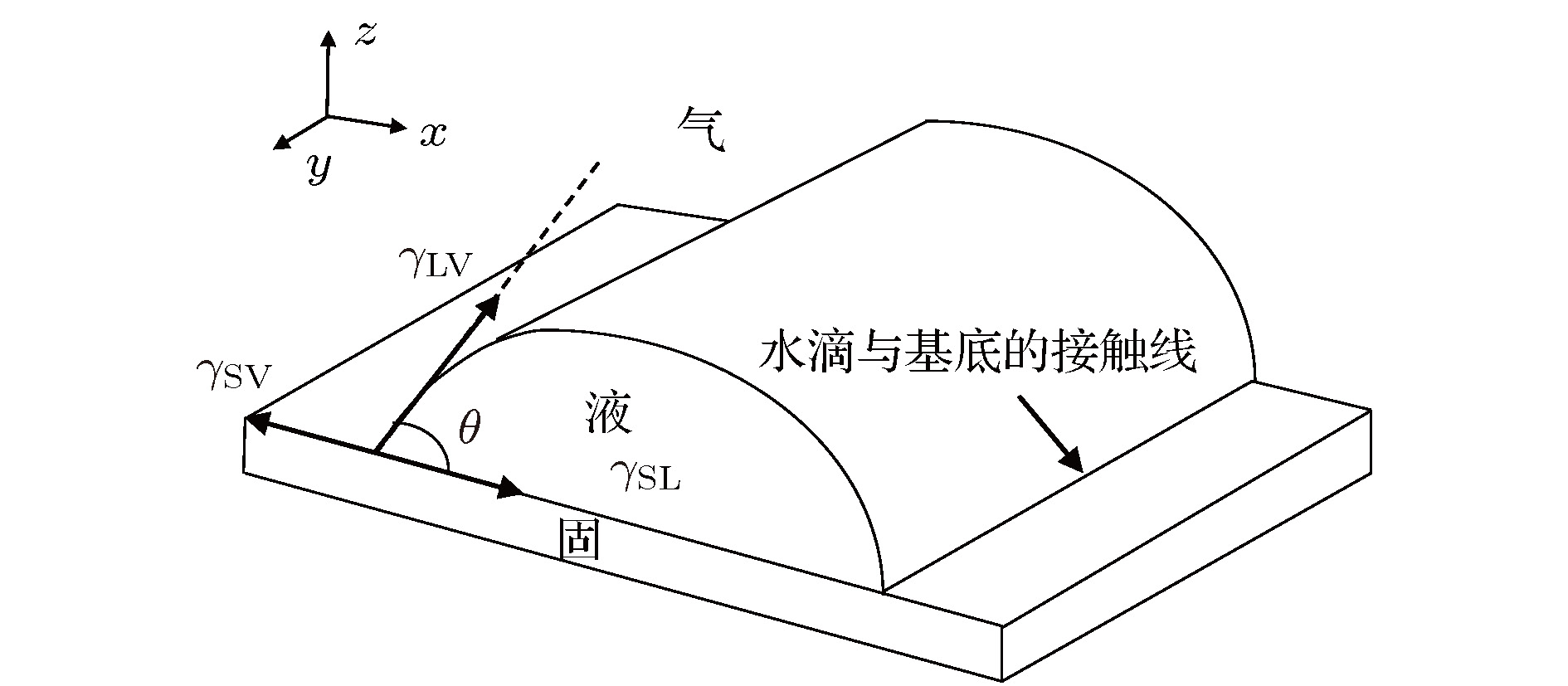
-
石墨烯因其独特的分子构型、卓越的物理化学性能而受到广泛关注. 本文首先利用分子动力学模拟比较了单层石墨烯、铜、二氧化硅三者表面的浸润性, 除了接触角的比较, 还分析了基底表面的水分子排布, 得到石墨烯表面的特征水分子排布为: 表面有两层密集的水分子层, 其中靠近基底的密集水分子层中O—H键与 垂直基底方向夹角集中在90°附近, 并且基底表面的氢键几乎都垂直于基底. 另一方面, 本文研究了石墨烯浸润透明特性, 发现在铜和二氧化硅上添加一层石墨烯, 对铜的浸润性影响较小, 对二氧化硅的浸润性影响很大, 不仅使其上接触角显著增大, 还使得基底表面的水分子排布呈现出类似单层石墨烯上的规律. 本文使用分子动力学模拟方法从微观尺度验证了文献的实验结果, 从基底表面水分子排布角度分析了石墨烯独特的浸润透明特性, 为进一步开发石墨烯在微结构设计上的应用提供了理论指导.Graphene has received a lot of attention for its excellent physical and chemical properties, and the unique wettability of graphene is still under investigation. Most of previous studies focused on graphene or carbon nanotubes, and less of them on the comparison of wettability between graphene and other materials to reveal the characteristic wettability of graphene. In the present study, the wettability of monolayer graphene, copper and silica are studied by using the molecular dynamics simulation, in which the contact angle and the water molecule arrangement (i.e. density distribution and angle distribution of water molecules) on the substrates are analyzed. The results show that although both copper and graphene are weak hydrophilic materials, there are two neat layers of water molecule structure on the surface of graphene, and water molecules are disordered on the surface of copper. Silica is a kind of strong hydrophilic material and graphene is a kind of weak hydrophilic material, but both of them have two layers of dense water molecule layer, which are in different states, on the surface. On the silica surface, the two layers of water molecules, whose densities are greatly different, are close to the substrate, and hydrogen bonds are randomly arranged, which is very different from the arrangement of water molecules on the graphene surface. By making a comparison of wettability among the three materials, the characteristic water molecule arrangement on graphene surface is obtained: there exist two layers of water molecules on the surface of graphene. Within the dense layer of water molecules near the substrate, the angles between the O−H bonds of water molecule and the vertical direction of substrate focus on 90°, while the hydrogen bonds on the surface are almost perpendicular to the substrate. Furthermore, it is found that adding a layer of graphene on copper (the main force between water molecules and copper is van der Waals force) will have a less influence on copper wettability. However, adding a layer of graphene on silica (the main force between water molecule and silica is from chemical bonds) will have a significant influence on the wettability of silica, i.e. not only the upper contact angle increases significantly, but also the arrangement of water molecules on the surface of the substrate becomes similar to that of graphene. These simulated results are found to be in agreement with the experimental results of Rafiee et al. [Rafiee J, Mi X, Gullapalli H, Thomas A V, Yavari F, Shi Y, Ajayan P M, Koratkar N A 2012 Nature 11 217]. This work can provide a theoretical guidance for further developing the applications of graphene in microstructure design.
-
Keywords:
- graphene /
- wettability /
- water /
- molecular simulation
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A J S 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[3] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[4] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau C N 2008 Nano Lett. 8 902
 Google Scholar
Google Scholar
[5] Rafiee J, Mi X, Gullapalli H, Thomas A V, Yavari F, Shi Y, Ajayan P M, Koratkar N A 2012 Nature 11 217
 Google Scholar
Google Scholar
[6] Feng L, Li S, Li Y, Li H, Zhang L, Zhai J, Song Y, Liu B, Jiang L, Zhu D 2002 Super-Hydrophobic Surfaces: From Natural to Artificial (Weinheim: Verlag GmbH & Co.KGaA) pp1857–1860
[7] Messinger J, Lubitz W, Shen J R 2014 Phys. Chem. Chem. Phys. 16 11810
 Google Scholar
Google Scholar
[8] 郭亚杰 2015 硕士学位论文 (南京: 东南大学)
Guo Y J 2015 M. S. Thesis (Nanjin: Dongnan Uinversity) (in Chinese)
[9] 王俊珺, 李涛, 李雄鹰, 李辉 2018 67 149601
 Google Scholar
Google Scholar
Wang J J, Li T, Li X Y, Li H 2018 Acta Phys. Sin. 67 149601
 Google Scholar
Google Scholar
[10] Wu Y, Aluru N R 2013 J. Phys. Chem. B 117 8802
 Google Scholar
Google Scholar
[11] Mattia D, Gogotsi Y 2008 Microfluid. Nanofluid. 5 289
 Google Scholar
Google Scholar
[12] Shin Y J, Wang Y, Huang H, Kalon G, Wee A T, Shen Z, Bhatia C S, Yang H 2010 Langmuir the Acs Journal of Surfaces & Colloids 26 3798
[13] Taherian F, Marcon V, Van Der Vegt N F A, Leroy F 2013 Langmuir the Acs Journal of Surfaces Colloids 29 1457
 Google Scholar
Google Scholar
[14] Jeong W J, Ha M Y, Yoon H S, Ambrosia M J L 2012 Langmuir 28 5360
 Google Scholar
Google Scholar
[15] Wang S, Zhang Y, Abidi N, Cabrales L 2009 Langmuir the Acs Journal of Surfaces & Colloids 25 11078
[16] Morcos I 1972 J. Chem. Phys. 57 1801
 Google Scholar
Google Scholar
[17] Schrader M E 1980 J. Phys. Chem 84 2774
 Google Scholar
Google Scholar
[18] Tadros M E, Hu P, Adamson A W 1974 J. Colloid Interface Sci. 49 184
 Google Scholar
Google Scholar
[19] Gordillo M C 2002 J. Chem. Phys. 117 3425
 Google Scholar
Google Scholar
[20] Luna M, Colchero J, Baró A M 1999 J. Phys. Chem. B 103 9576
 Google Scholar
Google Scholar
[21] Zhou H, Ganesh P, Presser V, Wander M C F, Fenter P, Kent P R C, Jiang D E, Chialvo A A, Mcdonough J, Shuford K L 2012 Phys. Rev. B 85 120
[22] Raj R, Maroo S C, Wang E N 2013 Nano Lett. 13 1509
 Google Scholar
Google Scholar
[23] 李帅 2014 硕士学位论文(西安: 西安电子科技大学)
Li S 2014 M. S. Thesis (XiAn:Xidian University) (in Chinese)
[24] Werder T, Walther J H, Jaffe R L, Halicioglu T, Koumoutsakos P 2003 J. Phys. Chem. B 107 1345
 Google Scholar
Google Scholar
[25] Akaishi A, Yonemaru T, Nakamura J 2017 ACS Omega 2 2184
 Google Scholar
Google Scholar
[26] Giulio S, Danilo S, Claudio D A, Alberto O 2011 Phys. Rev. E 84 061602
[27] Berendsen H J C, Grigera J R, Straatsma T P 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[28] Ryckaert J P, Ciccotti G, Berendsen H J C 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[29] Raina G, Gowthami T, Tamilselvi G 2017 Indian J. Sci. Technol. 9 48
[30] Munetoh S, Motooka T, Moriguchi K, Shintani A 2007 Comput. Mater. Sci. 39 334
 Google Scholar
Google Scholar
[31] Berthier J 2008 Microdrops and Digital Microfluidics (William Andrew Publishing) pp15−17
[32] Gennes P G D, Brochard-Wyart F, Quéré D 2004 Dyn. Triple Line 57 66
[33] Gordillo M C, Martí J 2008 Phys. Rev. B 78 7
[34] Gordillo M C, Martí J 2010 J. Phys. Condens. Matter 22 284111
 Google Scholar
Google Scholar
[35] Martí J, Nagy G, Guàrdia E, Gordillo M C 2006 J. Phys. Chem. B 110 23987
 Google Scholar
Google Scholar
[36] Nair R R, Blake P, Grigorenko A N, Novoselov K S, Booth T J, Stauber T, Peres N M R, Geim A K 2008 Science 320 1308
 Google Scholar
Google Scholar
[37] Malaspina D C, Schulz E P, Alarcón L M, Frechero M A, Appignanesi G A 2010 Eur. Phys. J. E 32 35
 Google Scholar
Google Scholar
[38] Homma Y, Chiashi S, Yamamoto T, Kono K, Matsumoto D, Shitaba J, Sato S 2013 Phys. Rev. Lett. 110 157402
 Google Scholar
Google Scholar
[39] 万骞 2014 硕士学位论文 (湖北: 武汉科技大学)
Wang Q 2014 M. S. Thesis (Hubei: Wuhan University of Science and Technology) (in Chinese)
[40] 陈聪, 李维仲 2009 物理化学学报 25 507
 Google Scholar
Google Scholar
Chen C, Li W Z 2009 Acta Phys. Chim. Sin. 25 507
 Google Scholar
Google Scholar
[41] 杨忠志, 崔宝秋 2007 物理化学学报 23 1332
Yang Z Z, Cui B Q 2007 Acta Phys. Chim. Sin. 23 1332
[42] Shih C J, Strano M S, Blankschtein D 2013 Nat. Mater. 12 866
 Google Scholar
Google Scholar
-
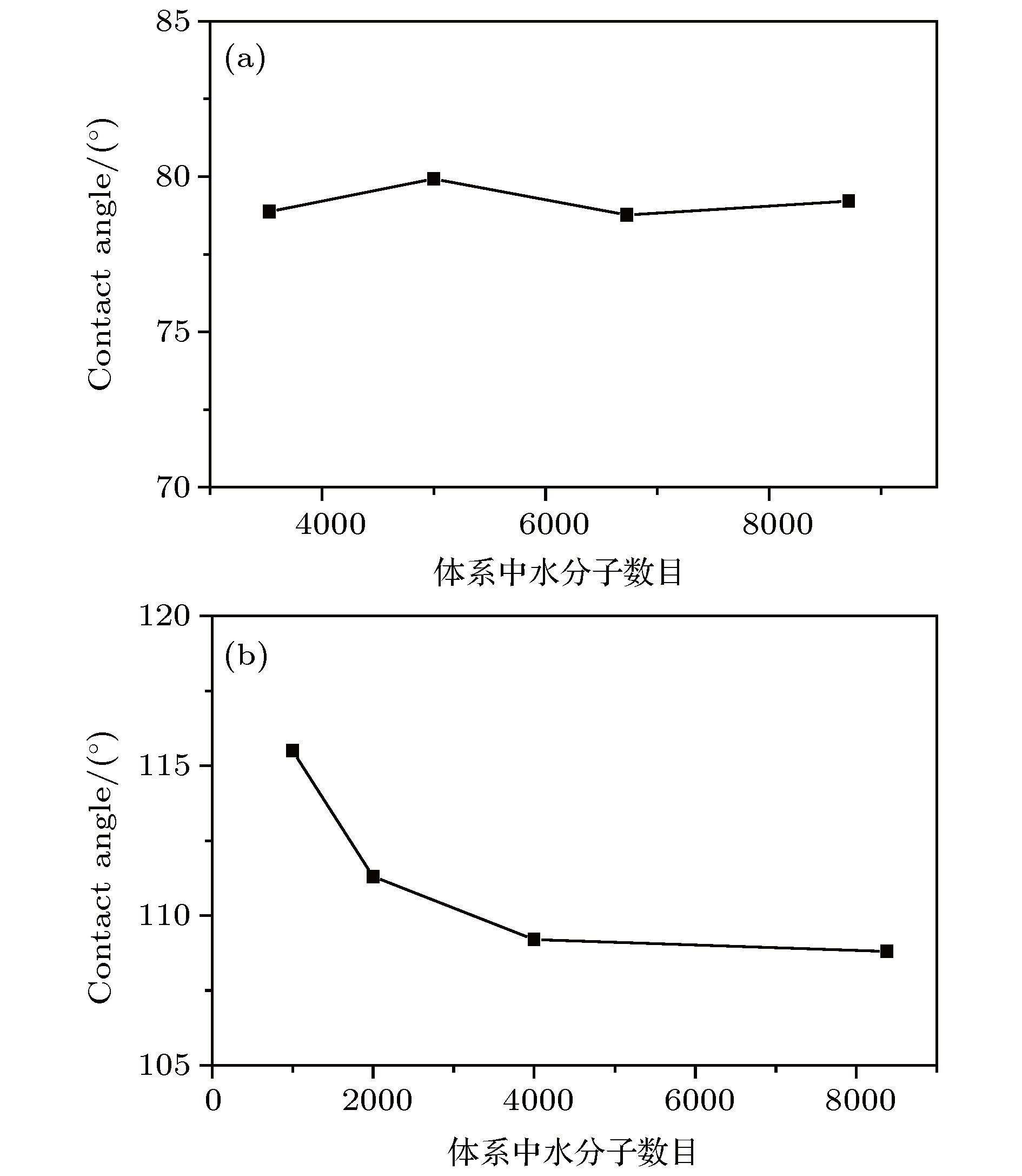
图 4 不同体系中接触角随水分子数目的变化 (a)无限长液柱状模拟体系中接触角与水分子数目的关系; (b)三维液滴状模拟体系中接触角与水分子数目的关系
Fig. 4. Changes of contact angles over number of water molecules inside systems: (a) The relationship between the contact angle and the number of water molecules in an infinite length liquid column system; (b) the relationship between the contact angle and the number of water molecules in three-dimensional droplet system.
图 6 水滴中心轴上沿z轴方向的水分子密度分布图 (a)水分子数目为3528; (b)水分子数目为5000; (c)水分子数目为6728; (d)水分子数目为8712
Fig. 6. Water molecule density distribution of the central axis of the water droplet along the z axis: (a) The system with 3528 water molecules; (b) the system with 5000 water molecules; (c) the system with 6728 water molecules; (d) the system with 8712 water molecules.
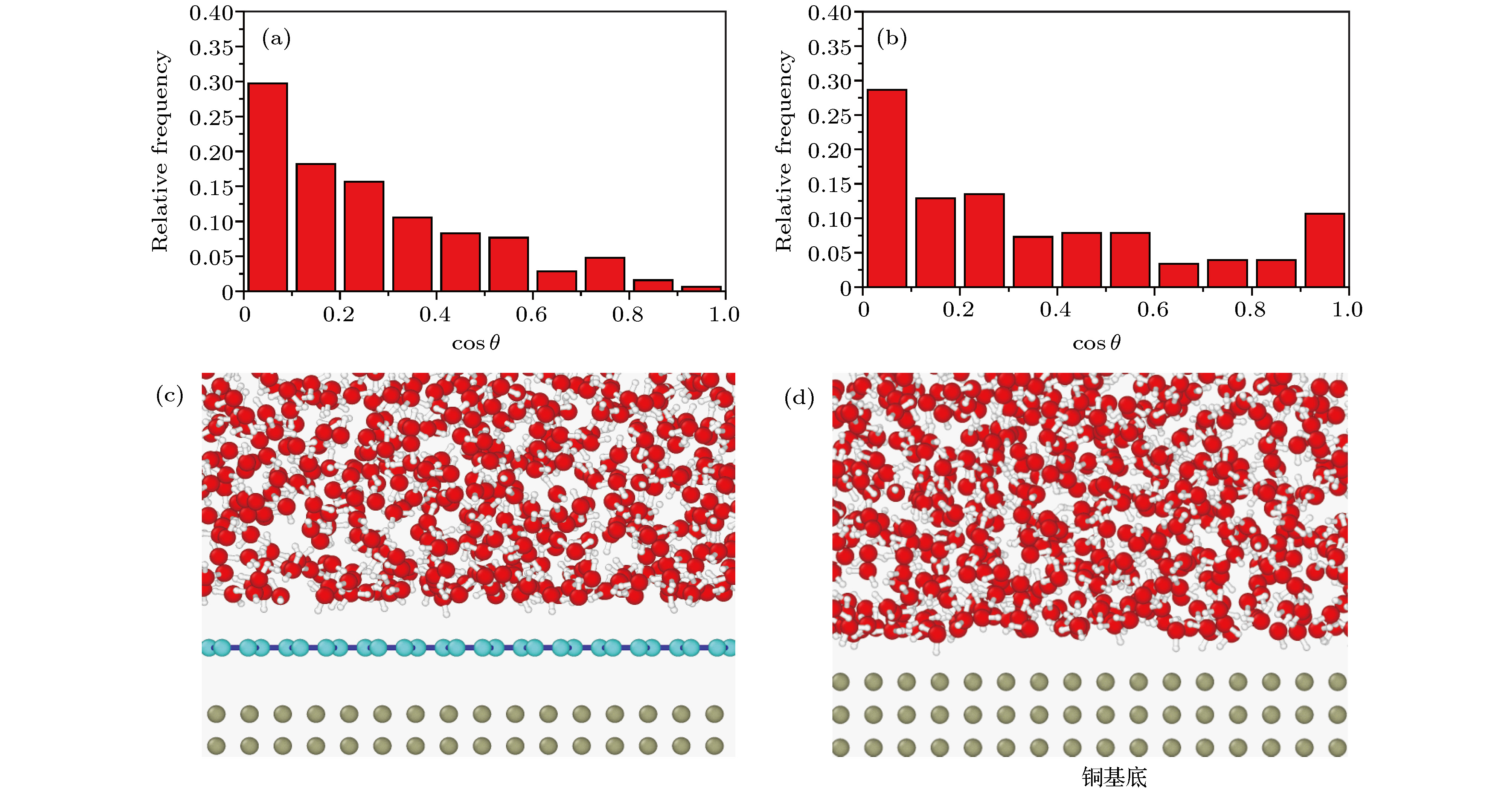
图 10 不同基底上水分子排布情况 (a)二氧化硅基底上水分子排布的局部视图; (b)单层石墨烯上水分子排布的局部视图
Fig. 10. The distribution of water molecules in different substrates: (a) A local view of the arrangement of water molecules on silicon dioxide substrate; (b) a local view of the arrangement of water molecules on a single graphene substrate.
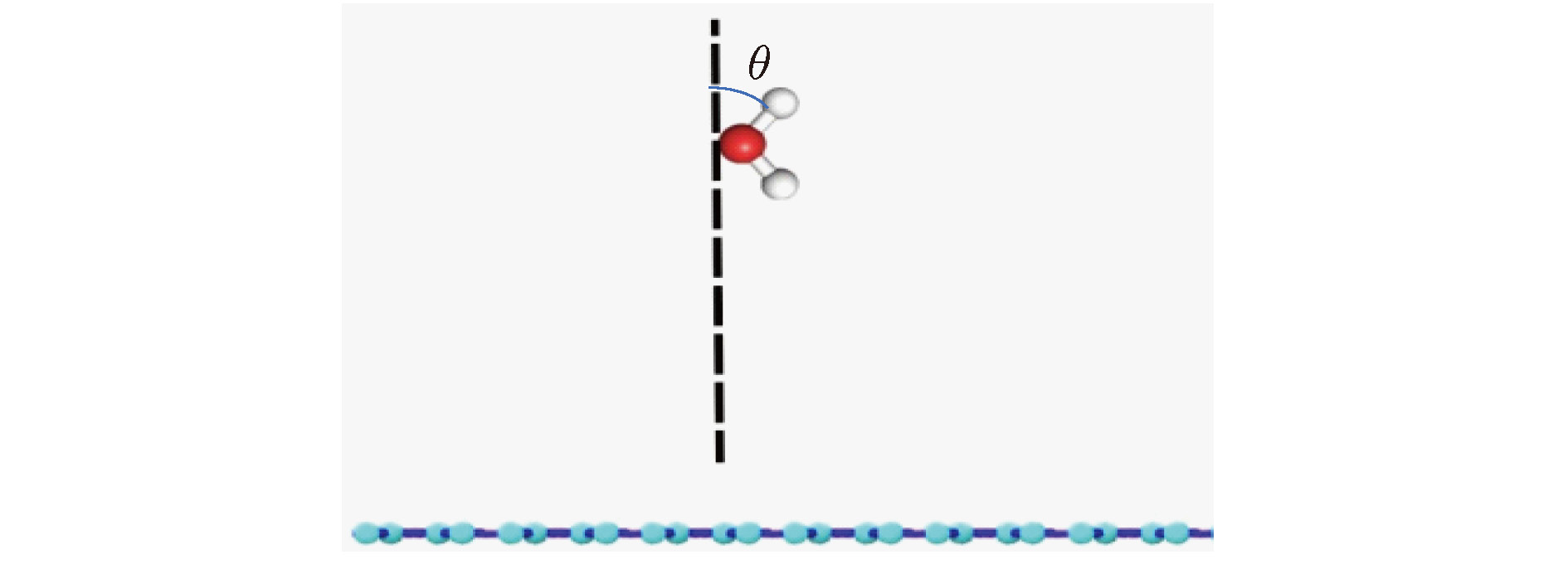
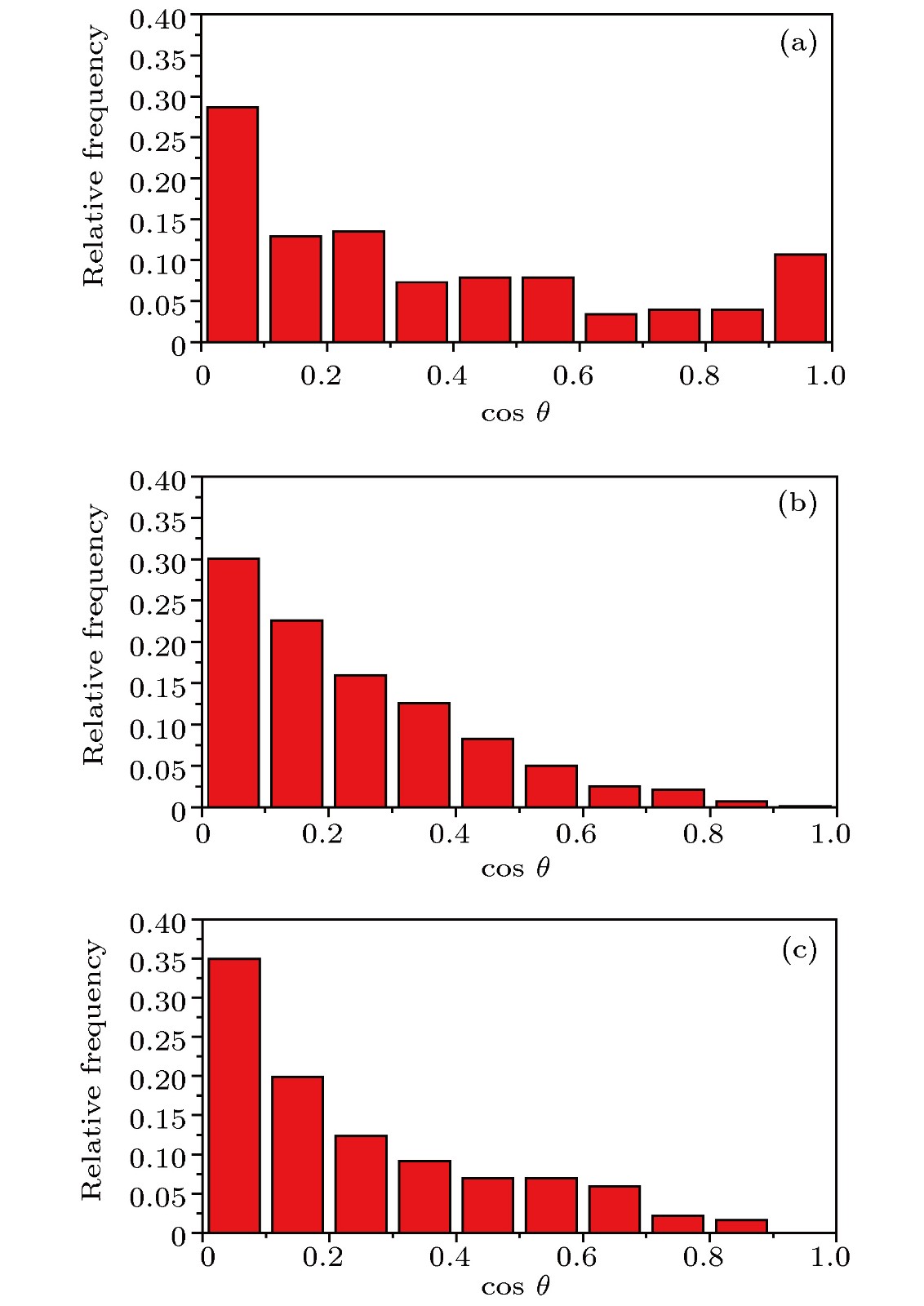
图 9 不同基底上水分子中O—H键与垂直基底方向夹角余弦cosθ的频率分布图 (a)基底为铜; (b)基底为二氧化硅; (c)基底为石墨烯
Fig. 9. Frequency distribution diagram of cosθ between O—H bond in water molecule and vertical base direction in systems with different bases: (a) The basement is copper; (b) the basement is silicon dioxide; (c) the basement is graphene.
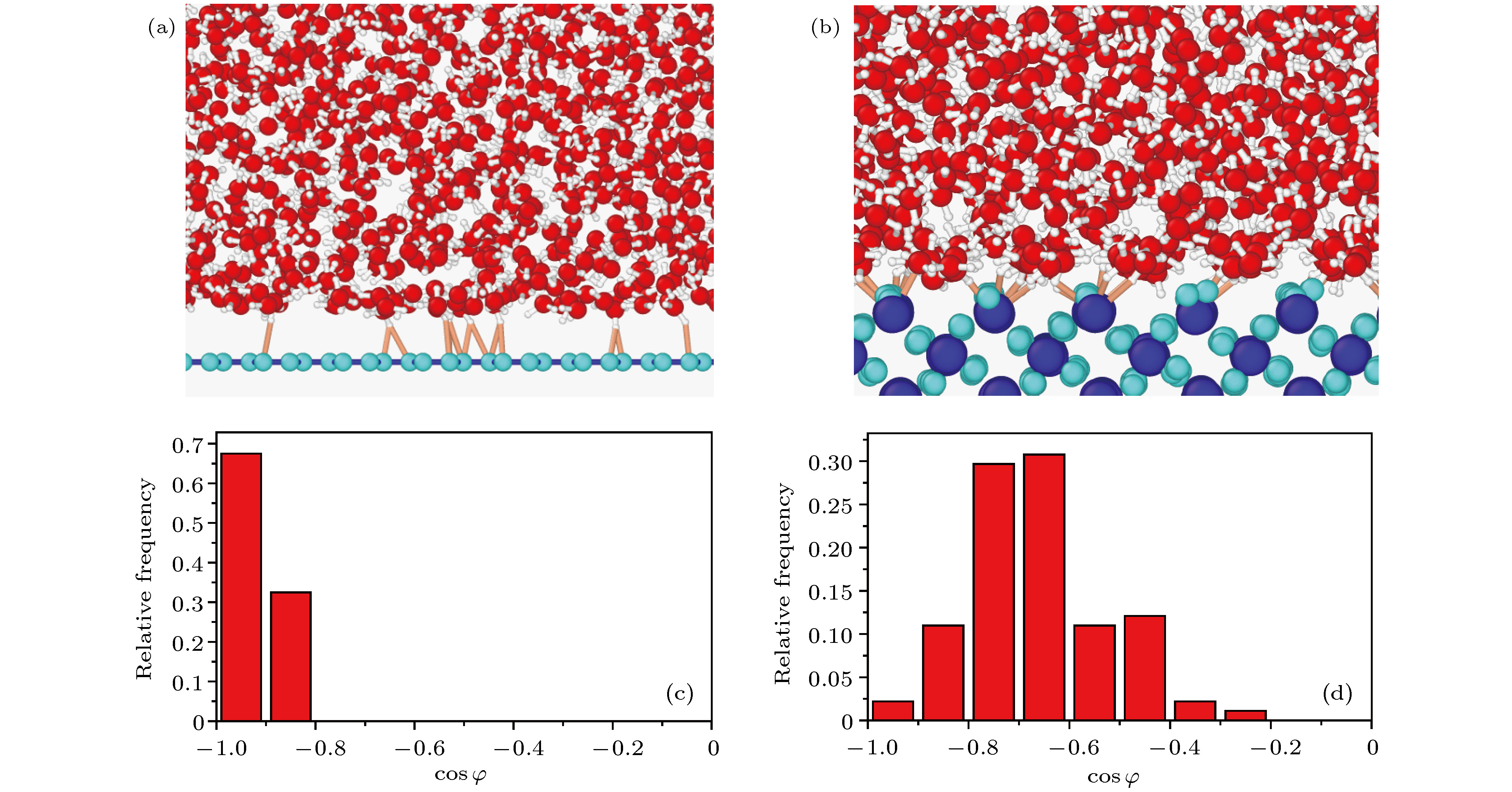
图 11 不同基底表面氢键的排布情况 (a)单层石墨烯与水分子之间的氢键; (b)二氧化硅与水分子之间的氢键; (c)单层石墨烯表面氢键与垂直基底方向夹角余弦cosφ的分布频率; (d)二氧化硅表面氢键与垂直基底方向夹角余弦cosφ的分布频率
Fig. 11. Frequency distribution diagram of the angle between hydrogen bond in water molecule and vertical base direction in systems with different bases: (a) The formation of hydrogen bonds between water molecules and a single graphene substrate; (b) the formation of hydrogen bonds between water molecules and silicon dioxide substrate; (c) cosine frequency of the angle between the hydrogen bond on a single graphene and the vertical direction of the base; (d) cosine frequency of the angle between the hydrogen bond on silicon dioxide and the vertical direction of the base.
图 14 铜添加石墨烯后基底表面水分子排布情况的变化 (a)铜添加石墨烯后最靠近基底1 Å范围内cosθ的分布频率; (b)铜表面最靠近基底1 Å范围内cosθ的分布频率; (c)铜添加石墨烯后表面水分子排布的局部视图; (d)铜表面水分子排布的局部视图
Fig. 14. The change of water molecule arrangement on the surface of substrate after adding graphene to copper: (a) Frequency distribution of cosθ in water molecule closest to the substrate and within a thickness of 1 Å after graphene added on the copper base; (b) frequency distribution of cosθ in water molecule closest to the substrate and within a thickness of 1 Å on copper base; (c) a local view of the arrangement of water molecules on copper substrate with graphene added to it; (d) a local view of the arrangement of water molecules on copper substrate.
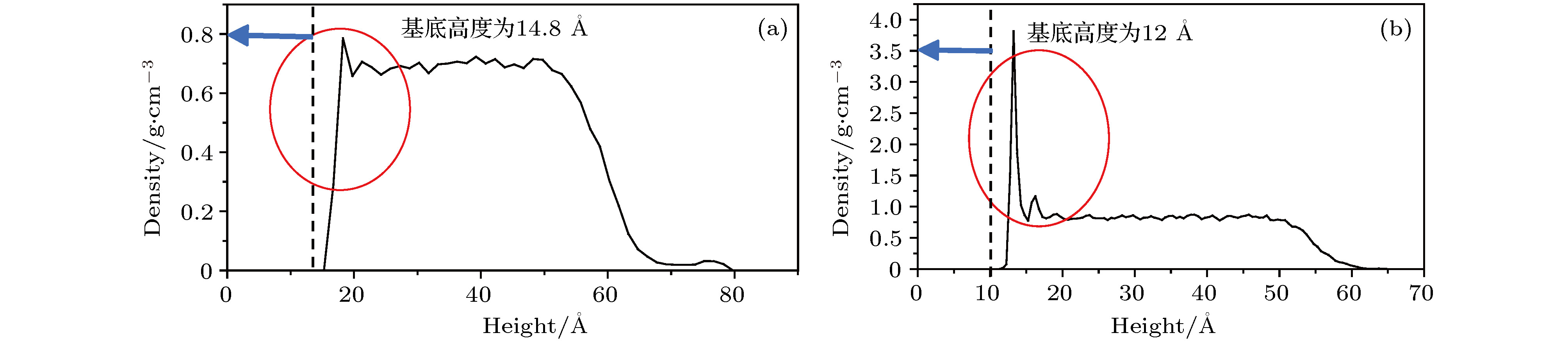
图 15 二氧化硅基底添加石墨烯前后水分子密度随高度分布的变化 (a)添加一层石墨烯; (b)未添加石墨烯
Fig. 15. Change of the distribution of water molecule density along the central axis of droplet before and after graphene added to silicon dioxide base: (a) With graphene added to silicon dioxide surface; (b) without graphene added to silicon dioxide surface.
图 16 二氧化硅添加石墨烯后基底表面水分子排布情况的变化 (a)二氧化硅添加石墨烯后最靠近基底1 Å范围内cosθ的分布频率; (b)二氧化硅表面最靠近基底1 Å范围内cosθ的分布频率; (c)二氧化硅添加石墨烯后表面水分子排布的局部视图; (d)二氧化硅表面水分子排布的局部视图
Fig. 16. Change of water molecule arrangement on the surface of substrate after adding graphene to silicon dioxide: (a) Frequency distribution of cosθ in water molecule closest to the substrate and within a thickness of 1 Å after graphene was added on silicon dioxide base; (b) frequency distribution of cosθ in water molecule closest to the substrate and within a thickness of 1 Å on silicon dioxide base; (c) a local view of the arrangement of water molecules and hydrogen bonds on silicon dioxide substrate with graphene added to it; (d) a local view of the arrangement of water molecules and hydrogen bonds on silicon dioxide substrate.
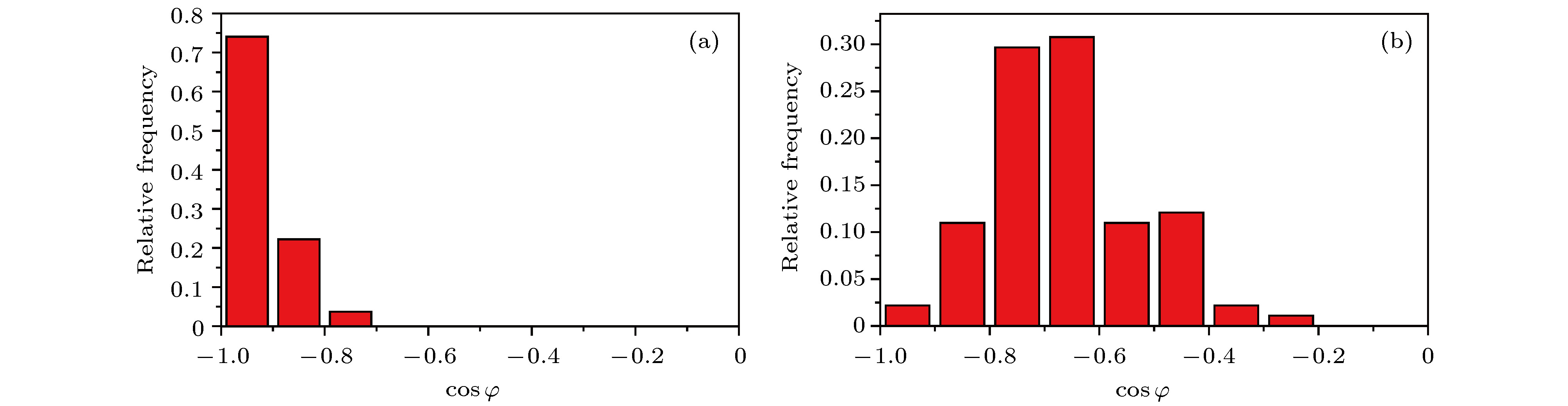
图 17 二氧化硅添加石墨烯前后基底表面氢键与垂直基底方向的夹角φ的余弦分布频率 (a)基底为二氧化硅表面添加一层石墨烯 (d)基底为二氧化硅
Fig. 17. The change of cosine frequency of the angle between the hydrogen bond on the base surface and the vertical direction of the base before and after adding graphene to silicon dioxide: (a) Silicon dioxide with a single graphene above as the basement; (b) silicon dioxide as the basement.
表 1 不同基底表面所测接触角的平均值与标准差
Table 1. Mean and standard deviation of contact angles measured on different base surfaces.
基底 三次测量所得的
接触角/(°)平均值/(°) 标准差/(°) 铜基底 79.87 78.24 81.23 79.78 1.50 二氧化硅基底 26.63 24.30 27.41 26.11 1.62 表 2 不同水分子数目的体系中所测接触角的平均值与标准差
Table 2. Mean and standard deviation of contact angles measured in systems with different numbers of water molecules.
体系中的
水分子数目三次测得的接触角/(°) 平均值/(°) 标准差/(°) 1000 78.87 77.06 79.58 78.50 1.30 2000 79.92 76.42 83.21 79.85 3.40 4000 78.76 77.56 79.37 78.56 0.92 8397 78.93 81.21 77.71 79.28 1.78 表 3 不同基底上水分子中O—H键与垂直基底方向的夹角余弦cosθ频率分布
Table 3. Frequency distribution of cosθ between O—H bond in water molecule and vertical base direction in systems with different bases.
cosθ的范围 占总体百分比/% 铜 二氧化硅 石墨烯 0—0.1 28.65 30.10 34.95 0.1—0.2 12.92 22.20 19.89 0.2—0.3 13.48 15.94 12.37 0.3—0.4 7.30 12.61 9.14 0.4—0.5 7.87 8.27 6.99 0.5—0.6 7.87 5.01 6.99 0.6—0.7 3.37 2.52 5.91 0.7—0.8 3.93 2.12 2.15 0.8—0.9 3.93 0.71 1.61 0.9—1.0 10.67 0.10 0.0 表 4 不同基底组合中所测接触角的平均值与标准差
Table 4. Mean and standard deviation of contact angles measured in different base combinations.
基底 三次测得的
接触角/(°)平均值/(°) 标准差/(°) 铜 + 石墨烯 81.99 83.14 82.05 82.39 0.65 二氧化硅 +
石墨烯57.23 55.62 60.02 57.62 2.23 表 5 铜添加石墨烯后基底表面水分子中O—H键与垂直基底方向的夹角余弦cosθ频率分布
Table 5. Frequency distribution of cosθ between O—H bond in water molecule and vertical base direction with and without graphene added on copper base.
cosθ的范围 占总体百分比/% 添加一层石墨烯 未添加石墨烯 0—0.1 29.71 28.65 0.1—0.2 18.21 12.92 0.2—0.3 15.66 13.48 0.3—0.4 10.54 7.30 0.4—0.5 8.31 7.87 0.5—0.6 7.67 7.87 0.6—0.7 2.88 3.37 0.7—0.8 4.79 3.93 0.8—0.9 1.60 3.93 0.9—1.0 0.64 10.67 表 6 二氧化硅添加石墨烯后基底表面水分子中O—H键与垂直基底方向的夹角余弦cosθ频率分布
Table 6. Frequency distribution of cosθ between O—H bond in water molecule and vertical base direction with and without graphene added on silicon dioxide base.
cosθ的范围 占总体百分比/% 添加一层石墨烯 二氧化硅 石墨烯 0—0.1 34.98 30.10 34.95 0.1—0.2 17.94 22.20 19.89 0.2—0.3 14.35 15.94 12.37 0.3—0.4 12.56 12.61 9.14 0.4—0.5 6.28 8.27 6.99 0.5—0.6 4.93 5.01 6.99 0.6—0.7 4.04 2.52 5.91 0.7—0.8 3.14 2.12 2.15 0.8—0.9 0.90 0.71 1.61 0.9—1.0 0.90 0.10 0 -
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A J S 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[3] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[4] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau C N 2008 Nano Lett. 8 902
 Google Scholar
Google Scholar
[5] Rafiee J, Mi X, Gullapalli H, Thomas A V, Yavari F, Shi Y, Ajayan P M, Koratkar N A 2012 Nature 11 217
 Google Scholar
Google Scholar
[6] Feng L, Li S, Li Y, Li H, Zhang L, Zhai J, Song Y, Liu B, Jiang L, Zhu D 2002 Super-Hydrophobic Surfaces: From Natural to Artificial (Weinheim: Verlag GmbH & Co.KGaA) pp1857–1860
[7] Messinger J, Lubitz W, Shen J R 2014 Phys. Chem. Chem. Phys. 16 11810
 Google Scholar
Google Scholar
[8] 郭亚杰 2015 硕士学位论文 (南京: 东南大学)
Guo Y J 2015 M. S. Thesis (Nanjin: Dongnan Uinversity) (in Chinese)
[9] 王俊珺, 李涛, 李雄鹰, 李辉 2018 67 149601
 Google Scholar
Google Scholar
Wang J J, Li T, Li X Y, Li H 2018 Acta Phys. Sin. 67 149601
 Google Scholar
Google Scholar
[10] Wu Y, Aluru N R 2013 J. Phys. Chem. B 117 8802
 Google Scholar
Google Scholar
[11] Mattia D, Gogotsi Y 2008 Microfluid. Nanofluid. 5 289
 Google Scholar
Google Scholar
[12] Shin Y J, Wang Y, Huang H, Kalon G, Wee A T, Shen Z, Bhatia C S, Yang H 2010 Langmuir the Acs Journal of Surfaces & Colloids 26 3798
[13] Taherian F, Marcon V, Van Der Vegt N F A, Leroy F 2013 Langmuir the Acs Journal of Surfaces Colloids 29 1457
 Google Scholar
Google Scholar
[14] Jeong W J, Ha M Y, Yoon H S, Ambrosia M J L 2012 Langmuir 28 5360
 Google Scholar
Google Scholar
[15] Wang S, Zhang Y, Abidi N, Cabrales L 2009 Langmuir the Acs Journal of Surfaces & Colloids 25 11078
[16] Morcos I 1972 J. Chem. Phys. 57 1801
 Google Scholar
Google Scholar
[17] Schrader M E 1980 J. Phys. Chem 84 2774
 Google Scholar
Google Scholar
[18] Tadros M E, Hu P, Adamson A W 1974 J. Colloid Interface Sci. 49 184
 Google Scholar
Google Scholar
[19] Gordillo M C 2002 J. Chem. Phys. 117 3425
 Google Scholar
Google Scholar
[20] Luna M, Colchero J, Baró A M 1999 J. Phys. Chem. B 103 9576
 Google Scholar
Google Scholar
[21] Zhou H, Ganesh P, Presser V, Wander M C F, Fenter P, Kent P R C, Jiang D E, Chialvo A A, Mcdonough J, Shuford K L 2012 Phys. Rev. B 85 120
[22] Raj R, Maroo S C, Wang E N 2013 Nano Lett. 13 1509
 Google Scholar
Google Scholar
[23] 李帅 2014 硕士学位论文(西安: 西安电子科技大学)
Li S 2014 M. S. Thesis (XiAn:Xidian University) (in Chinese)
[24] Werder T, Walther J H, Jaffe R L, Halicioglu T, Koumoutsakos P 2003 J. Phys. Chem. B 107 1345
 Google Scholar
Google Scholar
[25] Akaishi A, Yonemaru T, Nakamura J 2017 ACS Omega 2 2184
 Google Scholar
Google Scholar
[26] Giulio S, Danilo S, Claudio D A, Alberto O 2011 Phys. Rev. E 84 061602
[27] Berendsen H J C, Grigera J R, Straatsma T P 1987 J. Phys. Chem. 91 6269
 Google Scholar
Google Scholar
[28] Ryckaert J P, Ciccotti G, Berendsen H J C 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[29] Raina G, Gowthami T, Tamilselvi G 2017 Indian J. Sci. Technol. 9 48
[30] Munetoh S, Motooka T, Moriguchi K, Shintani A 2007 Comput. Mater. Sci. 39 334
 Google Scholar
Google Scholar
[31] Berthier J 2008 Microdrops and Digital Microfluidics (William Andrew Publishing) pp15−17
[32] Gennes P G D, Brochard-Wyart F, Quéré D 2004 Dyn. Triple Line 57 66
[33] Gordillo M C, Martí J 2008 Phys. Rev. B 78 7
[34] Gordillo M C, Martí J 2010 J. Phys. Condens. Matter 22 284111
 Google Scholar
Google Scholar
[35] Martí J, Nagy G, Guàrdia E, Gordillo M C 2006 J. Phys. Chem. B 110 23987
 Google Scholar
Google Scholar
[36] Nair R R, Blake P, Grigorenko A N, Novoselov K S, Booth T J, Stauber T, Peres N M R, Geim A K 2008 Science 320 1308
 Google Scholar
Google Scholar
[37] Malaspina D C, Schulz E P, Alarcón L M, Frechero M A, Appignanesi G A 2010 Eur. Phys. J. E 32 35
 Google Scholar
Google Scholar
[38] Homma Y, Chiashi S, Yamamoto T, Kono K, Matsumoto D, Shitaba J, Sato S 2013 Phys. Rev. Lett. 110 157402
 Google Scholar
Google Scholar
[39] 万骞 2014 硕士学位论文 (湖北: 武汉科技大学)
Wang Q 2014 M. S. Thesis (Hubei: Wuhan University of Science and Technology) (in Chinese)
[40] 陈聪, 李维仲 2009 物理化学学报 25 507
 Google Scholar
Google Scholar
Chen C, Li W Z 2009 Acta Phys. Chim. Sin. 25 507
 Google Scholar
Google Scholar
[41] 杨忠志, 崔宝秋 2007 物理化学学报 23 1332
Yang Z Z, Cui B Q 2007 Acta Phys. Chim. Sin. 23 1332
[42] Shih C J, Strano M S, Blankschtein D 2013 Nat. Mater. 12 866
 Google Scholar
Google Scholar
计量
- 文章访问数: 20515
- PDF下载量: 253
- 被引次数: 0













 下载:
下载: