-
采用第一性原理方法, 研究了三元Hf-C-N体系的空位有序结构及其力学性质和电子性质. 首先采用第一性原理和进化算法, 预测得到8种可能存在的热力学稳定的Hf-C-N空位有序结构; 这些结构都具有岩盐结构, 与实验发现的无序固溶体的结构类型一致. 本文的预测结果证明了Hf-C-N空位化合物能够以有序结构形式存在, 空位与C, N原子都位于[Hf6]八面体间隙, 这一结构特点与HfCx的相同. 然后采用第一性原理方法, 计算了Hf-C-N空位有序结构的力学性质, 发现除C∶N = 1∶4外, 相同C/N下, 随着空位浓度的增大, Hf-C-N的体模量、剪切模量、弹性模量、Pugh比、维氏硬度等降低; 而Hf6CN4 (空位浓度为1/6)的维氏硬度高于Hf5CN4 (无空位), 表现出空位硬化现象. 最后, 计算了Hf-C-N空位有序结构的态密度和晶体轨道哈密顿分布, 发现其具有强共价性和金属性; 且随着空位浓度增大, 总体键强减弱, 因而模量减小.
-
关键词:
- Hf-C-N空位有序结构 /
- 空位 /
- 维氏硬度 /
- 第一性原理方法
The thermal-mechanical properties of transition metal carbonitrides can be affected by the concentration and ordering of vacancies besides the C/N atomic ratio. However, there are few reports on the vacancy ordered structure of ternary transition metal carbonitrides. In the present paper, the first-principles method is used to study the vacancy ordered structures, mechanical properties, electronic properties and the effect of vacancies on the ternary Hf-C-N system. Firstly, the crystal structures of Hf-C-N system is examined by the first-principles and evolutionary algorithms implemented in USPEX under ambient pressure, and eight thermodynamical stable vacancy ordered structures are found, each of which has a rock-salt structure, and is also dynamical and mechanical stable, which are verified by the calculations of their phonon dispersion curves and elastic constants. The vacancies are occupied at the [Hf6] octahedral interstices, which replace the positions of non-metal atoms. Their crystallographic data such as space group, lattice constants are also predicted. To the best of our knowledge, there is no report on the Hf-C-N vacancy ordered structures and these structures investigated here in this work are all found for the first time. Then their mechanical properties are calculated. The Hf-C-N vacancy ordered structures all have very high bulk, shear and elastic modulus and hardness. It is found that except for C∶N = 1∶4, for the Hf-C-N system with the same C/N ratio the moduli, Vickers hardness values, and Pugh’s ratios decrease with the increase of the concentration of vacancy. However, the Vickers hardness of Hf6CN4 (the concentration of vacancy is equal to 1/6) is higher than that of Hf5CN4 (no vacancy), that is so-called vacancy hardening. Finally, the electronic density of states and the crystal orbital Hamilton populations are calculated. The chemical bonding of Hf-C-N vacancy ordered structure is analyzed, which is a mixture of covalence and metallic and is similar to that of binary transition metal carbides and nitrides. With the increase of the concentration of vacancy, the total bond strength decreases, and then the modulus decreases for Hf-C-N compound.[1] Squire T, Marschall J 2010 J. Eur. Ceram. Soc. 30 2239
 Google Scholar
Google Scholar
[2] Opeka M M, Talmy I G, Zaykosk J A 2004 J. Mater. Sci. 39 5887
 Google Scholar
Google Scholar
[3] Levine S R, Opila E J, Halbig M C, Kiser J D, Singh M, Salem J A 2002 J. Eur. Ceram. Soc. 22 2757
 Google Scholar
Google Scholar
[4] Ushakov SV, Navrotsky A 2012 J. Am. Ceram. Soc. 95 1463
 Google Scholar
Google Scholar
[5] Grill A, Aron P R 1983 Thin Solid Films 108 173
 Google Scholar
Google Scholar
[6] Helmersson U, Todorova S, Barnett S A, Sundgren J E, Markert L C, Greene J E 1987 J. Appl. Phys. 62 481
 Google Scholar
Google Scholar
[7] Mirkarimi P B, Hultman L, Barnett S A 1990 Appl. Phys. Lett. 57 2654
 Google Scholar
Google Scholar
[8] Veprek S, Veprek-Heijman M G J, Karvankova P, Prochazka J 2005 Thin Solid Films 476 1
 Google Scholar
Google Scholar
[9] Hultman L, Bareno J, Flink A, Soderberg H, Larsson K, Petrova V, Oden M, Greene J E, Petrov I 2007 Phys. Rev. B 75 155437
 Google Scholar
Google Scholar
[10] Shin C S, Gall D, Hellgren N, Patscheider J, Petrov I, Greene J E 2003 J. Appl. Phys. 93 6025
 Google Scholar
Google Scholar
[11] Jhi S H, Louie S G, Cohen M L, Ihm J 2001 Phys. Rev. Lett. 86 3348
 Google Scholar
Google Scholar
[12] Shin C S, Rudenja S, Gall D, Hellgren N, Lee T Y, Petrov I, Greene J E 2004 J. Appl. Phys. 95 356
 Google Scholar
Google Scholar
[13] Lee T, Ohmori K, Shin C S, Cahill D G, Petrov I, Greene J E 2005 Phys. Rev. B 71 144106
 Google Scholar
Google Scholar
[14] Holleck H 1986 J. Vac. Sci. Technol., A 4 2661
 Google Scholar
Google Scholar
[15] Yang Q, Lengauer W, Koch T, Scheerer M, Smid I 2000 J. Alloys Compd. 309 L5
 Google Scholar
Google Scholar
[16] Jhi S H, Ihm J, Louie S G, Cohen M L 1999 Nature 399 132
 Google Scholar
Google Scholar
[17] Feng W, Cui S, Hu H, Zhang G, Lü Z 2011 Physica B 406 3631
 Google Scholar
Google Scholar
[18] Balasubramanian K, Khare S V, Gall D 2018 Acta Mater. 152 175
 Google Scholar
Google Scholar
[19] Peng J, Tikhonov E 2021 Comput. Mater. Sci. 195 110464
 Google Scholar
Google Scholar
[20] Gusev A I, Rempel A A, Magerl A J 2001 Disorder and Order in Strongly Nonstoichiometric Compounds (Berlin Heidelberg: Springer) pp179−243
[21] Gusev A I 1991 Physical Chemistry of Non stoichiometric Refractory Compounds (Moscow: Nauka) (in Russian)
[22] Rudy E 1965 Ternary Phase Equilibria in Transition Metal-boron-carbon-silicon Systems. Part II. Ternary Systems. Vol. I. Ta-Hf-C system (Air Force Materials Laboratory, Wright-Patterson Air Force Base) pp38−60
[23] Lipatnikov V N, Lengauer W, Ettmayer P, Keil E, Groboth G, Kny E 1997 J. Alloys Compd. 261 192
 Google Scholar
Google Scholar
[24] Yu X X, Thompson G B, Weinberger C R 2015 J. Eur. Ceram. Soc. 35 95
 Google Scholar
Google Scholar
[25] Yu X X, Weinberger C R, Thompson G B 2016 Comput. Mater. Sci. 112 318
 Google Scholar
Google Scholar
[26] Yu X X, Weinberger C R, Thompson G B 2014 Acta Mater. 80 341
 Google Scholar
Google Scholar
[27] Xie C, Liu N, Cheng X, Li D, Zeng Q 2016 J. Eur. Ceram. Soc. 36 3593
 Google Scholar
Google Scholar
[28] Xie C, Oganov A R, Li D, Debela T T, Liu N, Dong D, Zeng Q 2016 Phys. Chem. Chem. Phys. 18 12299
 Google Scholar
Google Scholar
[29] Zhang Y, Liu B, Wang J 2016 Sci. Rep. 5 18098
 Google Scholar
Google Scholar
[30] Gunda N S H, Van der Ven A 2018 Phys. Rev. Mater. 2 083602
 Google Scholar
Google Scholar
[31] Connolly J W D, Williams A R 1983 Phy. Rev. B 27 5169
 Google Scholar
Google Scholar
[32] Weinberger C R, Thompson G B 2018 J. Am. Ceram. Soc. 101 4401
 Google Scholar
Google Scholar
[33] Gusev A I, Rempel A A 1994 J. Phys. Chem. Solids 55 299
 Google Scholar
Google Scholar
[34] Yu S, Zeng Q, Oganov A R, Frapper G, Zhang L 2015 Phys. Chem. Chem. Phys. 17 11763
 Google Scholar
Google Scholar
[35] Yu S, Zeng Q, Oganov A R, Frapper G, Huang B, Niu H, Zhang L 2017 RSC Adv. 7 4697
 Google Scholar
Google Scholar
[36] 樊涛, 曾庆丰, 于树印 2016 65 118102
 Google Scholar
Google Scholar
Fan T, Zeng Q F, Yu S Y 2016 Acta Phys. Sin. 65 118102
 Google Scholar
Google Scholar
[37] Zhao Z L, Bao K, Tian F B, Duan D F, Liu B B, Cui T 2015 Phys. Chem. Chem. Phys. 17 22837
 Google Scholar
Google Scholar
[38] Li D, Tian F B, Duan D F, Bao K, Chu B, Sha X, Liu B B, Cui T 2014 RSC Adv. 4 10133
 Google Scholar
Google Scholar
[39] Oganov A R, Glass C W 2006 J. Chem. Phys. 124 244704
 Google Scholar
Google Scholar
[40] Lyakhov A O, Oganov A R, Stokes H T, Zhu Q 2013 Comput. Phys. Commun. 184 1172
 Google Scholar
Google Scholar
[41] Oganov A R, Lyakhov A O, Valle M 2011 Acc. Chem. Res. 44 227
 Google Scholar
Google Scholar
[42] Rudy E 1970 J. Less-Common Met. 20 49
 Google Scholar
Google Scholar
[43] Erniraliev A, Karimov I, Faizullaev F, Patiev M 1978 Kristallografiya 33 778
[44] Karimov I, Em V T, Petrunin V F, Latergaus I S, Polishuk V S 1976 Materialy 12 1492
[45] Em V T, Karimov I, Latergaus I S 1987 Metallofizika 9 113
[46] Em V T, Tashmetov M Y 1996 Phys. Status Solidi B 198 571
 Google Scholar
Google Scholar
[47] Binder S, Lengauer W, Ettmayer P, Bauer J, Debuigne J, Bohn M 1995 J. Alloys Compd. 217 128
 Google Scholar
Google Scholar
[48] Hong Q J, van de Walle A 2015 Phys. Rev. B 92 020104
 Google Scholar
Google Scholar
[49] Buinevich V S, Nepapushev A A, Moskovskikh D O, Trusov G V, Kuskov K V, Vadchenko S G, Rogachev A S, Mukasyan A S 2020 Ceram. Int. 46 16068
 Google Scholar
Google Scholar
[50] Oganov A R 2011 Modern methods of crystal structure prediction (Weinheim: Wiley-VCR)
[51] Kresse G, Furthmüller J 1996 Phys. Rev. B:Condens. Matter 54 11169
 Google Scholar
Google Scholar
[52] Blöchl P E 1994 Phys. Rev. B:Condens. Matter 50 17953
 Google Scholar
Google Scholar
[53] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[54] Voigt W 1928 Lehrbuch der Kristallphysik (Leipzig, Germany: B. G. Teubner)
[55] Reuss A 1929 Z. Angew. Math. Mech. 9 49
 Google Scholar
Google Scholar
[56] Hill R W 1952 Proc. Phys. Soc. London, Sect. A 65 349
 Google Scholar
Google Scholar
[57] Chen X Q, Niu H, Li D, Li Y 2011 Intermetallics 19 1275
 Google Scholar
Google Scholar
[58] Pugh S F 1954 Philos. Mag. 45 823
 Google Scholar
Google Scholar
[59] Togo A, Oba F, Tanaka I 2008 Phys. Rev. B 78 134106
 Google Scholar
Google Scholar
[60] Momma K, Izumi F 2011 J. Appl. Crystallogr. 44 1272
 Google Scholar
Google Scholar
[61] Dronskowski R, Bloechl P E 1993 J. Phys. Chem. 97 8617
 Google Scholar
Google Scholar
[62] Hinuma Y, Pizzi G, Kumagai Y, Oba F, Tanaka I 2017 Comput. Mater. Sci. 128 140
 Google Scholar
Google Scholar
[63] Cowley R A 1976 Phys. Rev. B 13 4877
 Google Scholar
Google Scholar
-
图 1 (a) 常压下, 三元Hf-HfC-HfN体系的能量凸包图, 黑色球表示热力学稳定结构, 其他为亚稳结构; (b) Hf-C-N空位有序结构的X射线衍射模拟图谱, 衍射源为Cu Kα射线
Fig. 1. (a) Enthalpy convex-hull of ternary Hf-HfC-HfN system at ambient pressure. The black sphere indicates stable structure, and others are metastable structure. (b) The simulated X-ray diffractions of Hf-C-N vacancy ordered structures with a copper Kα X-ray source.
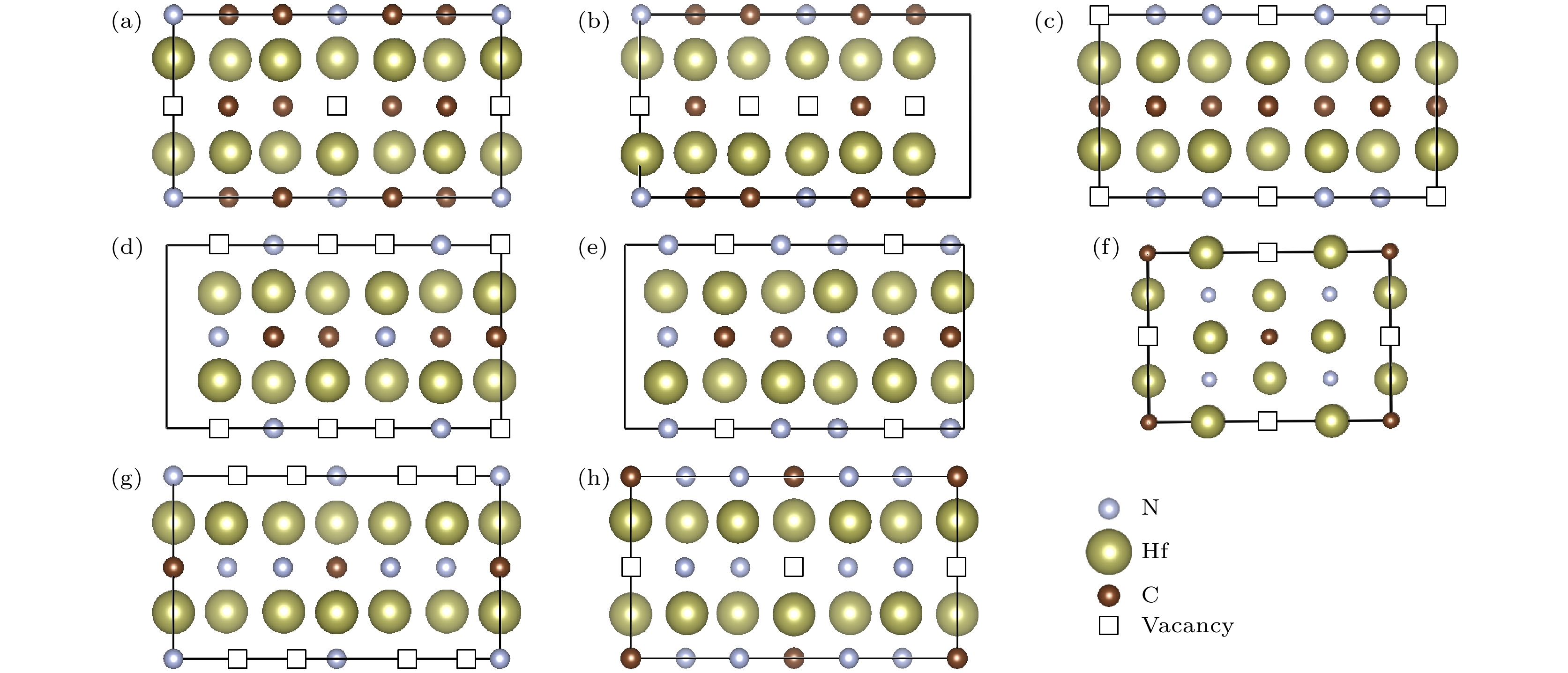
图 2 Hf-C-N空位有序结构在某一晶面上的空位分布 (a) Hf6C4N-
$C2 $ /m (0 0 1); (b) Hf6C3N-$C2 $ (1 0 0); (c) Hf6C3N2-$C2 $ /m (1 0 0); (d) Hf3CN-$C2 $ (1 0 0); (e) Hf6C2N3-$C2 $ (1 0 0); (f) Hf4CN2-Cmmm (0 0 1); (g) Hf6CN3-$C2 $ /m (1 0 0); (h) Hf6CN4-$C2 $ /m (0 0 1)Fig. 2. Vacancies on the crystallographic plane: (a) Hf6C4N-
$C2 $ /m (0 0 1); (b) Hf6C3N-$C2 $ (1 0 0); (c) Hf6C3N2-$C2 $ /m (1 0 0); (d) Hf3CN-$C2 $ (1 0 0); (e) Hf6C2N3-$C2 $ (1 0 0); (f) Hf4CN2-Cmmm (0 0 1); (g) Hf6CN3-$C2 $ /m (1 0 0); (h) Hf6CN4-$C2 $ /m (0 0 1).图 3 Hf-C-N空位有序结构的声子谱曲线 (a) Hf6C4N-
$C2 $ /m; (b) Hf6C3N-$C2 $ ; (c) Hf6C3N2-$C2 $ /m; (d) Hf3CN-$C2 $ ; (e) Hf6C2N3-$C2 $ ; (f) Hf4CN2-Cmmm; (g) Hf6CN3-$C2 $ /m; (h) Hf6CN4-$C2 $ /mFig. 3. Phonon dispersion curves of (a) Hf6C4N-
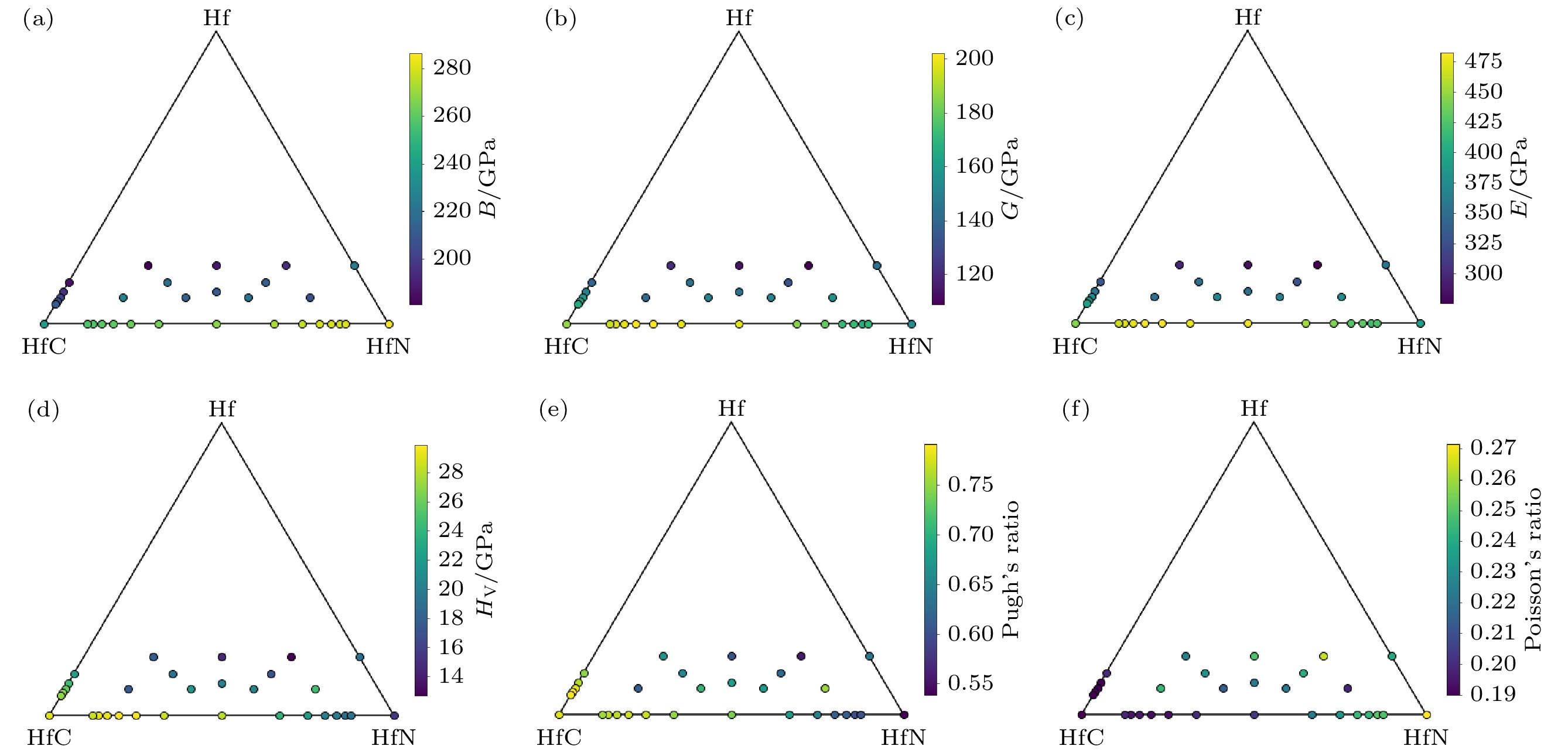
$C2 $ /m, (b) Hf6C3N-$C2 $ , (c) Hf6C3N2-$C2 $ /m, (d) Hf3CN-$C2 $ , (e) Hf6C2N3-$C2 $ , (f) Hf4CN2-Cmmm, (g) Hf6CN3-$C2 $ /m, (h) Hf6CN4-$C2 $ /m. They are all dynamical stable because no imaginary frequencies were found in Brillouin zone.图 4 三元Hf-HfC-HfN体系的力学性质-组分相图 (a) 体模量(B); (b) 剪切模量(G ); (c) 弹性模量(E ); (d) 维氏硬度(HV); (e) Pugh比(G/B); (f) 泊松比(μ)
Fig. 4. Mechanical properties-composition diagrams of ternary Hf-HfC-HfN system: (a) Bulk modulus (B); (b) shear modulus (G ); (c) elastic modulus (E ); (d) Vickers hardness (HV); (e) Pugh’s ratio (G/B); (f) Poisson’s ratio (μ).
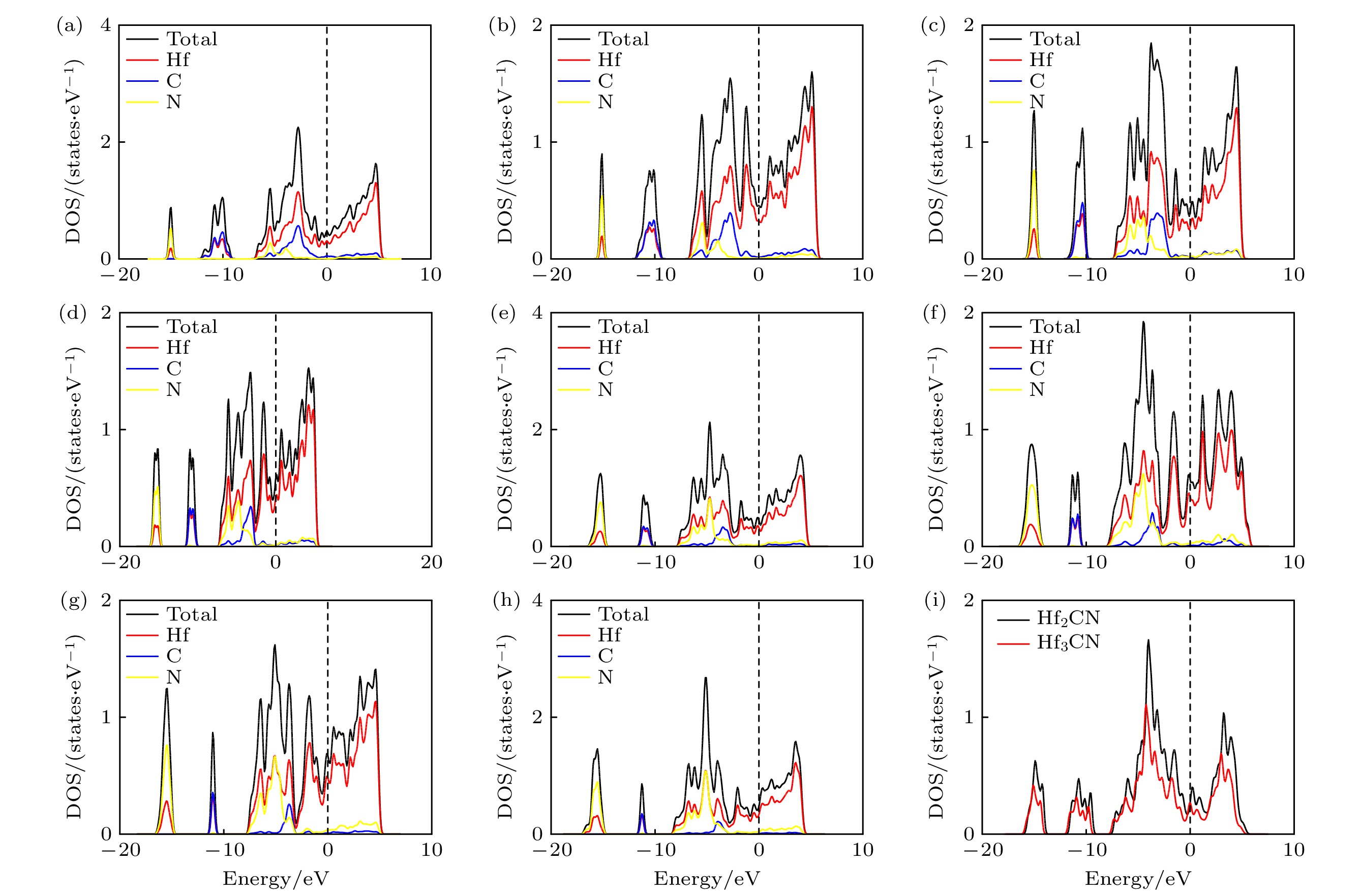
图 5 (a) Hf6C4N-
$C2 $ /m, (b) Hf6C3N-$C2 $ , (c) Hf6C3N2-$C2 $ /m, (d) Hf3CN-$C2 $ , (e) Hf6C2N3-$C2 $ , (f) Hf4CN2-Cmmm, (g) Hf6CN3-$C2 $ /m和(h) Hf6CN4-$C2 $ /m的态密度和分态密度; (i) Hf3CN和Hf2CN的总态密度对比; 其中Fermi能级位于0 eVFig. 5. Density of state (DOS) and partial density of state (PDOS) normalized by per HfCxNy of (a) Hf6C4N-
$C2 $ /m, (b) Hf6C3N-$C2 $ , (c) Hf6C3N2-$C2 $ /m, (d) Hf3CN-$C2 $ , (e) Hf6C2N3-$C2 $ , (f) Hf4CN2-Cmmm, (g) Hf6CN3-$C2 $ /m and (h) Hf6CN4-$C2 $ /m; (i) the total DOS of Hf3CN and Hf2CN normalized by per HfCxNy. The Fermi level is at 0 eV.表 1 Hf-C-N空位有序结构的空间群、晶格常数、反应焓ΔH (eV/atom)、Hf原子的配位数(CN) 和空位浓度(CV)
Table 1. Space group, lattice constants, the enthalpy of reaction ΔH (eV/atom), coordination number (CN) of Hf and the concentration of vacancy (CV) of Hf-C-N vacancy ordered structures.
Compound Space group Lattice constants/Å ΔH/(eV·atom–1) CN CV Hf6C4N $C2 $/m a = 5.679, b = 9.799, c = 5.671, β = 70.6o –0.0899 5 1/6 Hf6C3N $C2 $ a = 5.658, b = 9.763, c = 9.262, β = 144.8o –0.0980 4 1/3 Hf6C3N2 $C2 $m a = 5.660, b = 9.783, c = 5.619, β = 109.6o –0.1038 5 1/6 Hf3CN $C2 $ a = 5.632, b = 9.705, c = 5.625, β = 109.8o –0.1107 4 1/3 Hf6C2N3 $C2 $ a = 5.624, b = 9.725, c = 5.602, β = 109.6o –0.1047 5 1/6 Hf4CN2 Cmmm a = 6.427, b = 9.147, c = 3.235 –0.1082 4/5 1/4 Hf6CN3 $C2 $/m a = 5.592, b = 9.658, c = 6.455, β = 125.3o –0.0894 4 1/3 Hf6CN4 $C2 $/m a = 5.580, b = 9.681, c = 5.587, β = 70.3o –0.0815 5 1/6 表 2 Hf-C-N空位有序结构的弹性常数Cij (单位: GPa)
Table 2. Calculated elastic constants Cij (in GPa) of Hf-C-N vacancy ordered structures.
Compounds C11 C22 C33 C44 C55 C66 C12 C13 C23 Hf6C4N-$C2 $/m 414.3 406.6 415.6 158.0 170.6 148.7 94.1 116.1 104.5 Hf6C3N-$C2 $ 358.5 362.8 352.2 100.0 114.3 132.3 87.5 98.3 91.6 Hf6C3N2-$C2 $/m 414.6 417.4 407.6 152.2 157.6 147.8 111.9 115.0 116.3 Hf3CN-$C2 $ 354.5 363.5 348.7 90.6 103.6 128.7 102.1 109.5 101.3 Hf6C2N3-$C2 $ 409.7 418.7 418.1 149.6 160.2 148.9 123.4 122.9 126.5 Hf4CN2-Cmmm 373.4 368.8 406.8 142.2 133.1 135.8 146.4 112.0 124.4 Hf6CN3-$C2 $/m 361.1 358.4 351.7 84.9 99.8 124.9 108.1 121.7 114.2 Hf6CN4-$C2 $/m 401.2 414.1 403.5 146.5 157.2 139.8 134.0 139.9 147.8 表 3 Hf-C-N空位有序结构和HfC1–xNx[19]的力学性质—体模量(B)、剪切模量(G )、弹性模量(E )、泊松比(μ)、Pugh比(G/B)、维氏硬度(HV)等
Table 3. Mechanical properties—bulk modulus (B), shear modulus (G ), elastic modulus (E ), Poisson’s ratio (μ), Pugh’s ratio (G/B), Vickers hardness (HV) of Hf-C-N vacancy ordered structures and HfC1–xNx[19].
Compound B /GPa G /GPa E /GPa μ G/B HV /GPa Hf6C4N 229.0 140.8 350.6 0.2449 0.6148 17.5 Hf5C4N[19] 260.6 201.3 480.3 0.1928 0.7727 29.9 Hf6C3N 180.9 121.5 297.9 0.2256 0.6717 17.8 Hf4C3N[19] 262.2 202.1 482.4 0.1934 0.7707 29.9 Hf6C3N2 214.0 151.1 366.9 0.2143 0.7059 22.1 Hf3CN 188.0 113.4 283.3 0.2489 0.6031 14.6 Hf2CN[19] 268.1 198.5 477.6 0.2031 0.7403 28.1 Hf6C2N3 221.3 149.7 366.6 0.2239 0.6766 20.7 Hf4CN2 212.7 132.8 329.7 0.2417 0.6242 17.1 Hf3CN2[19] 272.8 185.1 452.8 0.2233 0.6786 23.9 Hf6CN3 195.4 108.9 275.6 0.2650 0.5574 12.7 Hf4CN3[19] 276.2 179.6 442.8 0.2328 0.6504 22.2 Hf6CN4 207.2 156.1 374.4 0.1989 0.7535 24.6 Hf5CN4[19] 279.0 171.5 427.0 0.2449 0.6147 20.0 表 4 Hf-C-N化合物的晶体轨道哈密顿分布的积分值(–ICOHP)
Table 4. Integrated crystal orbital Hamilton populations (–ICOHP) of Hf-C-N compounds.
Compound –ICOHP Compound –ICOHP Hf—C Hf—N Hf—Hf Hf—C Hf—N Hf—Hf Hf6C4N 3.373 3.567 0.529 Hf6C3N2 3.181 2.990 0.571 Hf5C4N 3.373 3.033 0.459 Hf4CN2 3.474 3.111 0.650 Hf6C3N 3.350 3.067 0.718 Hf3CN2 3.551 3.091 0.541 Hf4C3N 3.319 3.029 0.454 Hf6CN3 3.408 3.211 0.570 Hf6C3N2 3.607 3.103 0.530 Hf4CN3 3.321 3.159 0.520 Hf3CN 3.277 3.211 0.737 Hf6CN4 3.675 3.179 0.591 Hf2CN 3.483 2.802 0.490 Hf5CN4 3.319 3.017 0.500 -
[1] Squire T, Marschall J 2010 J. Eur. Ceram. Soc. 30 2239
 Google Scholar
Google Scholar
[2] Opeka M M, Talmy I G, Zaykosk J A 2004 J. Mater. Sci. 39 5887
 Google Scholar
Google Scholar
[3] Levine S R, Opila E J, Halbig M C, Kiser J D, Singh M, Salem J A 2002 J. Eur. Ceram. Soc. 22 2757
 Google Scholar
Google Scholar
[4] Ushakov SV, Navrotsky A 2012 J. Am. Ceram. Soc. 95 1463
 Google Scholar
Google Scholar
[5] Grill A, Aron P R 1983 Thin Solid Films 108 173
 Google Scholar
Google Scholar
[6] Helmersson U, Todorova S, Barnett S A, Sundgren J E, Markert L C, Greene J E 1987 J. Appl. Phys. 62 481
 Google Scholar
Google Scholar
[7] Mirkarimi P B, Hultman L, Barnett S A 1990 Appl. Phys. Lett. 57 2654
 Google Scholar
Google Scholar
[8] Veprek S, Veprek-Heijman M G J, Karvankova P, Prochazka J 2005 Thin Solid Films 476 1
 Google Scholar
Google Scholar
[9] Hultman L, Bareno J, Flink A, Soderberg H, Larsson K, Petrova V, Oden M, Greene J E, Petrov I 2007 Phys. Rev. B 75 155437
 Google Scholar
Google Scholar
[10] Shin C S, Gall D, Hellgren N, Patscheider J, Petrov I, Greene J E 2003 J. Appl. Phys. 93 6025
 Google Scholar
Google Scholar
[11] Jhi S H, Louie S G, Cohen M L, Ihm J 2001 Phys. Rev. Lett. 86 3348
 Google Scholar
Google Scholar
[12] Shin C S, Rudenja S, Gall D, Hellgren N, Lee T Y, Petrov I, Greene J E 2004 J. Appl. Phys. 95 356
 Google Scholar
Google Scholar
[13] Lee T, Ohmori K, Shin C S, Cahill D G, Petrov I, Greene J E 2005 Phys. Rev. B 71 144106
 Google Scholar
Google Scholar
[14] Holleck H 1986 J. Vac. Sci. Technol., A 4 2661
 Google Scholar
Google Scholar
[15] Yang Q, Lengauer W, Koch T, Scheerer M, Smid I 2000 J. Alloys Compd. 309 L5
 Google Scholar
Google Scholar
[16] Jhi S H, Ihm J, Louie S G, Cohen M L 1999 Nature 399 132
 Google Scholar
Google Scholar
[17] Feng W, Cui S, Hu H, Zhang G, Lü Z 2011 Physica B 406 3631
 Google Scholar
Google Scholar
[18] Balasubramanian K, Khare S V, Gall D 2018 Acta Mater. 152 175
 Google Scholar
Google Scholar
[19] Peng J, Tikhonov E 2021 Comput. Mater. Sci. 195 110464
 Google Scholar
Google Scholar
[20] Gusev A I, Rempel A A, Magerl A J 2001 Disorder and Order in Strongly Nonstoichiometric Compounds (Berlin Heidelberg: Springer) pp179−243
[21] Gusev A I 1991 Physical Chemistry of Non stoichiometric Refractory Compounds (Moscow: Nauka) (in Russian)
[22] Rudy E 1965 Ternary Phase Equilibria in Transition Metal-boron-carbon-silicon Systems. Part II. Ternary Systems. Vol. I. Ta-Hf-C system (Air Force Materials Laboratory, Wright-Patterson Air Force Base) pp38−60
[23] Lipatnikov V N, Lengauer W, Ettmayer P, Keil E, Groboth G, Kny E 1997 J. Alloys Compd. 261 192
 Google Scholar
Google Scholar
[24] Yu X X, Thompson G B, Weinberger C R 2015 J. Eur. Ceram. Soc. 35 95
 Google Scholar
Google Scholar
[25] Yu X X, Weinberger C R, Thompson G B 2016 Comput. Mater. Sci. 112 318
 Google Scholar
Google Scholar
[26] Yu X X, Weinberger C R, Thompson G B 2014 Acta Mater. 80 341
 Google Scholar
Google Scholar
[27] Xie C, Liu N, Cheng X, Li D, Zeng Q 2016 J. Eur. Ceram. Soc. 36 3593
 Google Scholar
Google Scholar
[28] Xie C, Oganov A R, Li D, Debela T T, Liu N, Dong D, Zeng Q 2016 Phys. Chem. Chem. Phys. 18 12299
 Google Scholar
Google Scholar
[29] Zhang Y, Liu B, Wang J 2016 Sci. Rep. 5 18098
 Google Scholar
Google Scholar
[30] Gunda N S H, Van der Ven A 2018 Phys. Rev. Mater. 2 083602
 Google Scholar
Google Scholar
[31] Connolly J W D, Williams A R 1983 Phy. Rev. B 27 5169
 Google Scholar
Google Scholar
[32] Weinberger C R, Thompson G B 2018 J. Am. Ceram. Soc. 101 4401
 Google Scholar
Google Scholar
[33] Gusev A I, Rempel A A 1994 J. Phys. Chem. Solids 55 299
 Google Scholar
Google Scholar
[34] Yu S, Zeng Q, Oganov A R, Frapper G, Zhang L 2015 Phys. Chem. Chem. Phys. 17 11763
 Google Scholar
Google Scholar
[35] Yu S, Zeng Q, Oganov A R, Frapper G, Huang B, Niu H, Zhang L 2017 RSC Adv. 7 4697
 Google Scholar
Google Scholar
[36] 樊涛, 曾庆丰, 于树印 2016 65 118102
 Google Scholar
Google Scholar
Fan T, Zeng Q F, Yu S Y 2016 Acta Phys. Sin. 65 118102
 Google Scholar
Google Scholar
[37] Zhao Z L, Bao K, Tian F B, Duan D F, Liu B B, Cui T 2015 Phys. Chem. Chem. Phys. 17 22837
 Google Scholar
Google Scholar
[38] Li D, Tian F B, Duan D F, Bao K, Chu B, Sha X, Liu B B, Cui T 2014 RSC Adv. 4 10133
 Google Scholar
Google Scholar
[39] Oganov A R, Glass C W 2006 J. Chem. Phys. 124 244704
 Google Scholar
Google Scholar
[40] Lyakhov A O, Oganov A R, Stokes H T, Zhu Q 2013 Comput. Phys. Commun. 184 1172
 Google Scholar
Google Scholar
[41] Oganov A R, Lyakhov A O, Valle M 2011 Acc. Chem. Res. 44 227
 Google Scholar
Google Scholar
[42] Rudy E 1970 J. Less-Common Met. 20 49
 Google Scholar
Google Scholar
[43] Erniraliev A, Karimov I, Faizullaev F, Patiev M 1978 Kristallografiya 33 778
[44] Karimov I, Em V T, Petrunin V F, Latergaus I S, Polishuk V S 1976 Materialy 12 1492
[45] Em V T, Karimov I, Latergaus I S 1987 Metallofizika 9 113
[46] Em V T, Tashmetov M Y 1996 Phys. Status Solidi B 198 571
 Google Scholar
Google Scholar
[47] Binder S, Lengauer W, Ettmayer P, Bauer J, Debuigne J, Bohn M 1995 J. Alloys Compd. 217 128
 Google Scholar
Google Scholar
[48] Hong Q J, van de Walle A 2015 Phys. Rev. B 92 020104
 Google Scholar
Google Scholar
[49] Buinevich V S, Nepapushev A A, Moskovskikh D O, Trusov G V, Kuskov K V, Vadchenko S G, Rogachev A S, Mukasyan A S 2020 Ceram. Int. 46 16068
 Google Scholar
Google Scholar
[50] Oganov A R 2011 Modern methods of crystal structure prediction (Weinheim: Wiley-VCR)
[51] Kresse G, Furthmüller J 1996 Phys. Rev. B:Condens. Matter 54 11169
 Google Scholar
Google Scholar
[52] Blöchl P E 1994 Phys. Rev. B:Condens. Matter 50 17953
 Google Scholar
Google Scholar
[53] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[54] Voigt W 1928 Lehrbuch der Kristallphysik (Leipzig, Germany: B. G. Teubner)
[55] Reuss A 1929 Z. Angew. Math. Mech. 9 49
 Google Scholar
Google Scholar
[56] Hill R W 1952 Proc. Phys. Soc. London, Sect. A 65 349
 Google Scholar
Google Scholar
[57] Chen X Q, Niu H, Li D, Li Y 2011 Intermetallics 19 1275
 Google Scholar
Google Scholar
[58] Pugh S F 1954 Philos. Mag. 45 823
 Google Scholar
Google Scholar
[59] Togo A, Oba F, Tanaka I 2008 Phys. Rev. B 78 134106
 Google Scholar
Google Scholar
[60] Momma K, Izumi F 2011 J. Appl. Crystallogr. 44 1272
 Google Scholar
Google Scholar
[61] Dronskowski R, Bloechl P E 1993 J. Phys. Chem. 97 8617
 Google Scholar
Google Scholar
[62] Hinuma Y, Pizzi G, Kumagai Y, Oba F, Tanaka I 2017 Comput. Mater. Sci. 128 140
 Google Scholar
Google Scholar
[63] Cowley R A 1976 Phys. Rev. B 13 4877
 Google Scholar
Google Scholar
计量
- 文章访问数: 8100
- PDF下载量: 146
- 被引次数: 0














 下载:
下载: