-
钙钛矿型铁电氧化物由于具有本征的、非易失的、可翻转的自发极化以及带有高电荷密度的极性表面等特性, 被认为是最有前途的功能材料之一. 研究钙钛矿型铁电氧化物的表面结构对理解其表面/界面能量转化、调控表面物质吸附和脱附、控制界面化学反应、以及设计稳定的低功耗电子器件具有重要意义. 本文首先概述了铁电相与其表面结构的关系, 并介绍了钙钛矿型铁电氧化物复杂表面结构的形成; 之后阐述了铁电表面/界面结构的调控机制, 为后续的钙钛矿型铁电氧化物的表面结构设计、表面性能与功能的控制提供了研究基础; 最后介绍了铁电氧化物表面/界面的功能调控和潜在器件的设计, 并结合目前铁电材料领域表面科学研究的局限性, 对今后基于钙钛矿型铁电氧化物表面结构的研究发展以及应用前景提出了展望.Over the past decades, exploration and artificial control of the surface and interfacial structure of the materials have played an important role in chemical catalyzing, energy conversion, information storage and medical field, and thus the finding of suitable materials with controllable surface/interface properties has attracted intense interest in recent years. Perovskite-type ferroelectric oxides are considered to be one of the most promising functional materials due to their intrinsic, non-volatile, reversible spontaneous polarization and controllable polar surface with high charge density. The investigating of the interaction between polarization and surface structure of perovskite-type ferroelectric oxide is very important for understanding the surface (interface) energy conversion, regulating the adsorption and desorption on the surface, controlling interfacial chemical reaction, and designing stable low-power electronic devices. In this paper, we summarize the theoretical mechanism and potential applications of the surface structures and functionality in perovskite-type ferroelectric oxide from three aspects. Firstly, we describe the inseparable relationship between the stabilized ferroelectric phase and surface structure of ferroelectric material, and illustrate the formation mechanism of complex surface structure of perovskite-type ferroelectric oxide. In order to reduce the surface energy to stabilize the polar surface of the material, perovskite-type ferroelectric oxide always needs to absorb foreign charged particles, change the stoichiometry and conduct electron orbital hybridization or surface relaxation, etc., which will cause the complexity of the surface structure of ferroelectric. Secondly, we outline the influence of ferroelectric polarization on the surface structure of ferroelectric and the behavior of changing ferroelectric polarization by controlling surface structure through adjusting the external environment, which provides an important basis for the subsequent regulation of the surface performance and functionality of perovskite-type ferroelectric oxide. Finally, we introduce the utilization of the controllable physical and chemical properties of ferroelectric surface (interface) into large area and into nanoscale (nanodomain), which has bright application prospects in many frontier fields, including non-volatile memory system, cell proliferation, microfluidic control system, catalysis, optical device and photodetector and so on. Furthermore, considering the limitations of current scientific research about the ferroelectric surface, we put forward the prospects for the future development of the ferroelectric material in the areas of information storage, controllable chemical reactions and new energy conversion.
[1] Valasek J 1920 Phys. Rev. 17 475
 Google Scholar
Google Scholar
[2] Setter N, Damjanovic D, Eng L, Fox G, Gevorgian S, Hong S, Kingon A, Kohlstedt H, Park N Y, Stephenson G B, Stolitchnov I, Taganstev A K, Taylor D V, Yamada T, Streiffer S 2006 J. Appl. Phys. 100 051606
 Google Scholar
Google Scholar
[3] Auciello O, Scott J F, Ramesh R 1998 Phys. Today 51 22
 Google Scholar
Google Scholar
[4] Dawber M, Rabe K M, Scott J F 2005 Rev. Mod. Phys. 77 1083
 Google Scholar
Google Scholar
[5] Li Y J, Wang J J, Ye J C, et al. 2015 Adv. Funct. Mater. 25 3405
 Google Scholar
Google Scholar
[6] Ishiwata S, Taguchi Y, Murakawa H, Onose Y, Tokura Y 2008 Science 319 1643
 Google Scholar
Google Scholar
[7] Li D, Zhao M H, Garra J, Kolpak A M, Rappe A M, Bonnell D A, Vohs J M 2008 Nat. Mater. 7 473
 Google Scholar
Google Scholar
[8] Song J, Kim T L, Lee J, et al. 2018 Nano Res. 11 642
 Google Scholar
Google Scholar
[9] Grilli S, Coppola S, Nasti G, Vespini V, Gentile G, Ambrogi V, Carfagna C, Ferraro P 2014 Rsc Adv. 4 2851
 Google Scholar
Google Scholar
[10] Liu Y, Zhang X, Cao C, et al. 2017 Adv. Funct. Mater. 27 1703771
 Google Scholar
Google Scholar
[11] Kakekhani A, Ismail-Beigi S 2015 ACS Catal. 5 4537
 Google Scholar
Google Scholar
[12] Guo J, Liu Y, Lin Y, Tian Y, Zhang J, Gong T, Cheng T, Huang W, Zhang X 2019 Nanoscale 11 20868
 Google Scholar
Google Scholar
[13] Goniakowski J, Finocchi F, Noguera C 2007 Reps. Prog. Phys. 71 016501
 Google Scholar
Google Scholar
[14] Damjanovic D 1998 Reps. Prog. Phys. 61 1267
 Google Scholar
Google Scholar
[15] Noguera C 2000 J. Phys.: Condens. Matter 12 R367
 Google Scholar
Google Scholar
[16] Garrity K, Kakekhani A, Kolpak A, Ismail-Beigi S 2013 Phys. Rev. B 88 045401
 Google Scholar
Google Scholar
[17] Spanier J E, Kolpak A M, Urban J J, Grinberg I, Ouyang L, Yun W S, Rappe A M, Park H 2006 Nano Lett. 6 735
 Google Scholar
Google Scholar
[18] Jia C L, Urban K 2004 Science 303 2001
 Google Scholar
Google Scholar
[19] Ebensperger C, Meyer B 2011 Phys. Status Solidi B 248 2229
 Google Scholar
Google Scholar
[20] Munkholm A, Streiffer S K, Ramana Murty M V, et al. 2002 Phys. Rev. Lett. 88 016101
 Google Scholar
Google Scholar
[21] Kakekhani A, Ismail-Beigi S, Altman E I 2016 Surf. Sci. 650 302
 Google Scholar
Google Scholar
[22] Kalinin S V, Johnson C Y, Bonnell D A 2002 J. Appl. Phys. 91 3816
 Google Scholar
Google Scholar
[23] Apostol N G, Stoflea L E, Lungu G A, Tache C A, Popescu D G, Pintilie L, Teodorescu C M 2013 Mater. Sci. Eng. B 178 1317
 Google Scholar
Google Scholar
[24] Fong D D, Kolpak A M, Eastman J A, et al. 2006 Phys. Rev. Lett. 96 127601
 Google Scholar
Google Scholar
[25] Gao P, Liu H J, Huang Y L, et al. 2016 Nat. Commun. 7 11318
 Google Scholar
Google Scholar
[26] Tian Y, Wei L, Zhang Q, Huang H, Zhang Y, Zhou H, Ma F, Gu L, Meng S, Chen L Q, Nan C W, Zhang J 2018 Nat. Commun. 9 3809
 Google Scholar
Google Scholar
[27] Kalinin S V, Bonnell D A 2001 Phys. Rev. B 63 125411
 Google Scholar
Google Scholar
[28] Ying X, Haitao Y 2014 Chem. Res. Chin. Univ. 30 794
 Google Scholar
Google Scholar
[29] Zhu L, Yao K L, Liu Z L, Zhang D H 2009 Phys. Lett. A 373 2374
 Google Scholar
Google Scholar
[30] Song C, Gao J, Liu J, Yang Y, Tian C, Hong J, Weng H, Zhang J 2020 ACS Appl. Mater. Interfaces 12 4150
 Google Scholar
Google Scholar
[31] Dai J Q, Xu J W, Zhu J H 2017 Appl. Surf. Sci. 392 135
 Google Scholar
Google Scholar
[32] Yun Y, Kampschulte L, Li M, Liao D, Altman E I 2007 J. Phys. Chem. C 111 13951
 Google Scholar
Google Scholar
[33] Yun Y, Altman E I 2007 J. Am. Chem. Soc. 129 15684
 Google Scholar
Google Scholar
[34] Kolpak A M, Grinberg I, Rappe A M 2007 Phys. Rev. Lett. 98 166101
 Google Scholar
Google Scholar
[35] Ferris R, Yellen B, Zauscher S 2012 Small 8 28
 Google Scholar
Google Scholar
[36] Israelachvili J N 2011 Intermolecular and Surface Forces (San Diego: Academic Press) pp293–294
[37] Ferris R J, Lin S, Therezien M, Yellen B B, Zauscher S 2013 ACS Appl. Mater. Interfaces 5 2610
 Google Scholar
Google Scholar
[38] Nishino R, Kozuka Y, Kagawa F, Uchida M, Kawasaki M 2018 Appl. Phys. Lett. 113 143501
 Google Scholar
Google Scholar
[39] Shin J, Nascimento V B, Geneste G, Rundgren J, Plummer E W, Dkhil B, Kalinin S V, Baddorf A P 2009 Nano Lett. 9 3720
 Google Scholar
Google Scholar
[40] Wang R V, Fong D D, Jiang F, et al. 2009 Phys. Rev. Lett. 102 047601
 Google Scholar
Google Scholar
[41] Jiang B, Bai Y, Chu W Y, Shi S Q, Qiao L J, Su Y J 2008 Appl. Surf. Sci. 254 5594
 Google Scholar
Google Scholar
[42] Sun X, Su Y J, Li X, Gao K W, Qiao L J 2012 J. Appl. Phys. 111 094110
 Google Scholar
Google Scholar
[43] Li X, Bai Y, Wang B C, Su Y J 2015 J. Appl. Phys. 118 094104
 Google Scholar
Google Scholar
[44] Lee H, Kim T H, Patzner J, Lu H, Lee J, Zhou H, Chang W, Mahanthappa M, Tsymbal E Y, Gruverman A, Eom C B 2016 Nano Lett. 16 2400
 Google Scholar
Google Scholar
[45] Du H, Lin X, Xu Z, Chu D 2015 J. Mater. Sci. 50 5641
 Google Scholar
Google Scholar
[46] Ito M, Matsubara Y, Kozuka Y, et al. 2014 Appl. Phys. Lett. 104 222101
 Google Scholar
Google Scholar
[47] Reiss B D, Bai G R, Auciello O, Ocola L E, Firestone M A 2006 Appl. Phys. Lett. 88 083903
 Google Scholar
Google Scholar
[48] Hong J, Kim B S, Char K, Hammond P T 2011 Biomacromolecules 12 2975
 Google Scholar
Google Scholar
[49] Yu Y, Wang X 2018 Adv. Mater. 30 1800154
 Google Scholar
Google Scholar
[50] Chen F, Huang H, Guo L, Zhang Y, Ma T 2019 Angew. Chem. Int. Ed. 58 10061
 Google Scholar
Google Scholar
[51] Cui Y, Briscoe J, Dunn S 2013 Chem. Mater. 25 4215
 Google Scholar
Google Scholar
[52] Giocondi J L, Rohrer G S 2001 J. Phys. Chem. B 105 8275
 Google Scholar
Google Scholar
[53] Bhardwaj A, Burbure N V, Gamalski A, Rohrer G S 2010 Chem. Mater. 22 3527
 Google Scholar
Google Scholar
[54] Tan C, Wang J, Zhong X, Jiang J, Zhang X, Ding Y 2017 Mater. Des. 129 186
 Google Scholar
Google Scholar
[55] Jones P M, Dunn S 2007 Nanotechnology 18 185702
 Google Scholar
Google Scholar
[56] Sun Y, Nemanich R J 2011 J. Appl. Phys. 109 104302
 Google Scholar
Google Scholar
[57] Ullah R, Pei M, Wu J, et al. 2020 ACS Appl. Energy Mater. 3 4149
 Google Scholar
Google Scholar
[58] Wang X, Yang D, Zhang H M, Song C, Wang J, Tan G, Zheng R, Dong S, Cheong S W, Zhang J 2019 Phys. Rev. B 99 054106
 Google Scholar
Google Scholar
[59] Medford A J, Vojvodic A, Hummelshøj J S, Voss J, Abild-Pedersen F, Studt F, Bligaard T, Nilsson A, Nørskov J K 2015 J. Catal. 328 36
 Google Scholar
Google Scholar
[60] Ostrovskii I V, Nadtochiy A B 2005 Appl. Phys. Lett. 86 222902
 Google Scholar
Google Scholar
[61] Kalinin S V, Bonnell D A, Alvarez T, Lei X, Hu Z, Shao R, Ferris J H 2004 Adv. Mater. 16 795
 Google Scholar
Google Scholar
[62] Christophis C, Cavalcanti-Adam E A, Hanke M, et al. 2013 Biointerphases 8 27
 Google Scholar
Google Scholar
[63] Li D, Bonnell D A 2008 Annu. Rev. Mater. Res. 38 351
 Google Scholar
Google Scholar
[64] Chen X, Karpinski P, Shvedov V, et al. 2015 Appl. Phys. Lett. 107 141102
 Google Scholar
Google Scholar
[65] Shur V Y, Lobov A I, Shur A G, et al. 2005 Appl. Phys. Lett. 87 022905
 Google Scholar
Google Scholar
-
图 2 (a) 束缚电荷引起的退极化场, E代表电场, P代表极化[21]; (b) 极化向外导致铁电体表面处能带向下弯曲; (c) 极化向内导致铁电体表面处能带向上弯曲, ECBM代表导带底的能量, EVBM代表价带顶的能量, EF代表费米能[23]; PZT薄膜的正极性表面(d)、负极性表面(e)和面内畴区域(f)的阳离子和阴离子之间的位移图[25]; (g) BWO (113)表面原子分辨率的STM图; (h) BWO薄膜从体态表面到边缘态的dI/dV曲线的演变[30]
Fig. 2. (a) Depolarization field caused by bound charge, E represents electric field, P represents polarization[21]; (b) the downward band bend at an ideal ferroelectric surface with outward (↑) polarization; (c) the upward band bend at an ideal ferroelectric surface with inward (↓) polarization, ECBM represents the energy of the conduction band minimum, EVBM the energy of the valence band maximum, EF Fermi energy[23]; vector maps of the displacement between the cation and anion columns of positively poled surface (d), negatively poled surface (e) and the domain with the in-plane polarization (f) about PZT thin films[25]; (g) atomic resolution STM image of BWO (113) surface; (h) evolution of dI/dV curves of BWO thin film when approaching from the terrace to the edge[30].
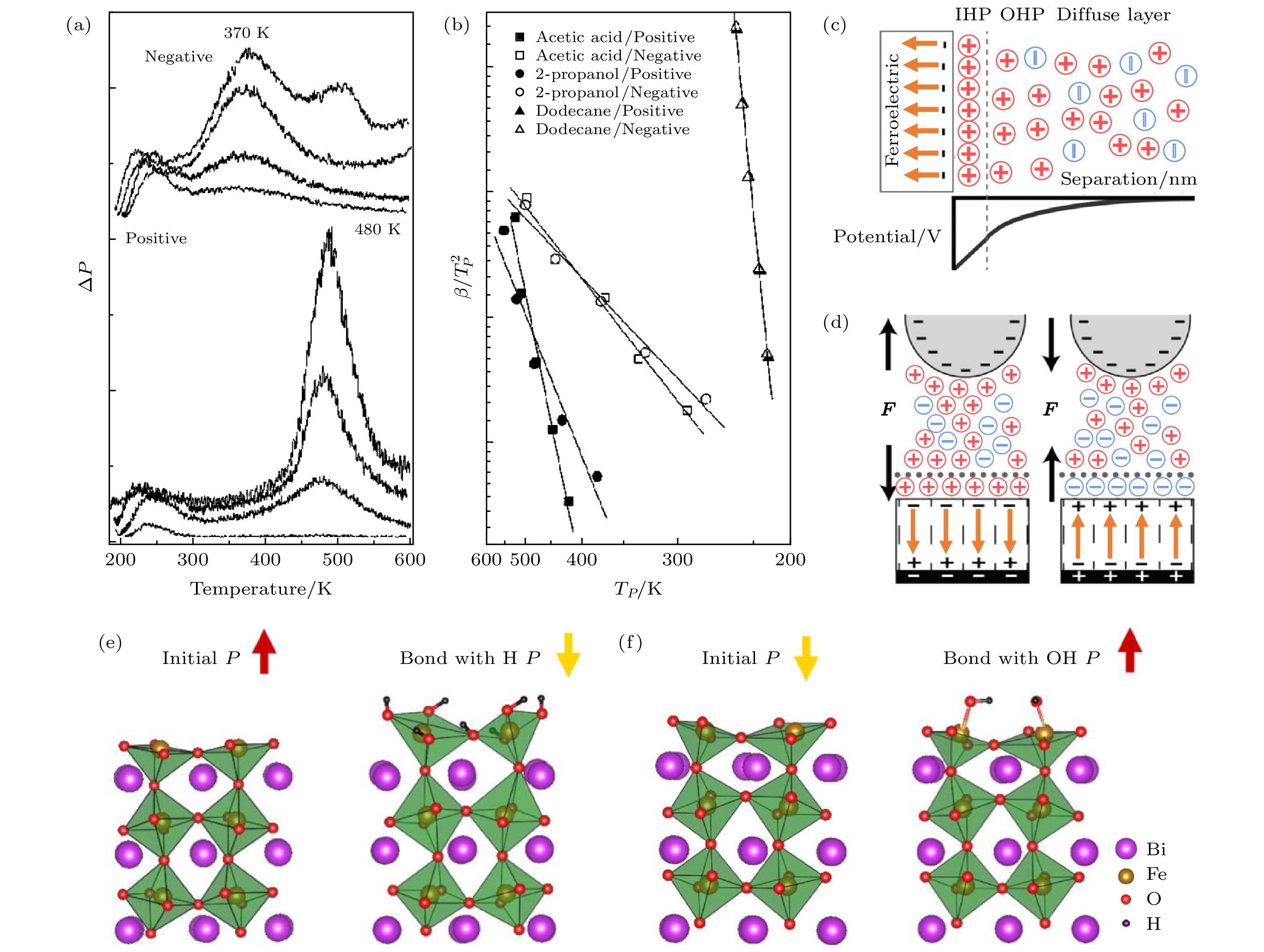
图 3 (a) LNO的正极性和负极性表面对异丙醇脱附的数据比较[32]; (b) LNO的正极性和负极性表面对乙酸、异丙醇和十二烷脱附的β/TP2-TP图[33]; (c) 电解质溶液中极化向内的铁电薄膜上方的双电层结构和对应电势的示意图; (d) 带负电荷的探针与极化向内或向外的铁电薄膜之间的双电层结构和相互作用力[37]; 极化向外(e) 和向内(f) 的BFO表面分别吸附 H+ (e) 和 OH– (f) 后极化翻转的示意图[26]
Fig. 3. (a) Comparison of 2-propanol desorption from positively and negatively poled LNO[32]; (b) plots of β/TP2 versus TP for desorption of acetic acid, 2-propanol and dodecane from positively and negatively poled LNO[33]; (c) schematic of the electric double layer structure above an inward-polarized ferroelectric thin film in an electrolyte solution and a corresponding sketch of the potential; (d) the electric double layer structure and interaction force between a negatively charged probe and a ferroelectric thin film with inward or outward polarization[37]; schematic diagrams show BFO polarizations are switched from outward/inward to inward/outward after surfaces adsorbed H+ (e) or OH– (f), respectively[26].
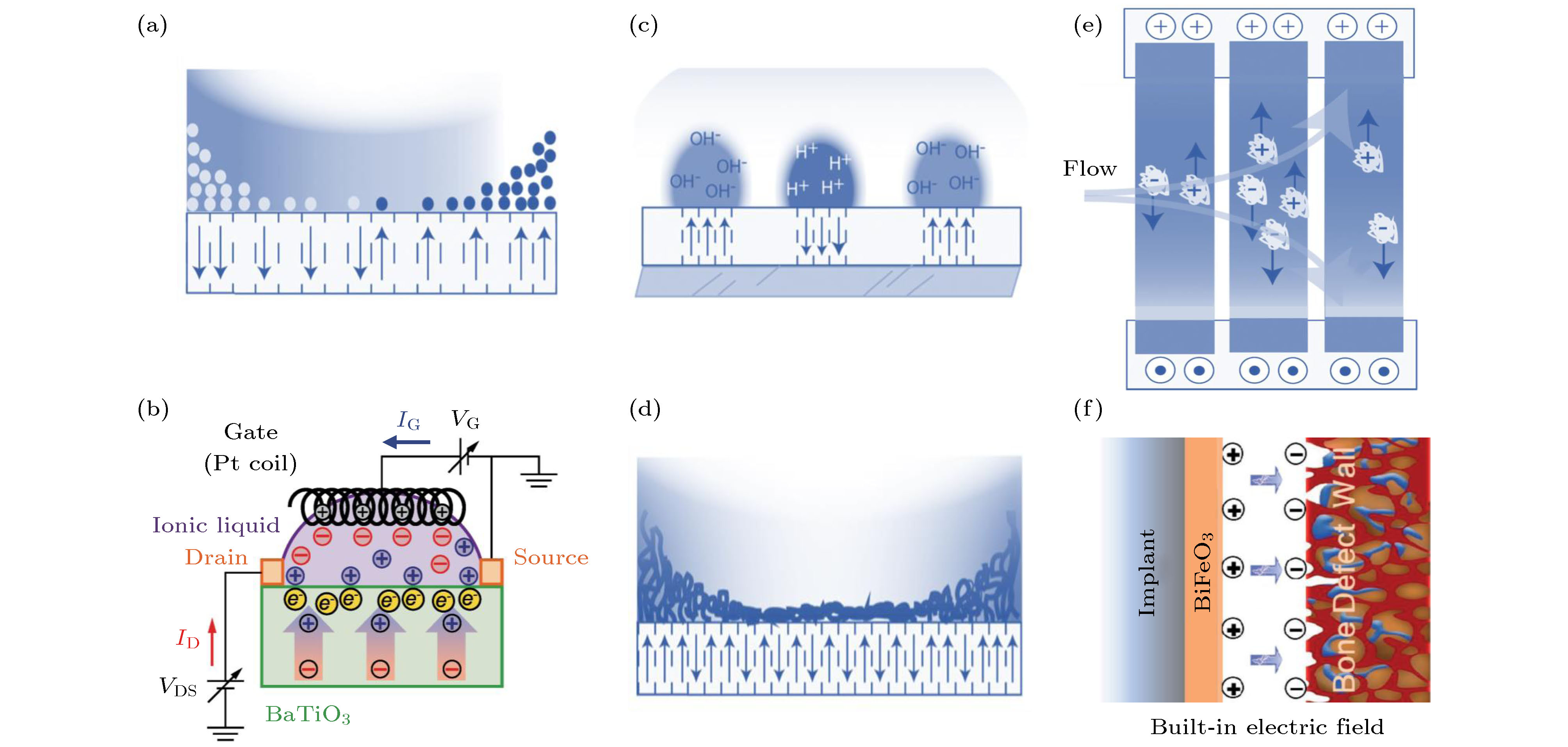
图 4 (a) 钙钛矿型铁电氧化物与液体界面形成离子梯度[35]; (b) 双电层晶体管示意图[46]; 示意图展示了钙钛矿型铁电氧化物表面电荷诱导的pH梯度(c)、极性大分子的高度变化(d), 以及利用铁电材料的表面电场控制溶液中带电粒子的流动方向(e) [35]; (f) 带正电的纳米膜植入体表面与带负电的内源性骨缺损壁之间形成内建电场[10]
Fig. 4. (a) Ionic gradient is formed in the interface between perovskite-type ferroelectric oxides and liquid[35]; (b) a schematic of the electric double-layer transistor[46]; schematic diagrams show pH gradients (c) and macromolecular height change (d) are induced by surface charges of perovskite-type ferroelectric oxides; and (e) the flow direction of charged particles in solution is controlled by the surface electric field of ferroelectric materials[35]; (f) a built-in electric field is formed between the electropositive nanofilm implant surface and electronegative endogenous bone defect wall[10].
图 5 极化向外(a)或极化向内(b)的BTO与Ag+还原电位、Pb2+氧化电位的能带结构图, EV代表价带顶的能量, EC代表导带底的能量, EF代表费米能, P代表极化[53]; (c)和(d) 分别展示了BWO(001)中带不同电荷畴壁的水分解示意图[57]; (e) 计算模拟通过动态翻转PTO的铁电极化可以实现将一氧化氮合成为氮气和氧气的过程示意图; (f) 铁电极化调控分子与表面相互作用的示意图[11]
Fig. 5. Energy band structure of BTO with outward (a) or inward (b) polarization and Ag+ reduction potential, Pb2+ oxidation potential, respectively, EV is the valence band edge, EC the conduction band edge, EF the Fermi level, P polarization[53]; (c) and (d) show the schematics of water splitting mechanism of different charged domain walls in BWO (001), respectively[57]; (e) simulation shows the decomposition process of nitric oxide into nitrogen and oxygen can be realized by dynamically switching ferroelectric polarization of PTO; (f) schematic of molecule-surface interaction controlled by ferroelectric polarization[11].
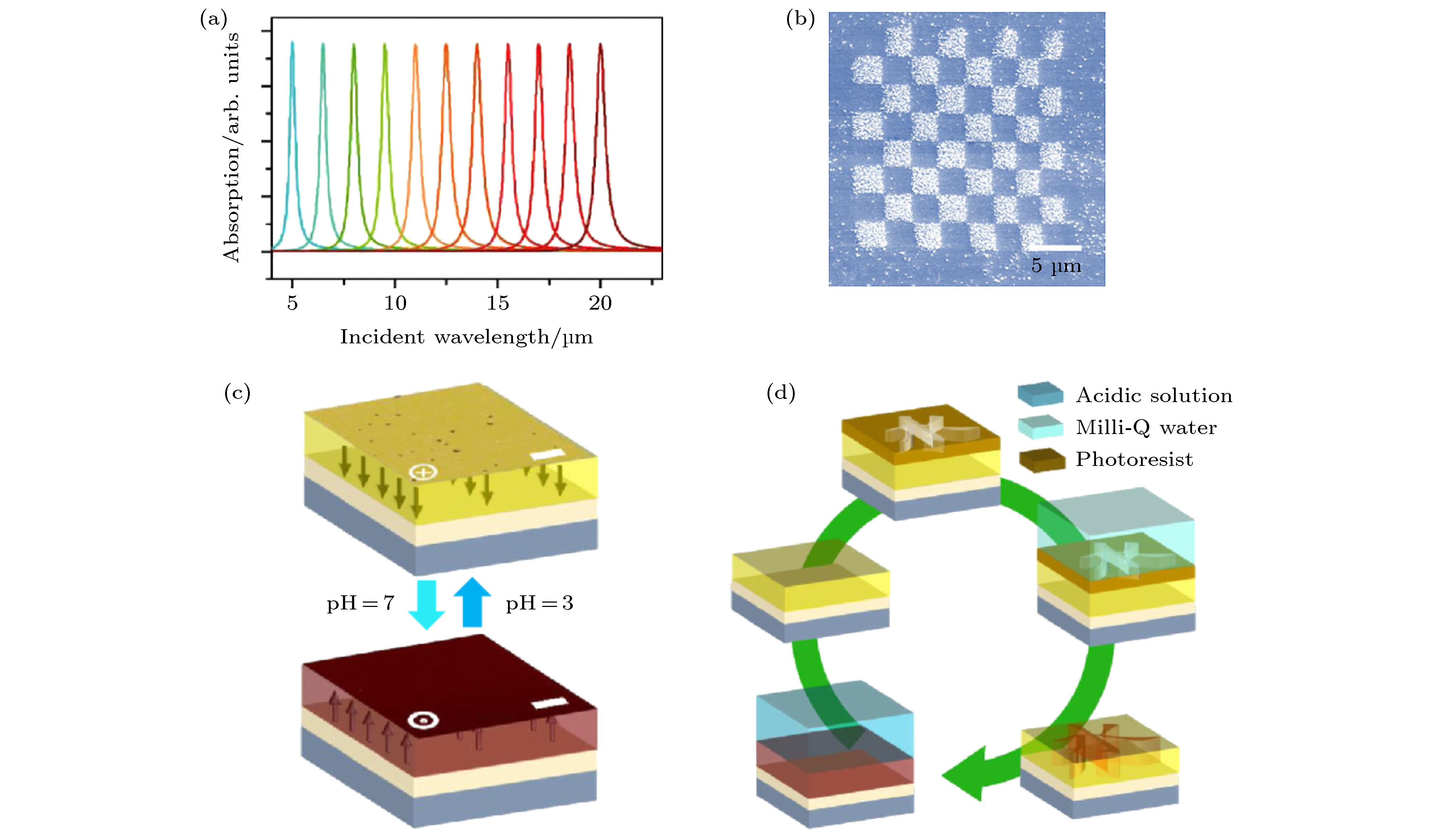
图 6 (a) 基于铁电畴调谐的石墨烯等离子体光电探测器的自驱动微型光谱仪的吸收特性, 目标光谱范围从5—20 μm[12]; (b) 扫描探针显微镜观察到的具有周期性畴结构的PZT薄膜上的Ag光沉积[61]; (c) BFO薄膜暴露在酸性溶液(pH = 3)后的铁电极化翻转为向内, 暴露在纯水溶液后极化翻转为向外; (d) 将BFO暴露在不同pH值的水溶液中, 印刷和消除铁电极化的原理图[26]
Fig. 6. (a) The absorption characteristics of a self-driven micro-spectrometer based on the graphene plasmonic photodetector tuned by ferroelectric domains, the target spectrum ranges from 5 to 20 μm[12]; (b) Ag photodeposition on the PZT thin film with periodic domain structure observed by scanning probe microscopy[61]; (c) the ferroelectric polarization of BFO thin film is switched to inward after being exposed to acidic solution (pH = 3) and then outward after being exposed to Milli-Q water; (d) the schematic of printing and erasing the ferroelectric polarization by exposing the BFO to aqueous solution with different pH value[26].
-
[1] Valasek J 1920 Phys. Rev. 17 475
 Google Scholar
Google Scholar
[2] Setter N, Damjanovic D, Eng L, Fox G, Gevorgian S, Hong S, Kingon A, Kohlstedt H, Park N Y, Stephenson G B, Stolitchnov I, Taganstev A K, Taylor D V, Yamada T, Streiffer S 2006 J. Appl. Phys. 100 051606
 Google Scholar
Google Scholar
[3] Auciello O, Scott J F, Ramesh R 1998 Phys. Today 51 22
 Google Scholar
Google Scholar
[4] Dawber M, Rabe K M, Scott J F 2005 Rev. Mod. Phys. 77 1083
 Google Scholar
Google Scholar
[5] Li Y J, Wang J J, Ye J C, et al. 2015 Adv. Funct. Mater. 25 3405
 Google Scholar
Google Scholar
[6] Ishiwata S, Taguchi Y, Murakawa H, Onose Y, Tokura Y 2008 Science 319 1643
 Google Scholar
Google Scholar
[7] Li D, Zhao M H, Garra J, Kolpak A M, Rappe A M, Bonnell D A, Vohs J M 2008 Nat. Mater. 7 473
 Google Scholar
Google Scholar
[8] Song J, Kim T L, Lee J, et al. 2018 Nano Res. 11 642
 Google Scholar
Google Scholar
[9] Grilli S, Coppola S, Nasti G, Vespini V, Gentile G, Ambrogi V, Carfagna C, Ferraro P 2014 Rsc Adv. 4 2851
 Google Scholar
Google Scholar
[10] Liu Y, Zhang X, Cao C, et al. 2017 Adv. Funct. Mater. 27 1703771
 Google Scholar
Google Scholar
[11] Kakekhani A, Ismail-Beigi S 2015 ACS Catal. 5 4537
 Google Scholar
Google Scholar
[12] Guo J, Liu Y, Lin Y, Tian Y, Zhang J, Gong T, Cheng T, Huang W, Zhang X 2019 Nanoscale 11 20868
 Google Scholar
Google Scholar
[13] Goniakowski J, Finocchi F, Noguera C 2007 Reps. Prog. Phys. 71 016501
 Google Scholar
Google Scholar
[14] Damjanovic D 1998 Reps. Prog. Phys. 61 1267
 Google Scholar
Google Scholar
[15] Noguera C 2000 J. Phys.: Condens. Matter 12 R367
 Google Scholar
Google Scholar
[16] Garrity K, Kakekhani A, Kolpak A, Ismail-Beigi S 2013 Phys. Rev. B 88 045401
 Google Scholar
Google Scholar
[17] Spanier J E, Kolpak A M, Urban J J, Grinberg I, Ouyang L, Yun W S, Rappe A M, Park H 2006 Nano Lett. 6 735
 Google Scholar
Google Scholar
[18] Jia C L, Urban K 2004 Science 303 2001
 Google Scholar
Google Scholar
[19] Ebensperger C, Meyer B 2011 Phys. Status Solidi B 248 2229
 Google Scholar
Google Scholar
[20] Munkholm A, Streiffer S K, Ramana Murty M V, et al. 2002 Phys. Rev. Lett. 88 016101
 Google Scholar
Google Scholar
[21] Kakekhani A, Ismail-Beigi S, Altman E I 2016 Surf. Sci. 650 302
 Google Scholar
Google Scholar
[22] Kalinin S V, Johnson C Y, Bonnell D A 2002 J. Appl. Phys. 91 3816
 Google Scholar
Google Scholar
[23] Apostol N G, Stoflea L E, Lungu G A, Tache C A, Popescu D G, Pintilie L, Teodorescu C M 2013 Mater. Sci. Eng. B 178 1317
 Google Scholar
Google Scholar
[24] Fong D D, Kolpak A M, Eastman J A, et al. 2006 Phys. Rev. Lett. 96 127601
 Google Scholar
Google Scholar
[25] Gao P, Liu H J, Huang Y L, et al. 2016 Nat. Commun. 7 11318
 Google Scholar
Google Scholar
[26] Tian Y, Wei L, Zhang Q, Huang H, Zhang Y, Zhou H, Ma F, Gu L, Meng S, Chen L Q, Nan C W, Zhang J 2018 Nat. Commun. 9 3809
 Google Scholar
Google Scholar
[27] Kalinin S V, Bonnell D A 2001 Phys. Rev. B 63 125411
 Google Scholar
Google Scholar
[28] Ying X, Haitao Y 2014 Chem. Res. Chin. Univ. 30 794
 Google Scholar
Google Scholar
[29] Zhu L, Yao K L, Liu Z L, Zhang D H 2009 Phys. Lett. A 373 2374
 Google Scholar
Google Scholar
[30] Song C, Gao J, Liu J, Yang Y, Tian C, Hong J, Weng H, Zhang J 2020 ACS Appl. Mater. Interfaces 12 4150
 Google Scholar
Google Scholar
[31] Dai J Q, Xu J W, Zhu J H 2017 Appl. Surf. Sci. 392 135
 Google Scholar
Google Scholar
[32] Yun Y, Kampschulte L, Li M, Liao D, Altman E I 2007 J. Phys. Chem. C 111 13951
 Google Scholar
Google Scholar
[33] Yun Y, Altman E I 2007 J. Am. Chem. Soc. 129 15684
 Google Scholar
Google Scholar
[34] Kolpak A M, Grinberg I, Rappe A M 2007 Phys. Rev. Lett. 98 166101
 Google Scholar
Google Scholar
[35] Ferris R, Yellen B, Zauscher S 2012 Small 8 28
 Google Scholar
Google Scholar
[36] Israelachvili J N 2011 Intermolecular and Surface Forces (San Diego: Academic Press) pp293–294
[37] Ferris R J, Lin S, Therezien M, Yellen B B, Zauscher S 2013 ACS Appl. Mater. Interfaces 5 2610
 Google Scholar
Google Scholar
[38] Nishino R, Kozuka Y, Kagawa F, Uchida M, Kawasaki M 2018 Appl. Phys. Lett. 113 143501
 Google Scholar
Google Scholar
[39] Shin J, Nascimento V B, Geneste G, Rundgren J, Plummer E W, Dkhil B, Kalinin S V, Baddorf A P 2009 Nano Lett. 9 3720
 Google Scholar
Google Scholar
[40] Wang R V, Fong D D, Jiang F, et al. 2009 Phys. Rev. Lett. 102 047601
 Google Scholar
Google Scholar
[41] Jiang B, Bai Y, Chu W Y, Shi S Q, Qiao L J, Su Y J 2008 Appl. Surf. Sci. 254 5594
 Google Scholar
Google Scholar
[42] Sun X, Su Y J, Li X, Gao K W, Qiao L J 2012 J. Appl. Phys. 111 094110
 Google Scholar
Google Scholar
[43] Li X, Bai Y, Wang B C, Su Y J 2015 J. Appl. Phys. 118 094104
 Google Scholar
Google Scholar
[44] Lee H, Kim T H, Patzner J, Lu H, Lee J, Zhou H, Chang W, Mahanthappa M, Tsymbal E Y, Gruverman A, Eom C B 2016 Nano Lett. 16 2400
 Google Scholar
Google Scholar
[45] Du H, Lin X, Xu Z, Chu D 2015 J. Mater. Sci. 50 5641
 Google Scholar
Google Scholar
[46] Ito M, Matsubara Y, Kozuka Y, et al. 2014 Appl. Phys. Lett. 104 222101
 Google Scholar
Google Scholar
[47] Reiss B D, Bai G R, Auciello O, Ocola L E, Firestone M A 2006 Appl. Phys. Lett. 88 083903
 Google Scholar
Google Scholar
[48] Hong J, Kim B S, Char K, Hammond P T 2011 Biomacromolecules 12 2975
 Google Scholar
Google Scholar
[49] Yu Y, Wang X 2018 Adv. Mater. 30 1800154
 Google Scholar
Google Scholar
[50] Chen F, Huang H, Guo L, Zhang Y, Ma T 2019 Angew. Chem. Int. Ed. 58 10061
 Google Scholar
Google Scholar
[51] Cui Y, Briscoe J, Dunn S 2013 Chem. Mater. 25 4215
 Google Scholar
Google Scholar
[52] Giocondi J L, Rohrer G S 2001 J. Phys. Chem. B 105 8275
 Google Scholar
Google Scholar
[53] Bhardwaj A, Burbure N V, Gamalski A, Rohrer G S 2010 Chem. Mater. 22 3527
 Google Scholar
Google Scholar
[54] Tan C, Wang J, Zhong X, Jiang J, Zhang X, Ding Y 2017 Mater. Des. 129 186
 Google Scholar
Google Scholar
[55] Jones P M, Dunn S 2007 Nanotechnology 18 185702
 Google Scholar
Google Scholar
[56] Sun Y, Nemanich R J 2011 J. Appl. Phys. 109 104302
 Google Scholar
Google Scholar
[57] Ullah R, Pei M, Wu J, et al. 2020 ACS Appl. Energy Mater. 3 4149
 Google Scholar
Google Scholar
[58] Wang X, Yang D, Zhang H M, Song C, Wang J, Tan G, Zheng R, Dong S, Cheong S W, Zhang J 2019 Phys. Rev. B 99 054106
 Google Scholar
Google Scholar
[59] Medford A J, Vojvodic A, Hummelshøj J S, Voss J, Abild-Pedersen F, Studt F, Bligaard T, Nilsson A, Nørskov J K 2015 J. Catal. 328 36
 Google Scholar
Google Scholar
[60] Ostrovskii I V, Nadtochiy A B 2005 Appl. Phys. Lett. 86 222902
 Google Scholar
Google Scholar
[61] Kalinin S V, Bonnell D A, Alvarez T, Lei X, Hu Z, Shao R, Ferris J H 2004 Adv. Mater. 16 795
 Google Scholar
Google Scholar
[62] Christophis C, Cavalcanti-Adam E A, Hanke M, et al. 2013 Biointerphases 8 27
 Google Scholar
Google Scholar
[63] Li D, Bonnell D A 2008 Annu. Rev. Mater. Res. 38 351
 Google Scholar
Google Scholar
[64] Chen X, Karpinski P, Shvedov V, et al. 2015 Appl. Phys. Lett. 107 141102
 Google Scholar
Google Scholar
[65] Shur V Y, Lobov A I, Shur A G, et al. 2005 Appl. Phys. Lett. 87 022905
 Google Scholar
Google Scholar
计量
- 文章访问数: 15953
- PDF下载量: 559
- 被引次数: 0














 下载:
下载: