-
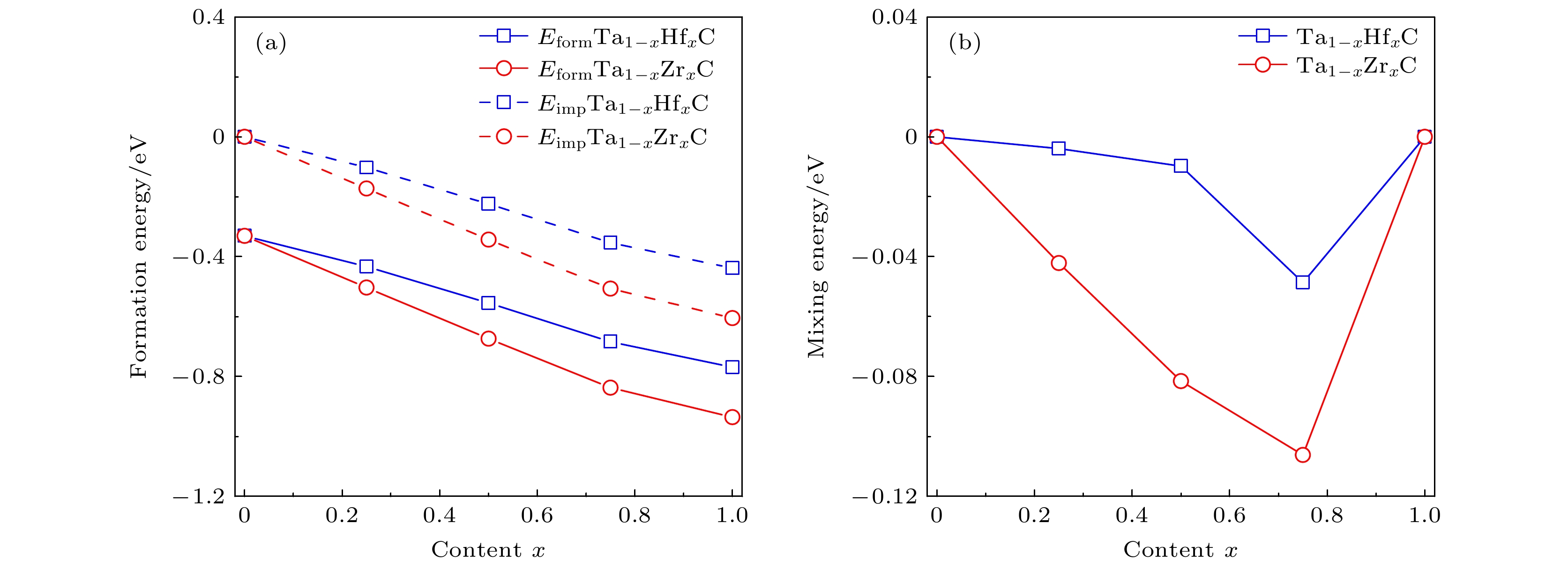
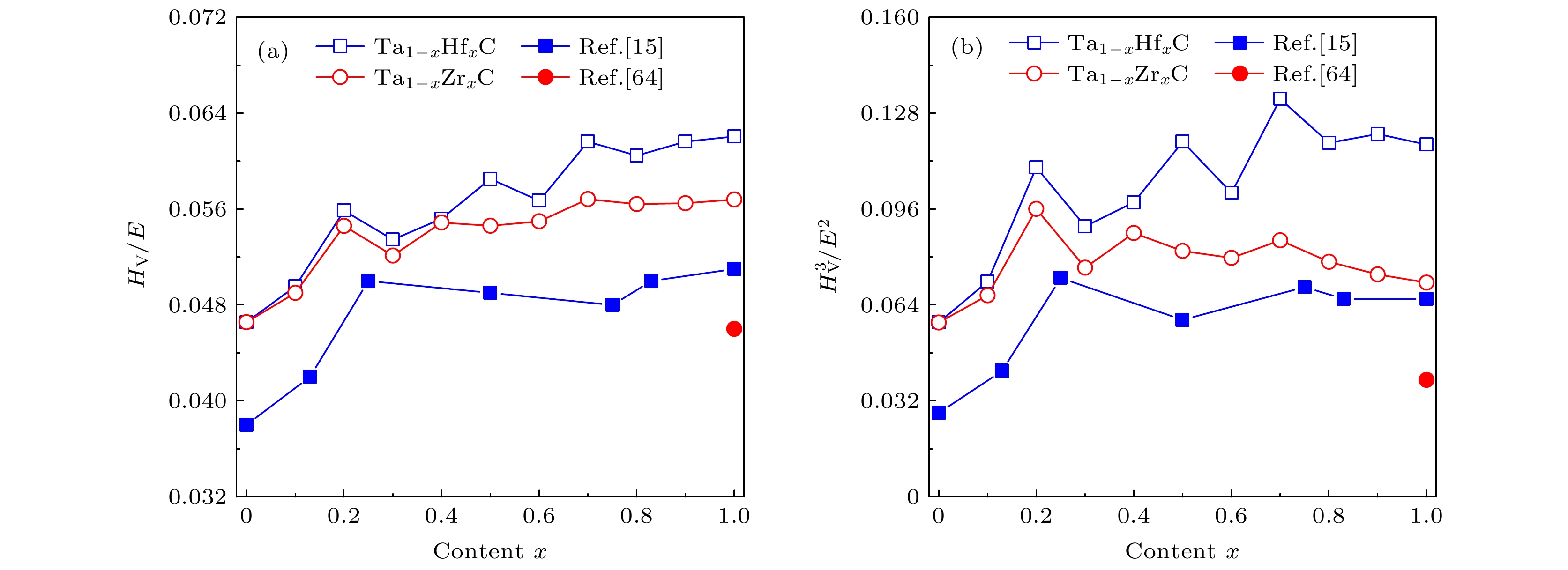
随着航空航天领域的飞速发展, 极端的环境要求超高温陶瓷材料具有更好的力学性能和超高熔点. 目前, 单金属碳化物的超高温陶瓷材料承受的压力日渐增大. 为了解决过渡金属单碳化物性能不足的问题, 我们基于密度泛函理论系统地研究了Ta1–xHfxC和Ta1–xZrxC (0 ≤ x ≤ 1)固溶体的物理性质. 通过调节Hf和Zr的浓度来研究它们的结构稳定性、晶格参数、力学性能、熔点和电子结构. 我们的计算结果表明碳化物固溶体的稳定性随Hf/Zr含量的增加而增加, 并且当Hf/Zr含量相同时, Ta1–xZrxC比Ta1–xHfxC的结构更稳定. 我们还发现随着Hf/Zr含量的增加, 固溶体的晶格常数和体积会膨胀. Ta1–xHfxC和Ta1–xZrxC固溶体的体积模量随着Hf/Zr含量的增加而减小, 而固溶体的熔点、杨氏模量、剪切模量、维氏硬度和断裂韧性在掺杂含量x = 0.2处会出现峰值. 而且, 添加Hf和Zr可以提高TaC的耐磨性. 电子态密度结果显示随着Hf/Zr含量的增加, 固溶体费米能级处的态密度值逐渐降低, 这表明固溶体的结构稳定性逐渐增强.With the rapid development of the aerospace field, the harsh environment requires ultra-high temperature ceramic materials with better mechanical properties and ultra-high melting points. At present, the ultra-high temperature ceramic materials of single metal carbides are required more and more urgently. In order to solve the problem about the insufficient performance of transition metal single carbides, we systematically study the various physical properties of Ta1–xHfxC and Ta1–xZrxC solid solutions in an entire content range (0 ≤ x ≤ 1) based on density functional theory, including the formation energy, impurity formation energy, mixing energy, lattice parameters, elastic constants, elastic modulus, Vickers hardness, fracture toughness, wear resistance, melting point and electronic density of states. The results of formation energy show that with the increase of Hf and Zr doping concentration, the structural stability of Ta1–xHfxC and Ta1–xZrxC solid solutions gradually increase. And the structure of Ta1–xZrxC solid solution is more stable than that of Ta1–xHfxC solid solution when the doping content of Hf and Zr are the same. The results of mixing energy indicate that the formation of binary metal carbides from single metal carbides is an exothermic process. Furthermore, we also find that with the increase of Hf and Zr doping content, the lattice constant and volume of Ta1–xHfxC and Ta1–xZrxC solid solutions can expand, which is mainly attributed to the atomic radii of Hf and Zr being larger than the radius of Ta. The results of mechanical properties show that the Ta1–xHfxC and Ta1–xZrxC solid solution are brittle materials in the entire Hf/Zr content range and have mechanical stability. The bulk modulus of Ta1–xHfxC and Ta1–xZrxC solid solutions decrease with the increase of Hf and Zr content, while the melting point, Young's modulus, shear modulus, Vickers hardness and fracture toughness of Ta1–xHfxC and Ta1–xZrxC solid solutions have peaks with the doping content x = 0.2. Moreover, the addition of Hf/Zr can enhance the wear resistance of TaC. The results of the electronic density of states show that as the doping content increases, the density of states at the Fermi level of Ta1–xHfxC and Ta1–xZrxC solid solutions decrease, which also indicates that the solid solution structure becomes more and more stable.
-
Keywords:
- metal carbides /
- first-principles /
- mechanical properties /
- electronic structure
[1] Kurbatkina V V, Patsrea E I, Vorotilo S A, Levashov E A, Timofeev A N 2016 Ceram. Int. 42 16491
 Google Scholar
Google Scholar
[2] Liu J X, Huang X, Zhang G J 2013 J. Am. Ceram. Soc. 96 1751
 Google Scholar
Google Scholar
[3] Patsrea E I, Levashov E A, Kurbatkina V V, Kovalev D Y 2015 Ceram. Int. 41 8885
 Google Scholar
Google Scholar
[4] Ghaffari S A, Faghihi-Sani M A, Golestani-Fard F, Mandal H 2013 J. Eur. Ceram. Soc. 33 1479
 Google Scholar
Google Scholar
[5] Hao W, Ni N, Guo F W, Cao F C, Jiang J, Zhao X F, Xiao P 2019 J. Am. Ceram. Soc. 102 997
 Google Scholar
Google Scholar
[6] Oyama S T 1996 The Chemistry of Transition Metal Carbides and Nitrides (Glasgow: Blackie Academic and Professional) pp1−27
[7] Pierson H O 1996 Handbook of Refractory Carbides and Nitrides (New Jersey: Noyes Publications) pp5−16
[8] Sciti D, Silvestroni L, Guicciardi S, Fabbriche D D, Bellosi A 2009 J. Mater. Res. 24 2056
 Google Scholar
Google Scholar
[9] Jiang D Y, Wang Q L, Hu W, Wei Z Q, Tong J B, Wan H Q 2016 J. Mater. Res. 31 3401
 Google Scholar
Google Scholar
[10] Adjaoud O, Steinle-Neumann G, Burton B P, Walle A 2009 Phys. Rev. B 80 134112
 Google Scholar
Google Scholar
[11] Wang X G, Liu J X, Kan Y M, Zhang G J 2012 J. Eur. Ceram. Soc. 32 1795
 Google Scholar
Google Scholar
[12] Simonenko E P, Ignatov N A, Simonenko N P, Ezhov Y S, Sevastyanov V G, Kuznetsov N T 2011 Russ. J. Inorg. Chem. 56 1681
 Google Scholar
Google Scholar
[13] Agte C, Alterthum H 1930 Z. Tech. Physik 11 182
[14] Barraza O C, Grasso S, Nasiri N A, Jayaseelan D D, Reece M J, Lee W E 2016 J. Eur. Ceram. Soc. 36 1539
 Google Scholar
Google Scholar
[15] Smith C J, Yu X, Guo Q, Weinberger C R 2018 Acta. Mater. 145 142
 Google Scholar
Google Scholar
[16] Gladyshevsky E I, Fedorov T F, Gorshkova L V 1964 Russ. J. Inorg. Chem. 9 639
[17] Avgustinik A I, Ordan’yan S S 1966 Zh. Prikl. Kim. 39 318
[18] Rudy E 1969 Techn. Rep. AFML-TR 65 334
[19] Yate L, Coy L E, Aperador W 2017 Sci. Rep. 7 3080
 Google Scholar
Google Scholar
[20] Segall M D, Lindan P L D, Probert M J, Pickard C J, Hasnip P J, Clark S J 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[21] Milman V, Winkler B, White J A, Pickard C J, Payne M C, Akhmatskaya E V, Nobes R H 2000 Int. J. Quantum Chem. 77 895
 Google Scholar
Google Scholar
[22] Li X, Chen X, Han L, Ruan C, Lu P, Guan P 2016 J. Mater. Res. 31 2956
 Google Scholar
Google Scholar
[23] Sun S, Fu H, Lin J, Guo G, Lei Y, Wang R 2018 J. Mater. Res. 33 495
 Google Scholar
Google Scholar
[24] Sun X W, Zhang X Y, Zhu Y Z, Zhang S H, Qin J Q, Ma M Z, Liu R P 2013 J. Mater. Sci. 48 7743
 Google Scholar
Google Scholar
[25] Hamann D R 1989 Phys. Rev. B 40 2980
 Google Scholar
Google Scholar
[26] Liu S Y, Liu S, Li D, Shen Y, Dang H, Liu Y, Xue W, Wang S 2014 J. Am. Ceram. Soc. 97 4019
 Google Scholar
Google Scholar
[27] Liu S Y, Zhang E, Liu S, Li D J, Li Y, Liu Y, Shen Y, Wang S 2016 J. Am. Ceram. Soc. 99 3336
 Google Scholar
Google Scholar
[28] Liu S Y, Meng Y, Liu S, Li D J, Li Y, Liu Y, Shen Y, Wang S 2017 J. Am. Ceram. Soc. 100 1221
 Google Scholar
Google Scholar
[29] Liu S Y, Meng Y, Liu S, Li D J, Li Y, Liu Y, Shen Y, Wang S 2017 Phys. Chem. Chem. Phys. 19 22190
 Google Scholar
Google Scholar
[30] Liu S Y, Chen Q Y, Liu S, Li D J, Li Y, Liu Y, Wang S 2018 J. Alloys Compd. 764 869
 Google Scholar
Google Scholar
[31] Liu S Y, Yu D S, Lv Y K, Li D J, Li Y, Cao M S 2013 Chin. Phys. B 22 017702
 Google Scholar
Google Scholar
[32] 邵庆生, 刘士余, 赵辉, 余大书, 曹茂盛 2012 61 047103
 Google Scholar
Google Scholar
Shao Q S, Liu S Y, Zhao H, Yu D S, Cao M S 2012 Acta Phys. Sin. 61 047103
 Google Scholar
Google Scholar
[33] 刘士余, 余大书, 吕跃凯, 李德军, 曹茂盛 2013 62 177102
 Google Scholar
Google Scholar
Liu S Y, Yu D S, Lv Y K, Li D J, Cao M S 2013 Acta Phys. Sin. 62 177102
 Google Scholar
Google Scholar
[34] Liu S Y, Shang J X, Wang F H, Zhang Y 2009 J. Phys. Condens. Matter. 21 225005
 Google Scholar
Google Scholar
[35] 尚家香, 喻显扬 2008 57 2380
 Google Scholar
Google Scholar
Shang J X, Yu X Y 2008 Acta Phys. Sin. 57 2380
 Google Scholar
Google Scholar
[36] 尚家香, 于潭波 2009 58 1179
 Google Scholar
Google Scholar
Shang J X, Yu X Y 2009 Acta Phys. Sin. 58 1179
 Google Scholar
Google Scholar
[37] Voigt W 1928 Lehrbuch der Kristallophysik Teuber-Leipzig (New York: Macmillan Publishers)
[38] Reuss A 1929 Z. Angew. Math. Mech. 9 49
 Google Scholar
Google Scholar
[39] Hill R 1952 Proc. Phys. Soc. A 65 349
 Google Scholar
Google Scholar
[40] Yang J, Gao F M 2012 Physica B: Condens. Matter 407 3527
 Google Scholar
Google Scholar
[41] Tian Y J, Xu B, Zhao Z H 2012 Int. J. Refract. Met. Hard. Mater 33 93
 Google Scholar
Google Scholar
[42] Niu H Y, Niu S W, Oganov A R 2019 J. Appl. Phys. 125 065105
 Google Scholar
Google Scholar
[43] Broek D 1982 Elementary Engineering Fracture Mechanics (3rd Ed.) (Netherlands: Martinus Nijhoff Publishers)
[44] Yan X L, Constantin L, Lu Y F, Silvain J F, Nastasi M, Cui B 2018 J. Am. Ceram. Soc. 101 4486
 Google Scholar
Google Scholar
[45] Yu X X, Thompson G B, Weinberger C R 2015 J. Eur. Ceram. Soc. 35 95
 Google Scholar
Google Scholar
[46] Wehr M R, Richards J A, Adair T W 1978 Physics of the Atom (Boston: Addison-Wesley Publishing Company)
[47] Ha D G, Kim J, Han J S, Kang S 2018 Ceram. Int. 44 19247
 Google Scholar
Google Scholar
[48] Vorotilo S, Sidnov K, Mosyagin I Y, Khvan A V, Levashov E A, Patsera E I, Abrikosov I A 2019 J. Alloys Compd. 778 480
 Google Scholar
Google Scholar
[49] Huang B, Duan Y H, Sun Y, Peng M J, Chen S 2015 J. Alloys Compd. 635 213
 Google Scholar
Google Scholar
[50] Weber W 1973 Phys. Rev. B 8 5082
 Google Scholar
Google Scholar
[51] Li H, Zhang L T, Zeng Q F, Guan K, Li K Y, Ren H T, Liu S H, Cheng L F 2011 Solid State Commun. 151 602
 Google Scholar
Google Scholar
[52] Gautam G S, Hari Kumar K C 2014 J. Alloys. Compd. 587 380
 Google Scholar
Google Scholar
[53] Fine M E, Brown L D, Marcus H L 1984 Scr. Metall. 18 951
 Google Scholar
Google Scholar
[54] Huang H M, Jiang Z Y and Luo S J 2017 Chin. Phys. B 26 096301
 Google Scholar
Google Scholar
[55] Fahrenholtz W G, Hilmas G E, Talmy I G, Zaykoski J A 2007 J. Am. Ceram. Soc. 90 1347
 Google Scholar
Google Scholar
[56] Ionescu E I, Bernard S, Lucas R, Kroll P, Ushakov S, Navrotsky A, Riedel R 2019 Adv. Eng. Mater. 21 1900269
 Google Scholar
Google Scholar
[57] Pugh S F 1954 Phiosl. Mag.J. Sci. 45 823
 Google Scholar
Google Scholar
[58] Liu Y Z, Jiang Y H, Zhou R, Feng J 2014 J. Alloys Compd. 582 500
 Google Scholar
Google Scholar
[59] Jiang X, Zhao J J, Jiang X 2011 Comput. Mater Sci. 50 2287
 Google Scholar
Google Scholar
[60] Frantsevich I N, Voronov F F, Bokuta S A 1983 Elastic Constants and Elastic Moduli of Metals and Insulators (Kiev: Naukova Dumka) pp60−180
[61] Yadav D S, Verma J, Singh D P 2016 J. Pure Appl. Ind. Phys. 6 212
[62] Brown H L, Kempter C P 1966 Phys. Stat. Sol. 18 K21
 Google Scholar
Google Scholar
[63] Zhang J, McMahon J M 2021 J. Mater Sci. 56 4266
 Google Scholar
Google Scholar
[64] Feng L, Fahrenholtz W G, Hilmas G E, Watts J, Zhou Y 2019 J. Am. Ceram. Soc. 102 5786
 Google Scholar
Google Scholar
[65] Valenccia D P, Yate L, Aperador W, Li Y G, Coy E 2018 J. Phys. Chem. C 122 25433
 Google Scholar
Google Scholar
[66] Silvestroni L, Pienti L, Guicciardi S, Sciti D 2015 Compos. Part B Eng. 72 10
 Google Scholar
Google Scholar
[67] He L F, Bao Y W, Wang J Y, Li M S, Zhou Y C 2009 Acta. Mater. 57 2765
 Google Scholar
Google Scholar
[68] Leyland A, Matthews A 2000 Wear 246 1
 Google Scholar
Google Scholar
[69] Carlsson A E 1990 Advances in Research and Applications (New York: Academic Press)
[70] Zhou J, Fu C L, Yoo M H, 1995 Phil. Mag. Lett. 71 45
 Google Scholar
Google Scholar
[71] 李荣, 罗小玲, 梁国明, 付文升 2011 60 117105
 Google Scholar
Google Scholar
Li R, Luo X L, Liang G M, Fu W S 2011 Acta Phys. Sin. 60 117105
 Google Scholar
Google Scholar
[72] Laverntyev A A, Gabrelian B V, Vorzhev V B, Nikiforov I Y, Khyzhun O Y, Rehr J J 2008 J. Alloys Compd. 462 4
 Google Scholar
Google Scholar
-
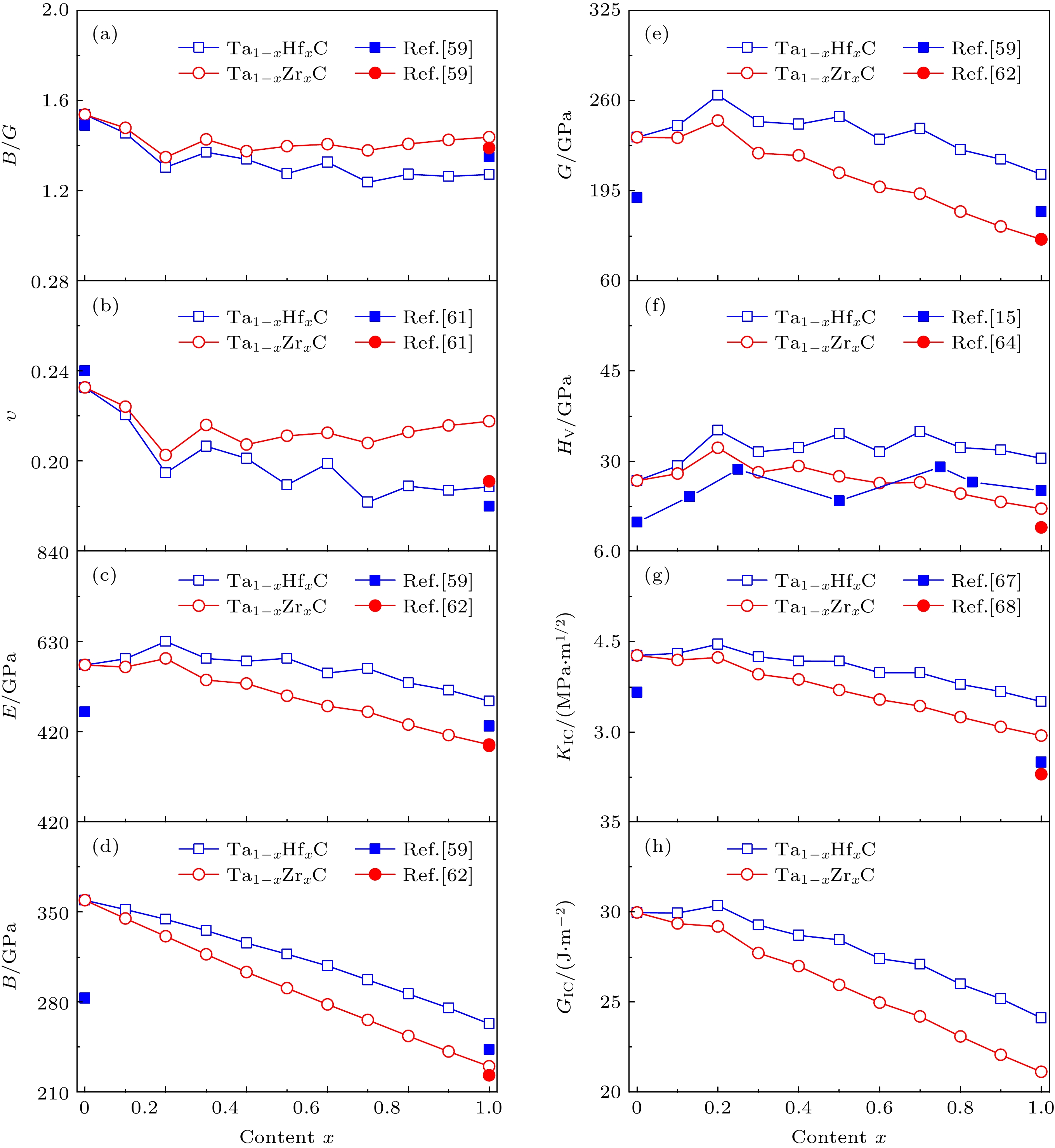
图 5 Ta1–xHfxC和Ta1–xZrxC固溶体的力学性质随Hf或Zr含量x的变化 (a) B/G比; (b)泊松比; (c) 杨氏模量; (d) 体积模量; (e) 剪切模量; (f) 维氏硬度; (g) 断裂韧性; (h) 临界能量释放率
Fig. 5. The mechanical properties of Ta1–xHfxC and Ta1–xZrxC solid solutions as a function of the Hf/Zr content: (a) B/G ratio; (b) Poisson’s ratio; (c) Young’s modulus; (d) bulk modulus; (e) shear modulus; (f) Vickers hardness; (g) fracture toughness; (h) critical energy release rate.
-
[1] Kurbatkina V V, Patsrea E I, Vorotilo S A, Levashov E A, Timofeev A N 2016 Ceram. Int. 42 16491
 Google Scholar
Google Scholar
[2] Liu J X, Huang X, Zhang G J 2013 J. Am. Ceram. Soc. 96 1751
 Google Scholar
Google Scholar
[3] Patsrea E I, Levashov E A, Kurbatkina V V, Kovalev D Y 2015 Ceram. Int. 41 8885
 Google Scholar
Google Scholar
[4] Ghaffari S A, Faghihi-Sani M A, Golestani-Fard F, Mandal H 2013 J. Eur. Ceram. Soc. 33 1479
 Google Scholar
Google Scholar
[5] Hao W, Ni N, Guo F W, Cao F C, Jiang J, Zhao X F, Xiao P 2019 J. Am. Ceram. Soc. 102 997
 Google Scholar
Google Scholar
[6] Oyama S T 1996 The Chemistry of Transition Metal Carbides and Nitrides (Glasgow: Blackie Academic and Professional) pp1−27
[7] Pierson H O 1996 Handbook of Refractory Carbides and Nitrides (New Jersey: Noyes Publications) pp5−16
[8] Sciti D, Silvestroni L, Guicciardi S, Fabbriche D D, Bellosi A 2009 J. Mater. Res. 24 2056
 Google Scholar
Google Scholar
[9] Jiang D Y, Wang Q L, Hu W, Wei Z Q, Tong J B, Wan H Q 2016 J. Mater. Res. 31 3401
 Google Scholar
Google Scholar
[10] Adjaoud O, Steinle-Neumann G, Burton B P, Walle A 2009 Phys. Rev. B 80 134112
 Google Scholar
Google Scholar
[11] Wang X G, Liu J X, Kan Y M, Zhang G J 2012 J. Eur. Ceram. Soc. 32 1795
 Google Scholar
Google Scholar
[12] Simonenko E P, Ignatov N A, Simonenko N P, Ezhov Y S, Sevastyanov V G, Kuznetsov N T 2011 Russ. J. Inorg. Chem. 56 1681
 Google Scholar
Google Scholar
[13] Agte C, Alterthum H 1930 Z. Tech. Physik 11 182
[14] Barraza O C, Grasso S, Nasiri N A, Jayaseelan D D, Reece M J, Lee W E 2016 J. Eur. Ceram. Soc. 36 1539
 Google Scholar
Google Scholar
[15] Smith C J, Yu X, Guo Q, Weinberger C R 2018 Acta. Mater. 145 142
 Google Scholar
Google Scholar
[16] Gladyshevsky E I, Fedorov T F, Gorshkova L V 1964 Russ. J. Inorg. Chem. 9 639
[17] Avgustinik A I, Ordan’yan S S 1966 Zh. Prikl. Kim. 39 318
[18] Rudy E 1969 Techn. Rep. AFML-TR 65 334
[19] Yate L, Coy L E, Aperador W 2017 Sci. Rep. 7 3080
 Google Scholar
Google Scholar
[20] Segall M D, Lindan P L D, Probert M J, Pickard C J, Hasnip P J, Clark S J 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[21] Milman V, Winkler B, White J A, Pickard C J, Payne M C, Akhmatskaya E V, Nobes R H 2000 Int. J. Quantum Chem. 77 895
 Google Scholar
Google Scholar
[22] Li X, Chen X, Han L, Ruan C, Lu P, Guan P 2016 J. Mater. Res. 31 2956
 Google Scholar
Google Scholar
[23] Sun S, Fu H, Lin J, Guo G, Lei Y, Wang R 2018 J. Mater. Res. 33 495
 Google Scholar
Google Scholar
[24] Sun X W, Zhang X Y, Zhu Y Z, Zhang S H, Qin J Q, Ma M Z, Liu R P 2013 J. Mater. Sci. 48 7743
 Google Scholar
Google Scholar
[25] Hamann D R 1989 Phys. Rev. B 40 2980
 Google Scholar
Google Scholar
[26] Liu S Y, Liu S, Li D, Shen Y, Dang H, Liu Y, Xue W, Wang S 2014 J. Am. Ceram. Soc. 97 4019
 Google Scholar
Google Scholar
[27] Liu S Y, Zhang E, Liu S, Li D J, Li Y, Liu Y, Shen Y, Wang S 2016 J. Am. Ceram. Soc. 99 3336
 Google Scholar
Google Scholar
[28] Liu S Y, Meng Y, Liu S, Li D J, Li Y, Liu Y, Shen Y, Wang S 2017 J. Am. Ceram. Soc. 100 1221
 Google Scholar
Google Scholar
[29] Liu S Y, Meng Y, Liu S, Li D J, Li Y, Liu Y, Shen Y, Wang S 2017 Phys. Chem. Chem. Phys. 19 22190
 Google Scholar
Google Scholar
[30] Liu S Y, Chen Q Y, Liu S, Li D J, Li Y, Liu Y, Wang S 2018 J. Alloys Compd. 764 869
 Google Scholar
Google Scholar
[31] Liu S Y, Yu D S, Lv Y K, Li D J, Li Y, Cao M S 2013 Chin. Phys. B 22 017702
 Google Scholar
Google Scholar
[32] 邵庆生, 刘士余, 赵辉, 余大书, 曹茂盛 2012 61 047103
 Google Scholar
Google Scholar
Shao Q S, Liu S Y, Zhao H, Yu D S, Cao M S 2012 Acta Phys. Sin. 61 047103
 Google Scholar
Google Scholar
[33] 刘士余, 余大书, 吕跃凯, 李德军, 曹茂盛 2013 62 177102
 Google Scholar
Google Scholar
Liu S Y, Yu D S, Lv Y K, Li D J, Cao M S 2013 Acta Phys. Sin. 62 177102
 Google Scholar
Google Scholar
[34] Liu S Y, Shang J X, Wang F H, Zhang Y 2009 J. Phys. Condens. Matter. 21 225005
 Google Scholar
Google Scholar
[35] 尚家香, 喻显扬 2008 57 2380
 Google Scholar
Google Scholar
Shang J X, Yu X Y 2008 Acta Phys. Sin. 57 2380
 Google Scholar
Google Scholar
[36] 尚家香, 于潭波 2009 58 1179
 Google Scholar
Google Scholar
Shang J X, Yu X Y 2009 Acta Phys. Sin. 58 1179
 Google Scholar
Google Scholar
[37] Voigt W 1928 Lehrbuch der Kristallophysik Teuber-Leipzig (New York: Macmillan Publishers)
[38] Reuss A 1929 Z. Angew. Math. Mech. 9 49
 Google Scholar
Google Scholar
[39] Hill R 1952 Proc. Phys. Soc. A 65 349
 Google Scholar
Google Scholar
[40] Yang J, Gao F M 2012 Physica B: Condens. Matter 407 3527
 Google Scholar
Google Scholar
[41] Tian Y J, Xu B, Zhao Z H 2012 Int. J. Refract. Met. Hard. Mater 33 93
 Google Scholar
Google Scholar
[42] Niu H Y, Niu S W, Oganov A R 2019 J. Appl. Phys. 125 065105
 Google Scholar
Google Scholar
[43] Broek D 1982 Elementary Engineering Fracture Mechanics (3rd Ed.) (Netherlands: Martinus Nijhoff Publishers)
[44] Yan X L, Constantin L, Lu Y F, Silvain J F, Nastasi M, Cui B 2018 J. Am. Ceram. Soc. 101 4486
 Google Scholar
Google Scholar
[45] Yu X X, Thompson G B, Weinberger C R 2015 J. Eur. Ceram. Soc. 35 95
 Google Scholar
Google Scholar
[46] Wehr M R, Richards J A, Adair T W 1978 Physics of the Atom (Boston: Addison-Wesley Publishing Company)
[47] Ha D G, Kim J, Han J S, Kang S 2018 Ceram. Int. 44 19247
 Google Scholar
Google Scholar
[48] Vorotilo S, Sidnov K, Mosyagin I Y, Khvan A V, Levashov E A, Patsera E I, Abrikosov I A 2019 J. Alloys Compd. 778 480
 Google Scholar
Google Scholar
[49] Huang B, Duan Y H, Sun Y, Peng M J, Chen S 2015 J. Alloys Compd. 635 213
 Google Scholar
Google Scholar
[50] Weber W 1973 Phys. Rev. B 8 5082
 Google Scholar
Google Scholar
[51] Li H, Zhang L T, Zeng Q F, Guan K, Li K Y, Ren H T, Liu S H, Cheng L F 2011 Solid State Commun. 151 602
 Google Scholar
Google Scholar
[52] Gautam G S, Hari Kumar K C 2014 J. Alloys. Compd. 587 380
 Google Scholar
Google Scholar
[53] Fine M E, Brown L D, Marcus H L 1984 Scr. Metall. 18 951
 Google Scholar
Google Scholar
[54] Huang H M, Jiang Z Y and Luo S J 2017 Chin. Phys. B 26 096301
 Google Scholar
Google Scholar
[55] Fahrenholtz W G, Hilmas G E, Talmy I G, Zaykoski J A 2007 J. Am. Ceram. Soc. 90 1347
 Google Scholar
Google Scholar
[56] Ionescu E I, Bernard S, Lucas R, Kroll P, Ushakov S, Navrotsky A, Riedel R 2019 Adv. Eng. Mater. 21 1900269
 Google Scholar
Google Scholar
[57] Pugh S F 1954 Phiosl. Mag.J. Sci. 45 823
 Google Scholar
Google Scholar
[58] Liu Y Z, Jiang Y H, Zhou R, Feng J 2014 J. Alloys Compd. 582 500
 Google Scholar
Google Scholar
[59] Jiang X, Zhao J J, Jiang X 2011 Comput. Mater Sci. 50 2287
 Google Scholar
Google Scholar
[60] Frantsevich I N, Voronov F F, Bokuta S A 1983 Elastic Constants and Elastic Moduli of Metals and Insulators (Kiev: Naukova Dumka) pp60−180
[61] Yadav D S, Verma J, Singh D P 2016 J. Pure Appl. Ind. Phys. 6 212
[62] Brown H L, Kempter C P 1966 Phys. Stat. Sol. 18 K21
 Google Scholar
Google Scholar
[63] Zhang J, McMahon J M 2021 J. Mater Sci. 56 4266
 Google Scholar
Google Scholar
[64] Feng L, Fahrenholtz W G, Hilmas G E, Watts J, Zhou Y 2019 J. Am. Ceram. Soc. 102 5786
 Google Scholar
Google Scholar
[65] Valenccia D P, Yate L, Aperador W, Li Y G, Coy E 2018 J. Phys. Chem. C 122 25433
 Google Scholar
Google Scholar
[66] Silvestroni L, Pienti L, Guicciardi S, Sciti D 2015 Compos. Part B Eng. 72 10
 Google Scholar
Google Scholar
[67] He L F, Bao Y W, Wang J Y, Li M S, Zhou Y C 2009 Acta. Mater. 57 2765
 Google Scholar
Google Scholar
[68] Leyland A, Matthews A 2000 Wear 246 1
 Google Scholar
Google Scholar
[69] Carlsson A E 1990 Advances in Research and Applications (New York: Academic Press)
[70] Zhou J, Fu C L, Yoo M H, 1995 Phil. Mag. Lett. 71 45
 Google Scholar
Google Scholar
[71] 李荣, 罗小玲, 梁国明, 付文升 2011 60 117105
 Google Scholar
Google Scholar
Li R, Luo X L, Liang G M, Fu W S 2011 Acta Phys. Sin. 60 117105
 Google Scholar
Google Scholar
[72] Laverntyev A A, Gabrelian B V, Vorzhev V B, Nikiforov I Y, Khyzhun O Y, Rehr J J 2008 J. Alloys Compd. 462 4
 Google Scholar
Google Scholar
计量
- 文章访问数: 7708
- PDF下载量: 161
- 被引次数: 0













 下载:
下载: