-
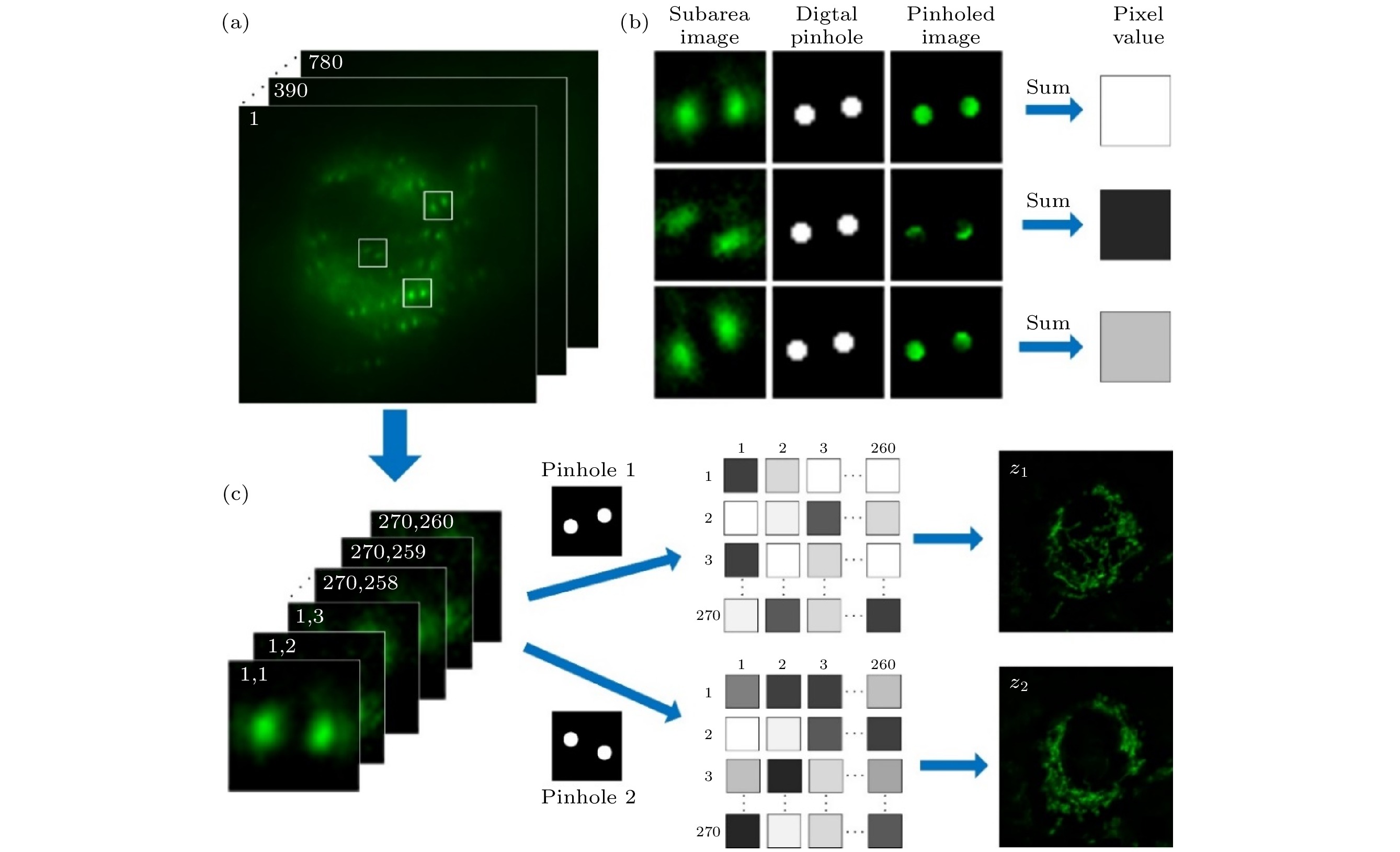
在传统共聚焦显微技术的基础上, 图像扫描显微技术使用面阵探测器来代替单点探测器, 结合虚拟数字针孔并利用像素重定位和解卷积图像重构算法将传统宽场显微镜的分辨率提高一倍, 实现了高信噪比的超分辨共焦成像. 但是, 由于采用逐点扫描的方式, 三维成像速度相对较慢, 限制了其在活体样品成像中的应用. 为了进一步提高图像扫描显微术的成像速度, 本文提出了一种基于双螺旋点扩散函数工程的多焦点图像扫描显微成像方法和系统. 在照明光路中, 利用高速数字微镜器件产生周期分布的聚焦点阵对样品进行并行激发和快速二维扫描; 在探测光路中, 利用双螺旋相位片将激发点荧光信号的强度分布转换为双螺旋的形式; 最终, 利用后期数字重聚焦处理, 从单次样品扫描数据中重构出多个样品层的超分辨宽场图像. 在此基础上, 利用搭建的系统分别对纤维状肌动蛋白和海拉细胞线粒体进行成像实验, 证明了该方法的超分辨能力和快速三维成像能力.Confocal laser scanning microscopy (CLSM) is a powerful imaging tool providing high resolution and optical sectioning. In its standard optical configuration, a pair of confocal pinholes is used to reject out-of-focus light. The diffraction limited resolution can be broken by reducing the confocal pinhole size. But this comes at the cost of extremely low signal-to-noise ratio (SNR). The limited SNR problem can be solved by image scanning microscopy (ISM), in which the single-point detector of a regular point-scanning confocal microscopy is substituted with an array detector such as CCD or CMOS, thus the two-fold super-resolution imaging can be achieved by pixel reassignment and deconvolution. However, the practical application of ISM is challenging due to its limited image acquisition speed. Here, we present a hybrid microscopy technique, named multifocal refocusing after scanning using helical phase engineering microscopy (MRESCH), which combines the double-helix point spread function (DH-PSF) engineering with multifocal structured illumination to dramatically improve the image acquisition speed. In the illumination path, sparse multifocal illumination patterns are generated by a digital micromirror device for parallel imaging information acquisition. In the detection path, a phase mask is introduced to modulate the conventional PSF to the DH-PSF, which provides volumetric information, and meanwhile, we also present a digital refocusing strategy for processing the collected raw data to recover the wild-filed image from different sample layers. To demonstrate imaging capabilities of MRESCH, we acquire the images of mitochondria in live HeLa cells and make a detailed comparison with those from the wide-field microscopy. In contrast to the conventional wide-field approach, the MRESCH can expand the imaging depth in a range from –1 μm to 1 μm. Next, we sample the F-actin of bovine pulmonary artery endothelial cells to characterize the lateral resolution of the MRESCH. The results show that the MRESCH has a better resolution capability than the conventional wide-field illumination microscopy. Finally, the proposed image scanning microscopy can record three-dimensional specimen information from a single multi-spot two-dimensional scan, which ensures faster data acquisition and larger field of view than ISM.
[1] Pawley J B 2006 Handbook of Biological Confocal Microscopy (USA: Springer) p16
[2] Denk W, Strickler J H, Webb W W 1990 Science 248 73
 Google Scholar
Google Scholar
[3] Yan J, Zhang Q L, Lin D Q, Yao S J 2016 Curr. Biochem. Eng. 3 56
 Google Scholar
Google Scholar
[4] Sheppard C J R 1988 Optik 80 53
[5] Müller C B, Enderlein J 2010 Phys. Rev. Lett. 104 198101
 Google Scholar
Google Scholar
[6] Ward E N, Pal R 2017 J. Microsc. 266 221
 Google Scholar
Google Scholar
[7] Sheppard C J R, Mehta S B, Heintzmann R 2013 Opt. Lett. 38 2889
 Google Scholar
Google Scholar
[8] Castello M, Sheppard C J R, Diaspro A, Vicidomini G 2015 Opt. Lett. 40 5355
 Google Scholar
Google Scholar
[9] Jesacher A, Ritschmarte M, Piestun R 2015 Optica 2 210
 Google Scholar
Google Scholar
[10] Roider C, Heintzmann R, Piestun R 2016 Opt. Express 24 15456
 Google Scholar
Google Scholar
[11] Roider C, Piestun R, Jesacher A 2017 Optica 4 1373
 Google Scholar
Google Scholar
[12] Wang Z J, Cai Y N, Liang Y S, Zhou X, Yan S H, Dan D, Bianco P R, Lei M, Yao B L 2017 Biomed. Opt. Express 8 5493
 Google Scholar
Google Scholar
[13] Li S W, Wu J J, Li H, Lin D Y, Yu B, Qu J L 2018 Opt. Express 26 23585
 Google Scholar
Google Scholar
[14] York A G, Parekh S H, Nogare D D, Fischer R S, Temprine K, Mione M, Chitnis A B, Combs C A, Shroff H 2012 Nat. Methods 9 749
 Google Scholar
Google Scholar
[15] Pavani S R P, Greengard A, Piestun R 2009 Appl. Phys. Lett. 95 021103
 Google Scholar
Google Scholar
[16] Grover G, Pavani S R P, Piestun R 2010 Opt. Lett. 35 3306
 Google Scholar
Google Scholar
[17] Grover G, Quirin S, Fiedler C, Piestun R 2011 Biomed. Opt. Express 2 3010
 Google Scholar
Google Scholar
[18] 于斌, 李恒, 陈丹妮, 牛憨笨 2013 62 154206
 Google Scholar
Google Scholar
Yu B, Li H, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 154206
 Google Scholar
Google Scholar
[19] Pavani S R P, Piestun R 2008 Opt. Express 16 3484
 Google Scholar
Google Scholar
[20] Grover G, DeLuca K, Quirin S 2012 Opt. Express 20 26681
 Google Scholar
Google Scholar
[21] Roider C, Jesacher A, Bernet S 2014 Opt. Express 22 4029
 Google Scholar
Google Scholar
[22] 李恒, 于斌, 陈丹妮, 牛憨笨 2013 62 144201
 Google Scholar
Google Scholar
Li H, Yu B, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 144201
 Google Scholar
Google Scholar
-
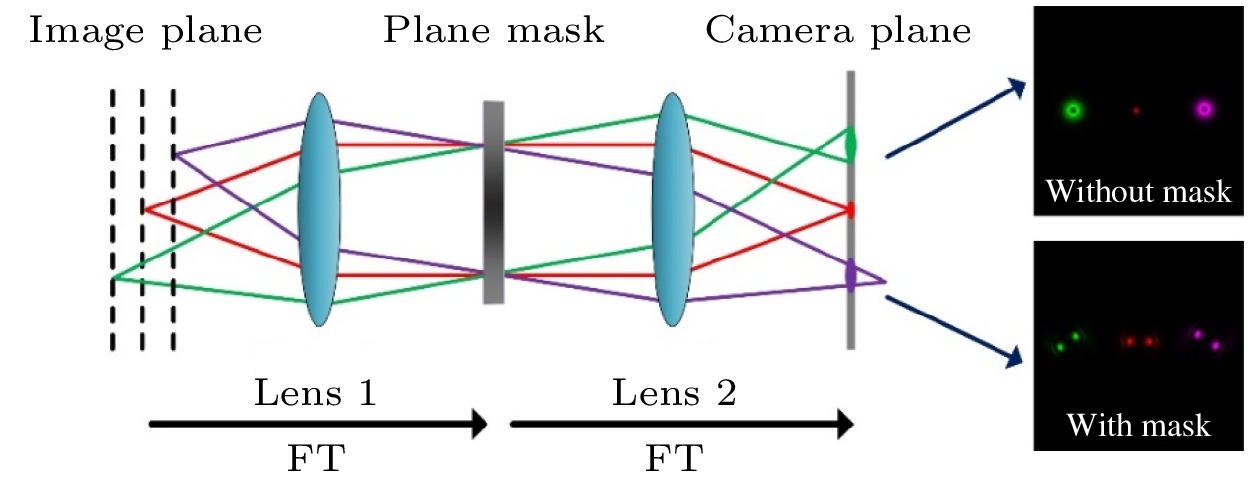
图 2 (a) DMD上载入的投影模式; (b) 激发罗丹明染料样品探测到的荧光点阵分布; (c) 存在相位片的条件下, 激发罗丹明染料样品探测到的双螺旋荧光点阵分布
Fig. 2. (a) Project pattern of DMD; (b) the fluorescence image of the excitation foci in a uniform solution of Rhodamine 6G at the sample plane; (c) the fluorescence image of the excitation foci in a uniform solution of Rhodamine 6G at the sample plane with DH phase mask.
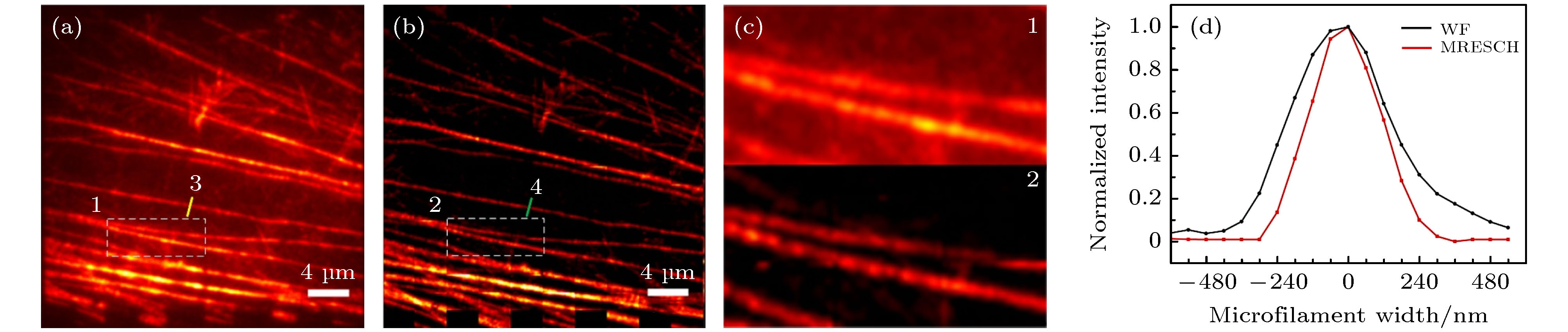
图 5 宽场照明和MRESCH对纤维状肌动蛋白的成像结果比较 (a) 纤维状肌动蛋白的宽场照明成像结果; (b) MRESCH的成像结果; (c) 图(a)和图(b)中白色方块区域的放大; (d)图(a)和图(b)中划线位置的横切面强度图(半高宽分别为: 宽场(WF)照明图像374 nm、MRESCH图像 277 nm)
Fig. 5. Comparison of F-actin imaging results with wide-field illumination and MRESCH: (a) Wide-field image of F-actin; (b) MRESCH image of F-actin; (c) magnification of white box region in panels (a) and (b); (d) plots of intensity along the colored lines in panels (a) and (b); the FWHM values are 374 nm and 277 nm for wide-field (WF) and MRESCH, respectively.
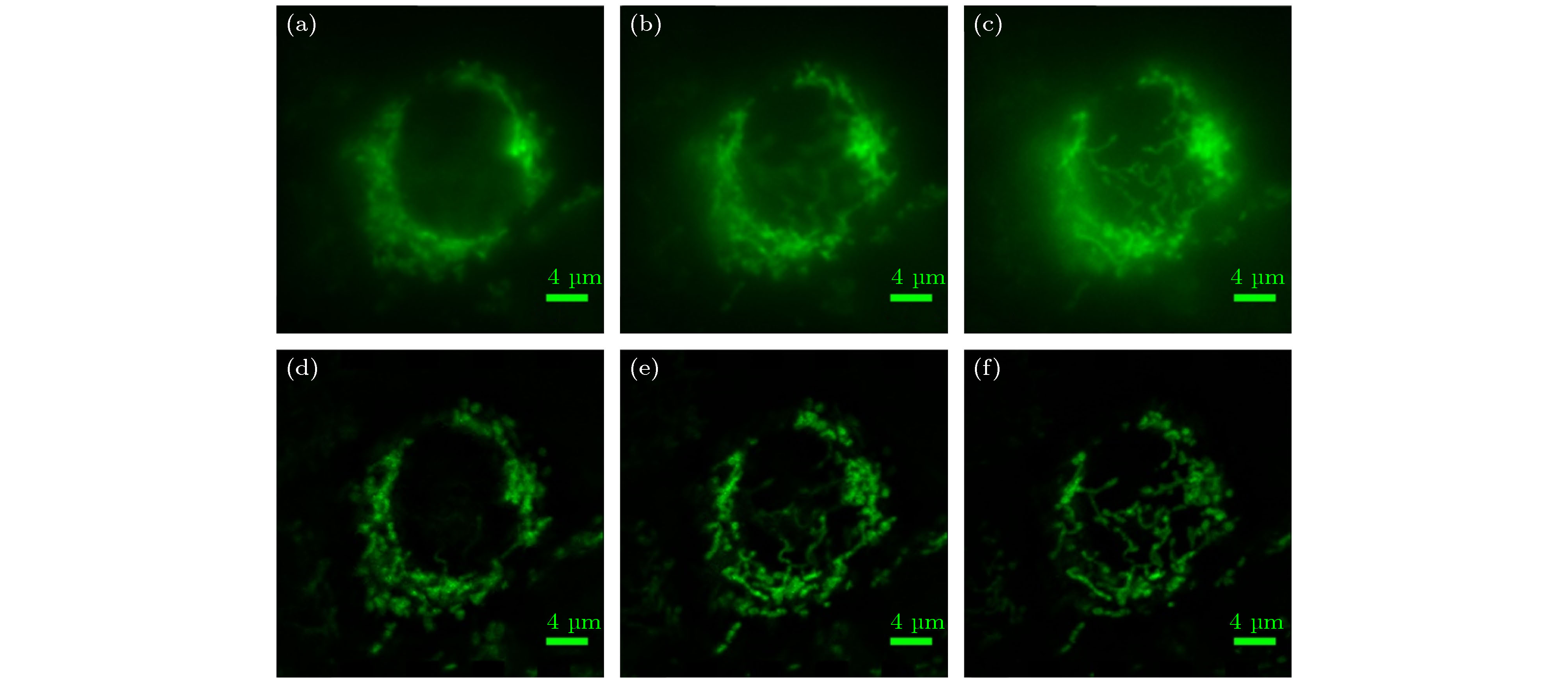
图 6 宽场照明和MRESCH对海拉细胞线粒体成像结果比较 (a) 线粒体在z = –1000 nm位置的宽场成像结果; (b) 线粒体在z = 0 nm位置的宽场成像结果; (c) 线粒体在z = 1000 nm位置的宽场成像结果; (d) 线粒体在z = –1000 nm位置的MRESCH成像结果; (e) 线粒体在z = 0 nm位置的MRESCH成像结果; (f) 线粒体在z = 1000 nm位置的MRESCH成像结果
Fig. 6. Comparison of mitochondrial imaging results of HeLa cells with wide-field illumination and MRESCH: (a) Wide-field image of mitochondria at z = –1000 nm; (b) wide-field image of mitochondrion at z = 0 nm; (c) wide-field image of mitochondria at z = 1000 nm; (d) image obtained via MRESCH at z = –1000 nm; (e) image obtained via MRESCH at z = 0 nm; (f) image obtained via MRESCH at z = 1000 nm.
-
[1] Pawley J B 2006 Handbook of Biological Confocal Microscopy (USA: Springer) p16
[2] Denk W, Strickler J H, Webb W W 1990 Science 248 73
 Google Scholar
Google Scholar
[3] Yan J, Zhang Q L, Lin D Q, Yao S J 2016 Curr. Biochem. Eng. 3 56
 Google Scholar
Google Scholar
[4] Sheppard C J R 1988 Optik 80 53
[5] Müller C B, Enderlein J 2010 Phys. Rev. Lett. 104 198101
 Google Scholar
Google Scholar
[6] Ward E N, Pal R 2017 J. Microsc. 266 221
 Google Scholar
Google Scholar
[7] Sheppard C J R, Mehta S B, Heintzmann R 2013 Opt. Lett. 38 2889
 Google Scholar
Google Scholar
[8] Castello M, Sheppard C J R, Diaspro A, Vicidomini G 2015 Opt. Lett. 40 5355
 Google Scholar
Google Scholar
[9] Jesacher A, Ritschmarte M, Piestun R 2015 Optica 2 210
 Google Scholar
Google Scholar
[10] Roider C, Heintzmann R, Piestun R 2016 Opt. Express 24 15456
 Google Scholar
Google Scholar
[11] Roider C, Piestun R, Jesacher A 2017 Optica 4 1373
 Google Scholar
Google Scholar
[12] Wang Z J, Cai Y N, Liang Y S, Zhou X, Yan S H, Dan D, Bianco P R, Lei M, Yao B L 2017 Biomed. Opt. Express 8 5493
 Google Scholar
Google Scholar
[13] Li S W, Wu J J, Li H, Lin D Y, Yu B, Qu J L 2018 Opt. Express 26 23585
 Google Scholar
Google Scholar
[14] York A G, Parekh S H, Nogare D D, Fischer R S, Temprine K, Mione M, Chitnis A B, Combs C A, Shroff H 2012 Nat. Methods 9 749
 Google Scholar
Google Scholar
[15] Pavani S R P, Greengard A, Piestun R 2009 Appl. Phys. Lett. 95 021103
 Google Scholar
Google Scholar
[16] Grover G, Pavani S R P, Piestun R 2010 Opt. Lett. 35 3306
 Google Scholar
Google Scholar
[17] Grover G, Quirin S, Fiedler C, Piestun R 2011 Biomed. Opt. Express 2 3010
 Google Scholar
Google Scholar
[18] 于斌, 李恒, 陈丹妮, 牛憨笨 2013 62 154206
 Google Scholar
Google Scholar
Yu B, Li H, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 154206
 Google Scholar
Google Scholar
[19] Pavani S R P, Piestun R 2008 Opt. Express 16 3484
 Google Scholar
Google Scholar
[20] Grover G, DeLuca K, Quirin S 2012 Opt. Express 20 26681
 Google Scholar
Google Scholar
[21] Roider C, Jesacher A, Bernet S 2014 Opt. Express 22 4029
 Google Scholar
Google Scholar
[22] 李恒, 于斌, 陈丹妮, 牛憨笨 2013 62 144201
 Google Scholar
Google Scholar
Li H, Yu B, Chen D N, Niu H B 2013 Acta Phys. Sin. 62 144201
 Google Scholar
Google Scholar
计量
- 文章访问数: 10278
- PDF下载量: 194
- 被引次数: 0














 下载:
下载: