He W, Luo Q, Wang L, Gu D, Qu B 2020 Energy Storage Mater. 25 76
 Google Scholar
Google Scholar
[26] Liu W, Oh P, Liu X, Lee M J, Cho W, Chae S, Kim Y, Cho J 2015 Angew Chem. Int. Ed. 54 4440
 Google Scholar
Google Scholar
[27] Gu M, Belharouak I, Genc A, Wang Z G, Wang D P, Amine K, Gao F, Zhou G W, Thevuthasan S, Baer D R, Zhang J G, Browning N D, Liu J, Wang C M 2012 Nano Lett. 12 5186
 Google Scholar
Google Scholar
[28] Kresse G, Furthmuller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[29] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[31] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[32] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[34] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[35] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[36] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[37] Chong S K, Liu Y N, Yan W W, Chen Y Z 2016 RSC. Adv. 6 53662
 Google Scholar
Google Scholar
[38] Sun G, Yu F D, Que L F, Deng L, Wang M J, Jiang Y S, Shao G, Wang Z B 2019 Nano Energy 66 104102
 Google Scholar
Google Scholar
[39] Wu S Q, Cai N L, Zhu Z Z, Yang Y 2008 Electrochim. Acta 53 7915
 Google Scholar
Google Scholar
[40] Hu C H, Yang Y, Zhu Z Z 2010 Solid State Commun. 150 669
 Google Scholar
Google Scholar
[41] Turner D E, Zhu Z Z, Chan C T, Mo K M 1997 Phys. Rev. B 55 13842
 Google Scholar
Google Scholar
-
图 3 (a) Li1.208Ni0.333Co0.042Mn0.417O2材料的总态密度和各原子分态密度; (b)—(d) 金属空位形成前后材料中空位周围的6个氧原子的2p电子态密度和, 分别用黑色和红色实线表示
Fig. 3. (a) Total and atomic-decomposed partial density of states for Li1.208Ni0.333Co0.042Mn0.417O2; (b)–(d) sum of the density of states of 2p electronic states of the six oxygen atoms around the M-vacancy before and after the formation of M vacancy, respectively. Black and red solid lines represent DOSs before and after the vacancy formation, respectively.
图 4 材料中三种过渡金属差分电荷密度对比 (a)—(c) 空位形成前; (d)—(f) 空位形成后. 所画的平面是金属附近4个O所在的平面. 图中实线和虚线分别表示电荷聚集
$ (\Delta \rho > 0) $ 与电荷移出$ (\Delta \rho < 0) $ Fig. 4. Contour plots of the charge density differences: (a)–(c) Before the M-vacancy formation; (d)–(f) after the M-vacancy formation. The solid lines and the dot lines represent the charge accumulation (∆ρ > 0) and the charge depletion (∆ρ < 0), respectively.
表 1 空位形成前后Li1.208Ni0.333Co0.042Mn0.417O2的晶格常数, 原胞体积和空位形成能的计算值
Table 1. Computational lattice constants, unit cell volume and vacancy formation energies of Li1.208Ni0.333Co0.042Mn0.417O2 before and after the defect formations.
材料体系 晶格常数 体积/Å3 体积变化量(Vvac – V0)/V0 空位形成能$ /\mathrm{e}\mathrm{V} $ a/Å b/Å c/Å 无空位时 5.786 11.552 14.302 822.285 — — 含Ni空位 5.778 11.492 14.268 815.131 –0.87% 5.31 含Co空位 5.796 11.434 14.343 821.290 –0.12% 6.52 含Mn空位 5.732 11.523 14.231 811.067 –1.36% 8.27 表 2 各原子的电子轨道对价带顶和导带底上电子态的贡献
Table 2. Contribution of electronic orbital of various types of atoms for the electronic states at VBM and CBM.
$ {\mathrm{p}}_{y} $ $ {\mathrm{p}}_{z} $ $ {\mathrm{p}}_{x} $ $ {\mathrm{d}}_{xy} $ $ {\mathrm{d}}_{yz} $ $ {\mathrm{d}}_{{z}^{2}} $ $ {\mathrm{d}}_{xz} $ $ {\mathrm{d}}_{{x}^{2}-{y}^{2}} $ VBM-O 0.138 0.206 0.268 0 0 0 0 0 CBM-O 0.134 0.164 0.097 0 0 0 0 0 VBM-Ni 0 0 0 0.048 0.029 0.004 0.095 0.01 CBM-Ni 0 0 0 0.028 0.141 0 0.068 0.102 CBM-Mn 0 0 0 0.003 0.015 0.007 0.003 0.034 -
[1] Whittingham M S 2004 Chem. Rev. 104 4271
 Google Scholar
Google Scholar
[2] Hong J, Gwon H, Jung S K, Ku K, Kang K 2015 J. Electrochem. Soc. 162 A2447
 Google Scholar
Google Scholar
[3] He P, Yu H J, Li D, Zhou H S 2012 J. Mater. Chem. 22 3680
 Google Scholar
Google Scholar
[4] Lu J, Chen Z H, Ma Z F, Pan F, Curtiss L A, Amine K 2016 Nat. Nanotechnol. 11 1031
 Google Scholar
Google Scholar
[5] Deng Y W, Feng C L, Jiaqiang E, Wei K X, Zhang B, Zhang Z Q, Han D D, Zhao X H, Xu W W 2019 Energy 183 869
 Google Scholar
Google Scholar
[6] Cano Z P, Banham D, Ye S Y, Hintennach A, Lu J, Fowler M, Chen Z W 2018 Nat. Energy 3 279
 Google Scholar
Google Scholar
[7] Zeng X Q, Li M, Abd El-Hady D, Alshitari W, Al-Bogami A S, Lu J, Amine K 2019 Adv. Energy Mater. 9 1900161
 Google Scholar
Google Scholar
[8] Goodenough J B, Park K S 2013 J. Am. Chem. Soc. 135 1167
 Google Scholar
Google Scholar
[9] Hou P Y, Zhang H Z, Zi Z Y, Zhang L Q, Xu X J 2017 J. Mater. Chem. A 5 4254
 Google Scholar
Google Scholar
[10] Hou P Y, Yin J M, Ding M, Huang J Z, Xu X J 2017 Small 13 1701802
 Google Scholar
Google Scholar
[11] Kim H, Lee E J, Sun Y K 2014 Mater. Today 17 285
 Google Scholar
Google Scholar
[12] Lee J K, Oh C, Kim N, Hwang J Y, Sun Y K 2016 J. Mater. Chem. A 4 5366
 Google Scholar
Google Scholar
[13] Lee J H, Yoon C S, Hwang J Y, Kim S J, Maglia F, Lamp P, Myung S T, Sun Y K 2016 Energy Environ. Sci. 9 2152
 Google Scholar
Google Scholar
[14] Lu Z H, MacNeil D D, Dahn J R 2001 Electrochem. Solid-State Lett. 4 A191
 Google Scholar
Google Scholar
[15] Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D 2011 Energy Environ. Sci. 4 3243
 Google Scholar
Google Scholar
[16] Lu Z H, MacNeil D D, Dahn J R 2001 Electrochem. Solid-State Lett. 4 A200
 Google Scholar
Google Scholar
[17] Thackeray M M, David W I F, Bruce P G, Goodenough J B 1983 Mater. Res. Bull. 18 461
 Google Scholar
Google Scholar
[18] Manthiram A, Chemelewski K, Lee E S 2014 Energy Environ. Sci. 7 1339
 Google Scholar
Google Scholar
[19] Padhi A K, Nanjundaswamy K S, Goodenough J B 1997 J. Electrochem. Soc. 144 1188
 Google Scholar
Google Scholar
[20] Yuan L X, Wang Z H, Zhang W X, Hu X L, Chen J T, Huang Y H, Goodenough J B 2011 Energy Environ. Sci. 4 269
 Google Scholar
Google Scholar
[21] Nyten A, Kamali S, Haggstrom L, Gustafsson T, Thomas J O 2006 J. Mater. Chem. 16 2266
 Google Scholar
Google Scholar
[22] Ohzuku T, Nagayama M, Tsuji K, Ariyoshi K 2011 J. Mater. Chem. 21 10179
 Google Scholar
Google Scholar
[23] Lee J, Zhang Q, Kim J, Dupre N, Avdeev M, Jeong M, Yoon W S, Gu L, Kang B 2020 Adv. Energy Mater. 10 1902231
 Google Scholar
Google Scholar
[24] Wang J, He X, Paillard E, Laszczynski N, Li J, Passerini S 2016 Adv. Energy Mater. 6 1600906
 Google Scholar
Google Scholar
[25] Zheng H, Hu Z, Liu P, Xu W, Xie Q, He W, Luo Q, Wang L, Gu D, Qu B 2020 Energy Storage Mater. 25 76
 Google Scholar
Google Scholar
[26] Liu W, Oh P, Liu X, Lee M J, Cho W, Chae S, Kim Y, Cho J 2015 Angew Chem. Int. Ed. 54 4440
 Google Scholar
Google Scholar
[27] Gu M, Belharouak I, Genc A, Wang Z G, Wang D P, Amine K, Gao F, Zhou G W, Thevuthasan S, Baer D R, Zhang J G, Browning N D, Liu J, Wang C M 2012 Nano Lett. 12 5186
 Google Scholar
Google Scholar
[28] Kresse G, Furthmuller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[29] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[30] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[31] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[32] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[34] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[35] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[36] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[37] Chong S K, Liu Y N, Yan W W, Chen Y Z 2016 RSC. Adv. 6 53662
 Google Scholar
Google Scholar
[38] Sun G, Yu F D, Que L F, Deng L, Wang M J, Jiang Y S, Shao G, Wang Z B 2019 Nano Energy 66 104102
 Google Scholar
Google Scholar
[39] Wu S Q, Cai N L, Zhu Z Z, Yang Y 2008 Electrochim. Acta 53 7915
 Google Scholar
Google Scholar
[40] Hu C H, Yang Y, Zhu Z Z 2010 Solid State Commun. 150 669
 Google Scholar
Google Scholar
[41] Turner D E, Zhu Z Z, Chan C T, Mo K M 1997 Phys. Rev. B 55 13842
 Google Scholar
Google Scholar
计量
- 文章访问数: 9488
- PDF下载量: 150
- 被引次数: 0


















 下载:
下载:





