-
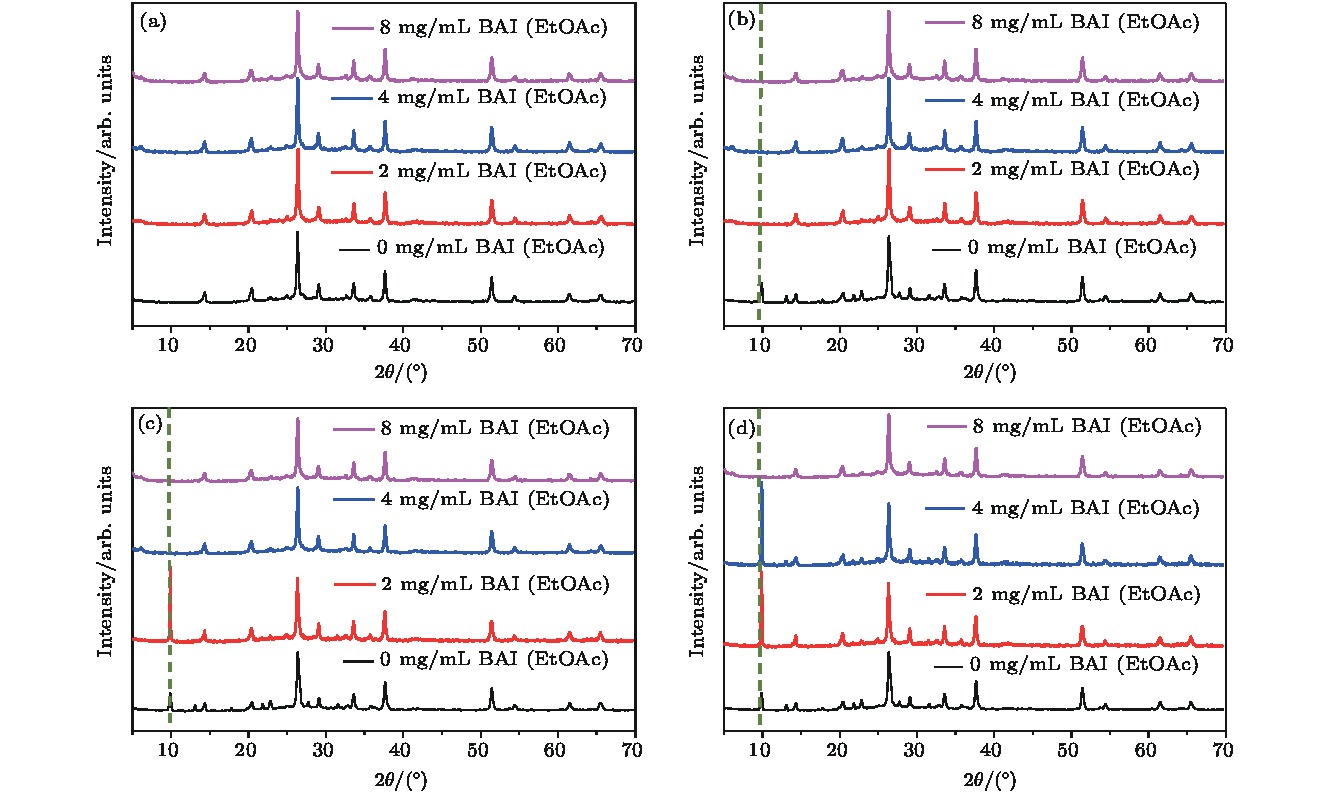
有机-无机杂化钙钛矿中的有机阳离子组分具有在光照和加热条件下本征的化学不稳定性, 而全无机钙钛矿有望从根本上解决组分稳定性问题. 但是, 全无机钙钛矿在湿度条件下, 极易相变为非光学活性的δ相. 本文以CsPbI2Br全无机钙钛矿为对象, 研究不同碳链长度的有机铵盐表面处理对于钙钛矿湿稳定性和器件光电性能的影响. 实验结果表明, 有机铵盐碳链的增长显著改善钙钛矿相稳定性. 其中, 当用碘化丁铵处理时, CsPbI2Br全无机钙钛矿表现出最佳的湿稳定性. 随着碘化丁铵的处理浓度的增加, 钙钛矿的湿稳定性进一步改善. 当用适宜浓度的碘化丁铵处理CsPbI2Br薄膜时, 钙钛矿层表层的丁铵阳离子对电荷传输不会有明显阻碍, 可以获得优良的器件效率. 总之, 适宜的有机阳离子层既能提高全无机钙钛矿的湿稳定性, 又能改善其光伏性能.All-inorganic perovskite cesium lead halides with superior stability, suitable bandgap and high absorption efficient have become a promising candidate for photovoltaic application. In all-inorganic cesium lead halide perovskites, CsPbX3 (X = Br, I) exhibits excellent photoelectric properties, which are similar to those of organic-inorganic hybrid perovskites. The CsPbI3 faces a challenge of unideal tolerant factor for perovskite phase while CsPbI3–xBrx has better tolerant factor. Among them, CsPbI2Br is one of most popular candidates because of its good thermal stability. Nevertheless, CsPbI2Br shows instability due to the phase transition caused by moisture and lower efficiency because of defects. For all inorganic perovskite devices, the alkyl chain length of surface treatment agent should be taken into account when using organic cationic passivation method. In this paper, CsPbI2Br perovskite is treated with different organic ammonium salts to enhance its phase stability. The experimental results show that α-phase CsPbI2Br is more stable with the increase of the alkyl chain length. Butylamine iodine (BAI) among three kinds of surface treating agents is proved to have the best defect passivation and phase stabilization effect. With the increase of alkyl chain length, the hydrophobicity of the organic molecular layer increases, which plays a crucial role in protecting optically active CsPbI2Br. Meanwhile, it is found that the stability of perovskite is enhanced with the concentration of the BAI solution increasing. This should be related to the organic cation termination formed on the surface of CsPbI2Br film. Solar cell devices based on the CsPbI2Br thin films treated with different concentrations of BAI are assembled and then the effect of organic ion surface treatment on the photoelectric performance of batteries is further explored. The experimental results show that when the concentration of BAI is relatively high (4 mg/mL and 8 mg/mL), the device’s photovoltaic performance decreases especially the photocurrent obviously decreases, while the post-treatment process using 2 mg/mL BAI will enhance not only the phase stability but also the photovoltaic parameters after defect passivation. Considering both humidity resistance and device efficiency, this work demonstrates that the CsPbI2Br thin film with suitable BAI treatment can improve the wet stability of perovskite, and enhance the photovoltaic performance.
[1] Stranks S D, Eperon G E, Grancini G, Menelaou C, Alcocer M J P, Leijtens T, Herz L M, Petrozza A, Snaith H J 2013 Science 342 341
 Google Scholar
Google Scholar
[2] Lin Q Q, Armin A, Nagiri R C R, Burn P L, Meredith P 2015 Nat. Photon. 9 106
 Google Scholar
Google Scholar
[3] Fang Z M, Wang S Z, Yang S F, Ding L M 2018 Inorg. Chem. Front. 5 1690
 Google Scholar
Google Scholar
[4] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[5] Im J H, Lee C R, Lee J W, Park S W, Park N G 2011 Nanoscale 3 4088
 Google Scholar
Google Scholar
[6] Kim H S, Lee C R, Im J H, Lee K B, Moehl T, Marchioro A, Moon S J, Humphry-Baker R, Yum J H, Moser J E, Graetzel M, Park N G 2012 Sci. Rep. 2 591
 Google Scholar
Google Scholar
[7] Burschka J, Pellet N, Moon S J, Humphry-Baker R, Gao P, Nazeeruddin M K, Gratzel M 2013 Nature 499 316
 Google Scholar
Google Scholar
[8] Zhou H P, Chen Q, Li G, Luo S, Song T B, Duan H S, Hong Z R, You J B, Liu Y S, Yang Y 2014 Science 345 542
 Google Scholar
Google Scholar
[9] Yang W S, Park B W, Jung E H, Jeon N J, Kim Y C, Lee D U, Shin S S, Seo J W, Kim E K, Noh J H, Seok S I 2017 Science 356 1376
 Google Scholar
Google Scholar
[10] Liu M, Johnston M B, Snaith H J 2013 Nature 501 395
 Google Scholar
Google Scholar
[11] Laboratory NREL https://www.nrel.gov/pv/assets/pdfs/pv-efficiency-chart.20190103.pdf [2019-03-04]
[12] Zuo C T, Bolink H J, Han H W, Huang J S, Cahen D, Ding L M 2016 Adv. Sci. 3 1500324
 Google Scholar
Google Scholar
[13] Nenon D P, Christians J A, Wheeler L M, Blackburn J L, Sanehira E M, Dou B J, Olsen M L, Zhu K, Berrya J J, Luther J M 2016 Energ. Environ. Sci. 9 2072
 Google Scholar
Google Scholar
[14] Sutton R J, Eperon G E, Miranda L, Parrott E S, Kamino B A, Patel J B, Horantner M T, Johnston M B, Haghighirad A A, Moore D T, Snaith H J 2016 Adv. Energy Mater. 6 1502458
 Google Scholar
Google Scholar
[15] Frolova L A, Anokhin D V, Piryazev A A, Luchkin S Y, Dremova N N, Stevenson K J, Troshin P A 2017 J. Phys. Chem. Lett. 8 67
 Google Scholar
Google Scholar
[16] Eames C, Frost J M, Barnes P R F, O'Regan B C, Walsh A, Islam M S 2015 Nat. Commun. 6 7497
 Google Scholar
Google Scholar
[17] Liang J, Wang C X, Wang Y R, Xu Z R, Lu Z P, Ma Y, Zhu H F, Hu Y, Xiao C C, Yi X, Zhu G Y, Lv H L, Ma L B, Chen T, Tie Z X, Jin Z, Liu J 2016 J. Am. Chem. Soc. 138 15829
 Google Scholar
Google Scholar
[18] Lau C F J, Deng X F, Ma Q S, Zheng J H, Yun J S, Green M A, Huang S J, Ho-Baillie A W Y 2016 ACS Energy Lett. 1 573
 Google Scholar
Google Scholar
[19] Niezgoda J S, Foley B J, Chen A Z, Choi J J 2017 ACS Energy Lett. 2 1043
 Google Scholar
Google Scholar
[20] Li W, Rothmann M U, Liu A, Wang Z Y, Zhang Y P, Pascoe A R, Lu J F, Jiang L C, Chen Y, Huang F Z, Peng Y, Bao Q L, Etheridge J, Bach U, Cheng Y B 2017 Adv. Energy Mater. 7 1700946
 Google Scholar
Google Scholar
[21] Liu C, Li W Z, Zhang C L, Ma Y P, Fan J D, Mai Y H 2018 J. Am. Chem. Soc. 140 3825
 Google Scholar
Google Scholar
[22] Moller C K 1958 Nature 182 1436
[23] Green M A, Ho-Baillie A, Snaith H J 2014 Nat. Photon. 8 506
 Google Scholar
Google Scholar
[24] Beal R E, Slotcavage D J, Leijtens T, Bowring A R, Belisle R A, Nguyen W H, Burkhard G F, Hoke E T, McGehee M D 2016 J. Phys. Chem. Lett. 7 746
 Google Scholar
Google Scholar
[25] Mariotti S, Hutter O S, Phillips L J, Yates P J, Kundu B, Durose K 2018 ACS Appl. Mater. Inter. 10 3750
 Google Scholar
Google Scholar
[26] Marronnier A, Roma G, Boyer-Richard S, Pedesseau L, Jancu J M, Bonnassieux Y, Katan C, Stoumpos C C, Kanatzidis M G, Even J 2018 ACS Nano 12 3477
 Google Scholar
Google Scholar
[27] Wang Y, Zhang T Y, Kan M, Li Y H, Wang T, Zhao Y X 2018 Joule 2 2065
 Google Scholar
Google Scholar
[28] Yoo H S, Park N G 2018 Sol. Energ. Mat. Sol. C. 179 57
 Google Scholar
Google Scholar
[29] Li N, Zhu Z L, Chueh C C, Liu H B, Peng B, Petrone A, Li X S, Wang L D, Jen A K Y 2017 Adv. Energy Mater. 7 1601307
 Google Scholar
Google Scholar
-
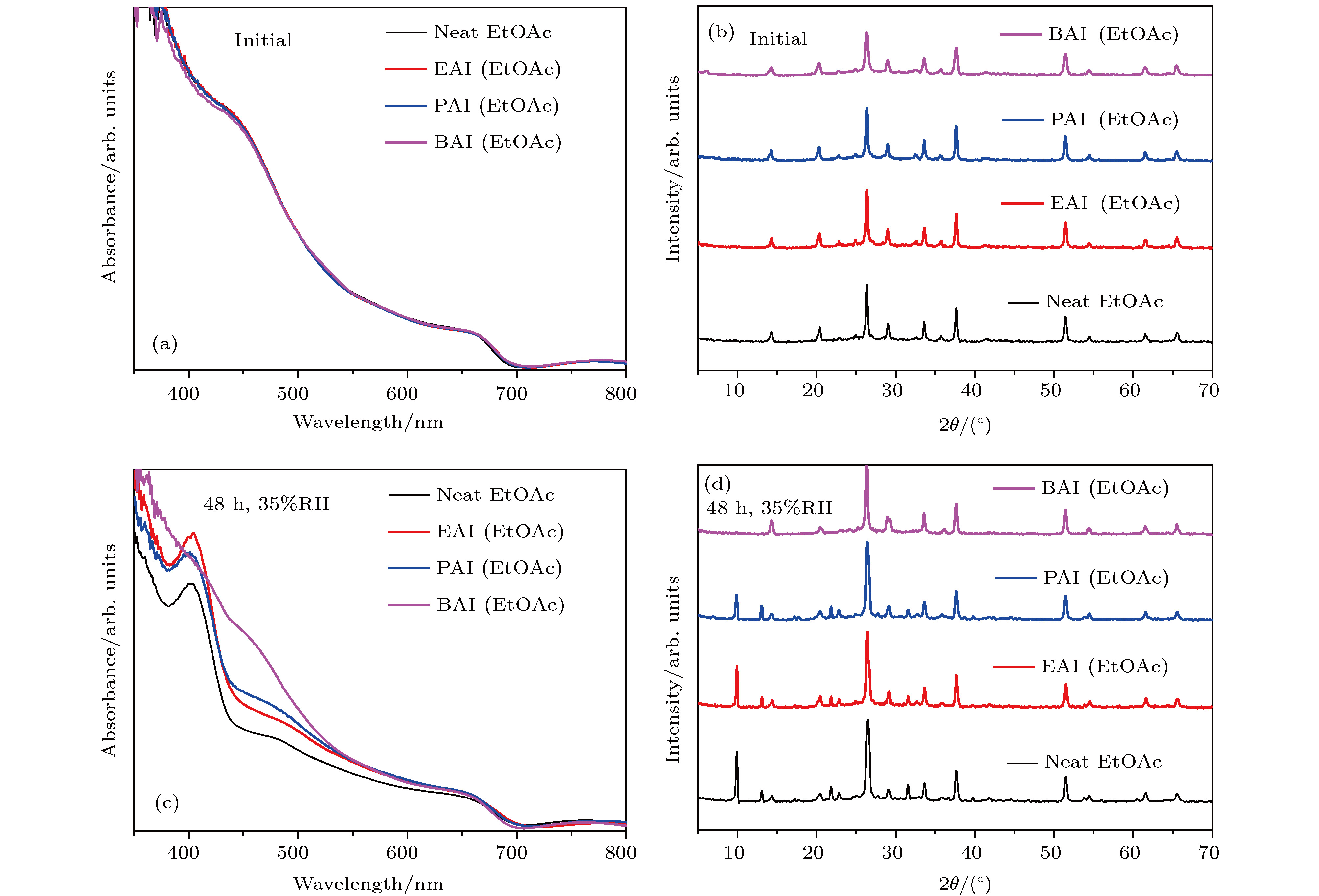
图 1 未处理、EAI、PAI和BAI处理后的CsPbI2Br薄膜 (a) 紫外可见吸收光谱(新制); (b) XRD图谱; (c) 紫外可见吸收光谱(35% RH, 48 h); (d) XRD图谱(35% RH, 48 h)
Fig. 1. (a) UV-vis spectra and (b) XRD patterns of CsPbI2Br films under EtOAc, EAI, PAI, BAI treatments, respectively. After placed in 35% RH for 48 h, (c) UV-vis spectra and (d) XRD patterns of CsPbI2Br films under EtOAc, EAI, PAI, BAI treatments, respectively.
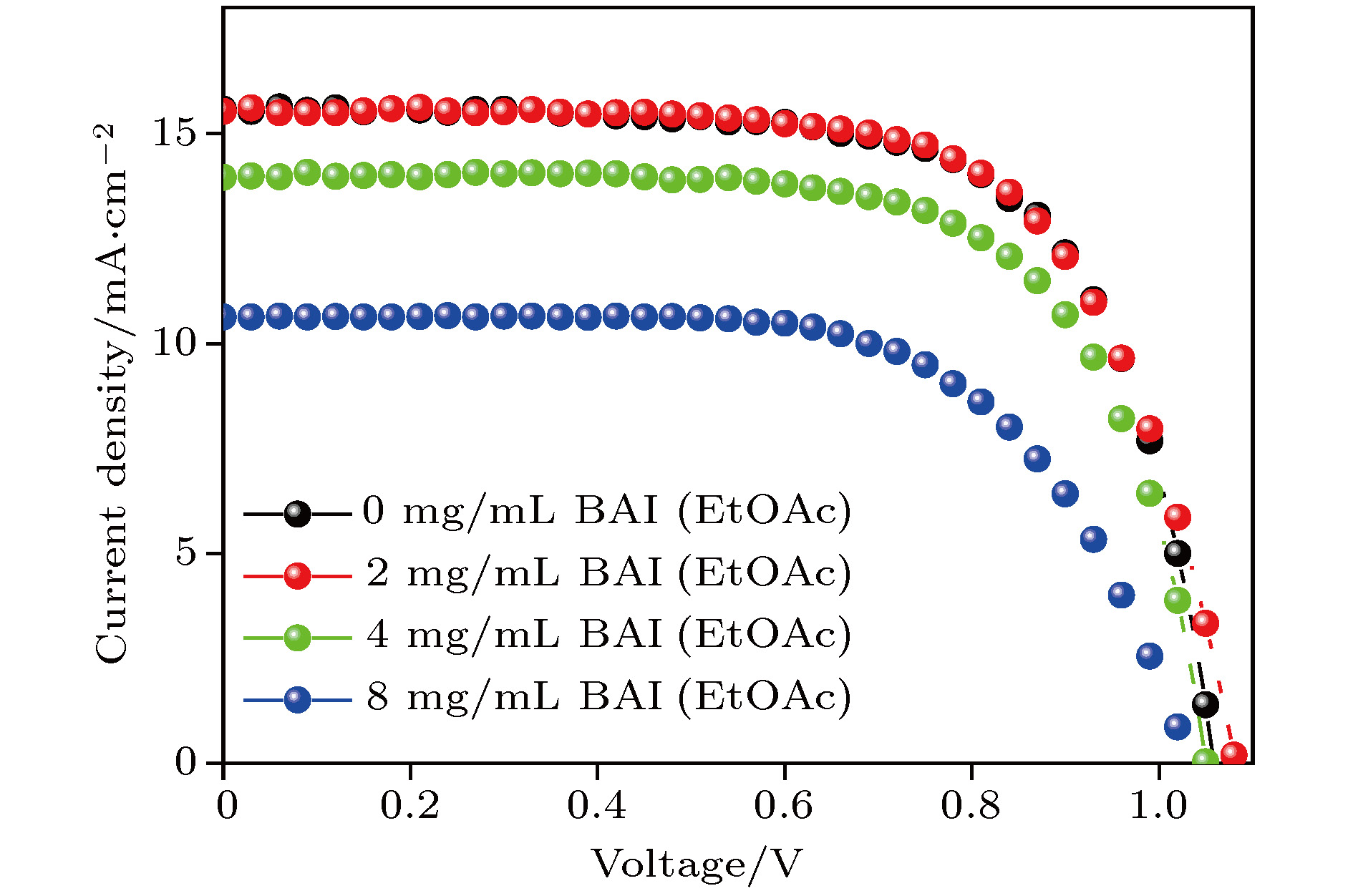
表 1 不同浓度BAI处理后CsPbI2Br钙钛矿太阳能电池的光伏参数(取32个样品均值)
Table 1. Photovoltaic parameters of CsPbI2Br perovskite solar cells under different BAI treatments (average of 32 devices)
处理方式 Jsc/mA·cm–2 Uoc/V FF/% PCE/% 0 mg/mL BAI 15.7 ± 0.13 1.05 ± 0.015 69 ± 3 11.4 ± 0.6 2 mg/mL BAI 15.8 ± 0.1 1.07 ± 0.01 68 ± 1.8 11.6 ± 0.4 4 mg/mL BAI 14.3 ± 0.1 1.05 ± 0.01 68.5 ± 2.2 10.3 ± 0.37 8 mg/mL BAI 10.7 ± 0.14 1.04 ± 0.013 66 ± 2.7 7.5 ± 0.5 -
[1] Stranks S D, Eperon G E, Grancini G, Menelaou C, Alcocer M J P, Leijtens T, Herz L M, Petrozza A, Snaith H J 2013 Science 342 341
 Google Scholar
Google Scholar
[2] Lin Q Q, Armin A, Nagiri R C R, Burn P L, Meredith P 2015 Nat. Photon. 9 106
 Google Scholar
Google Scholar
[3] Fang Z M, Wang S Z, Yang S F, Ding L M 2018 Inorg. Chem. Front. 5 1690
 Google Scholar
Google Scholar
[4] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[5] Im J H, Lee C R, Lee J W, Park S W, Park N G 2011 Nanoscale 3 4088
 Google Scholar
Google Scholar
[6] Kim H S, Lee C R, Im J H, Lee K B, Moehl T, Marchioro A, Moon S J, Humphry-Baker R, Yum J H, Moser J E, Graetzel M, Park N G 2012 Sci. Rep. 2 591
 Google Scholar
Google Scholar
[7] Burschka J, Pellet N, Moon S J, Humphry-Baker R, Gao P, Nazeeruddin M K, Gratzel M 2013 Nature 499 316
 Google Scholar
Google Scholar
[8] Zhou H P, Chen Q, Li G, Luo S, Song T B, Duan H S, Hong Z R, You J B, Liu Y S, Yang Y 2014 Science 345 542
 Google Scholar
Google Scholar
[9] Yang W S, Park B W, Jung E H, Jeon N J, Kim Y C, Lee D U, Shin S S, Seo J W, Kim E K, Noh J H, Seok S I 2017 Science 356 1376
 Google Scholar
Google Scholar
[10] Liu M, Johnston M B, Snaith H J 2013 Nature 501 395
 Google Scholar
Google Scholar
[11] Laboratory NREL https://www.nrel.gov/pv/assets/pdfs/pv-efficiency-chart.20190103.pdf [2019-03-04]
[12] Zuo C T, Bolink H J, Han H W, Huang J S, Cahen D, Ding L M 2016 Adv. Sci. 3 1500324
 Google Scholar
Google Scholar
[13] Nenon D P, Christians J A, Wheeler L M, Blackburn J L, Sanehira E M, Dou B J, Olsen M L, Zhu K, Berrya J J, Luther J M 2016 Energ. Environ. Sci. 9 2072
 Google Scholar
Google Scholar
[14] Sutton R J, Eperon G E, Miranda L, Parrott E S, Kamino B A, Patel J B, Horantner M T, Johnston M B, Haghighirad A A, Moore D T, Snaith H J 2016 Adv. Energy Mater. 6 1502458
 Google Scholar
Google Scholar
[15] Frolova L A, Anokhin D V, Piryazev A A, Luchkin S Y, Dremova N N, Stevenson K J, Troshin P A 2017 J. Phys. Chem. Lett. 8 67
 Google Scholar
Google Scholar
[16] Eames C, Frost J M, Barnes P R F, O'Regan B C, Walsh A, Islam M S 2015 Nat. Commun. 6 7497
 Google Scholar
Google Scholar
[17] Liang J, Wang C X, Wang Y R, Xu Z R, Lu Z P, Ma Y, Zhu H F, Hu Y, Xiao C C, Yi X, Zhu G Y, Lv H L, Ma L B, Chen T, Tie Z X, Jin Z, Liu J 2016 J. Am. Chem. Soc. 138 15829
 Google Scholar
Google Scholar
[18] Lau C F J, Deng X F, Ma Q S, Zheng J H, Yun J S, Green M A, Huang S J, Ho-Baillie A W Y 2016 ACS Energy Lett. 1 573
 Google Scholar
Google Scholar
[19] Niezgoda J S, Foley B J, Chen A Z, Choi J J 2017 ACS Energy Lett. 2 1043
 Google Scholar
Google Scholar
[20] Li W, Rothmann M U, Liu A, Wang Z Y, Zhang Y P, Pascoe A R, Lu J F, Jiang L C, Chen Y, Huang F Z, Peng Y, Bao Q L, Etheridge J, Bach U, Cheng Y B 2017 Adv. Energy Mater. 7 1700946
 Google Scholar
Google Scholar
[21] Liu C, Li W Z, Zhang C L, Ma Y P, Fan J D, Mai Y H 2018 J. Am. Chem. Soc. 140 3825
 Google Scholar
Google Scholar
[22] Moller C K 1958 Nature 182 1436
[23] Green M A, Ho-Baillie A, Snaith H J 2014 Nat. Photon. 8 506
 Google Scholar
Google Scholar
[24] Beal R E, Slotcavage D J, Leijtens T, Bowring A R, Belisle R A, Nguyen W H, Burkhard G F, Hoke E T, McGehee M D 2016 J. Phys. Chem. Lett. 7 746
 Google Scholar
Google Scholar
[25] Mariotti S, Hutter O S, Phillips L J, Yates P J, Kundu B, Durose K 2018 ACS Appl. Mater. Inter. 10 3750
 Google Scholar
Google Scholar
[26] Marronnier A, Roma G, Boyer-Richard S, Pedesseau L, Jancu J M, Bonnassieux Y, Katan C, Stoumpos C C, Kanatzidis M G, Even J 2018 ACS Nano 12 3477
 Google Scholar
Google Scholar
[27] Wang Y, Zhang T Y, Kan M, Li Y H, Wang T, Zhao Y X 2018 Joule 2 2065
 Google Scholar
Google Scholar
[28] Yoo H S, Park N G 2018 Sol. Energ. Mat. Sol. C. 179 57
 Google Scholar
Google Scholar
[29] Li N, Zhu Z L, Chueh C C, Liu H B, Peng B, Petrone A, Li X S, Wang L D, Jen A K Y 2017 Adv. Energy Mater. 7 1601307
 Google Scholar
Google Scholar
计量
- 文章访问数: 13947
- PDF下载量: 295
- 被引次数: 0













 下载:
下载: