-
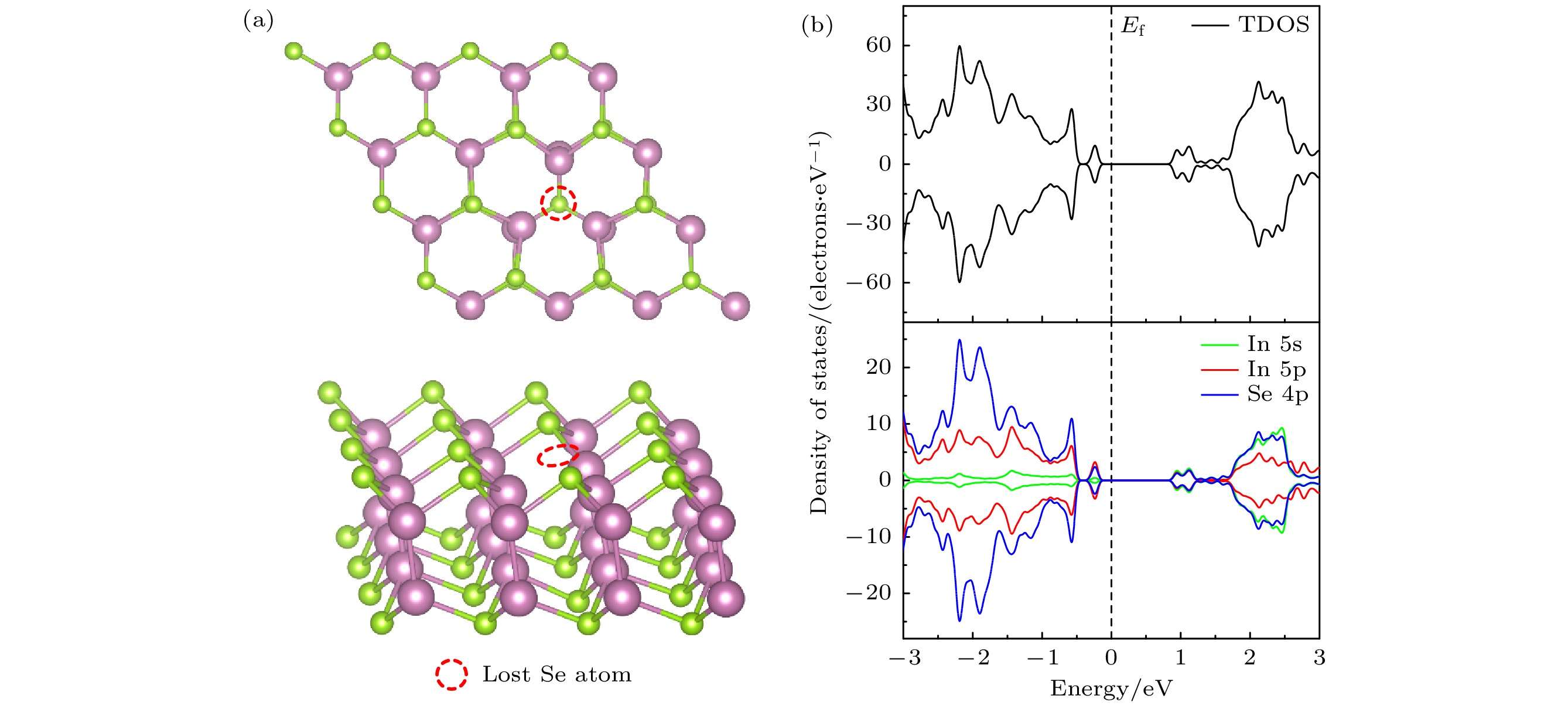
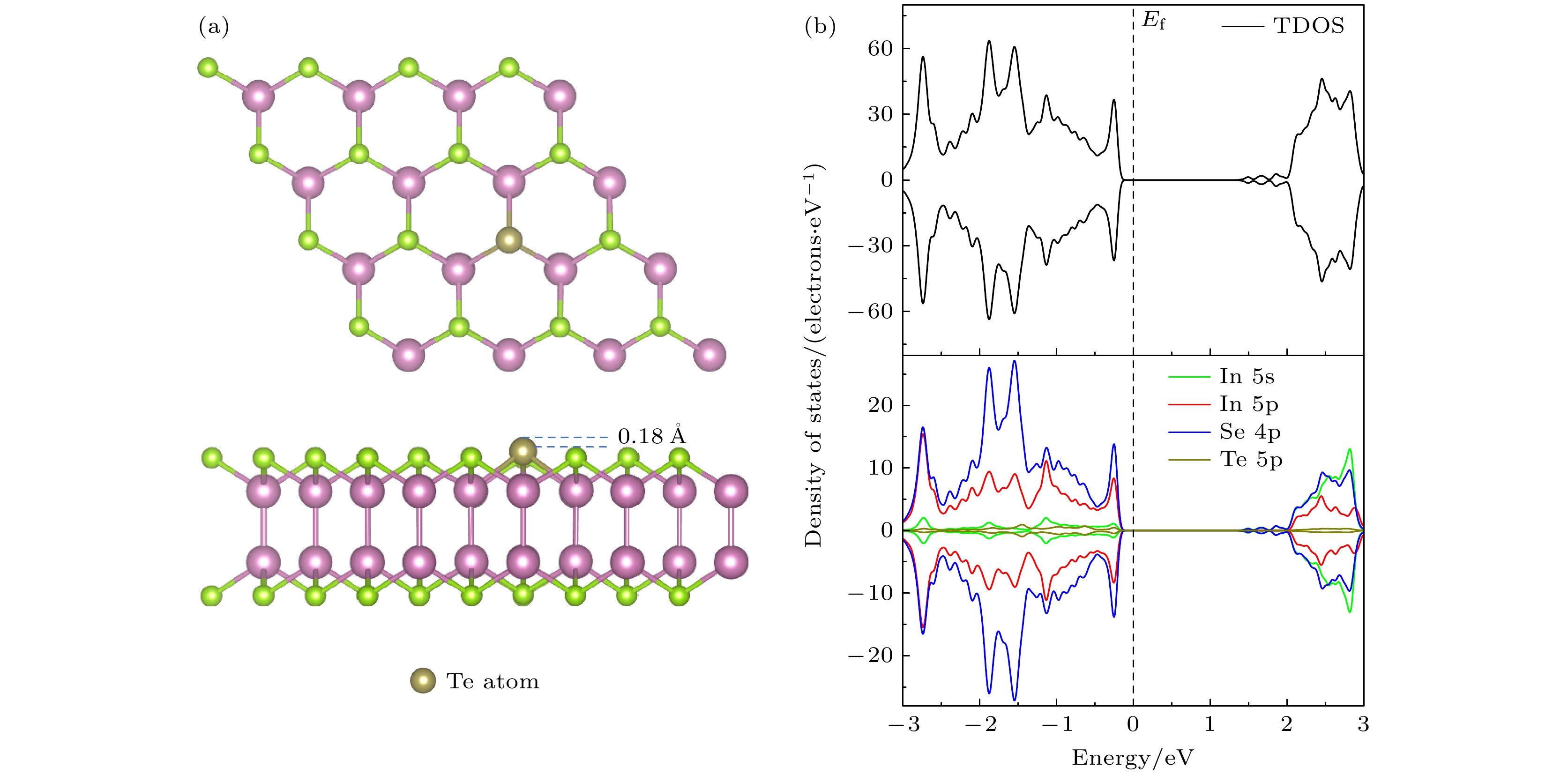
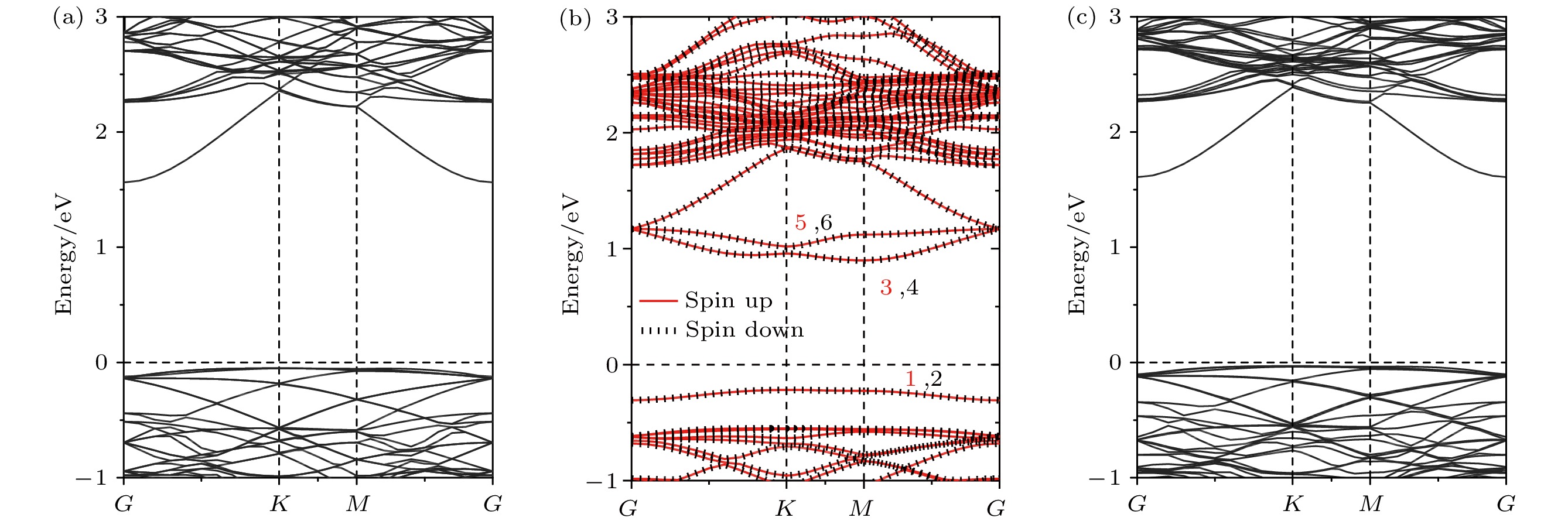
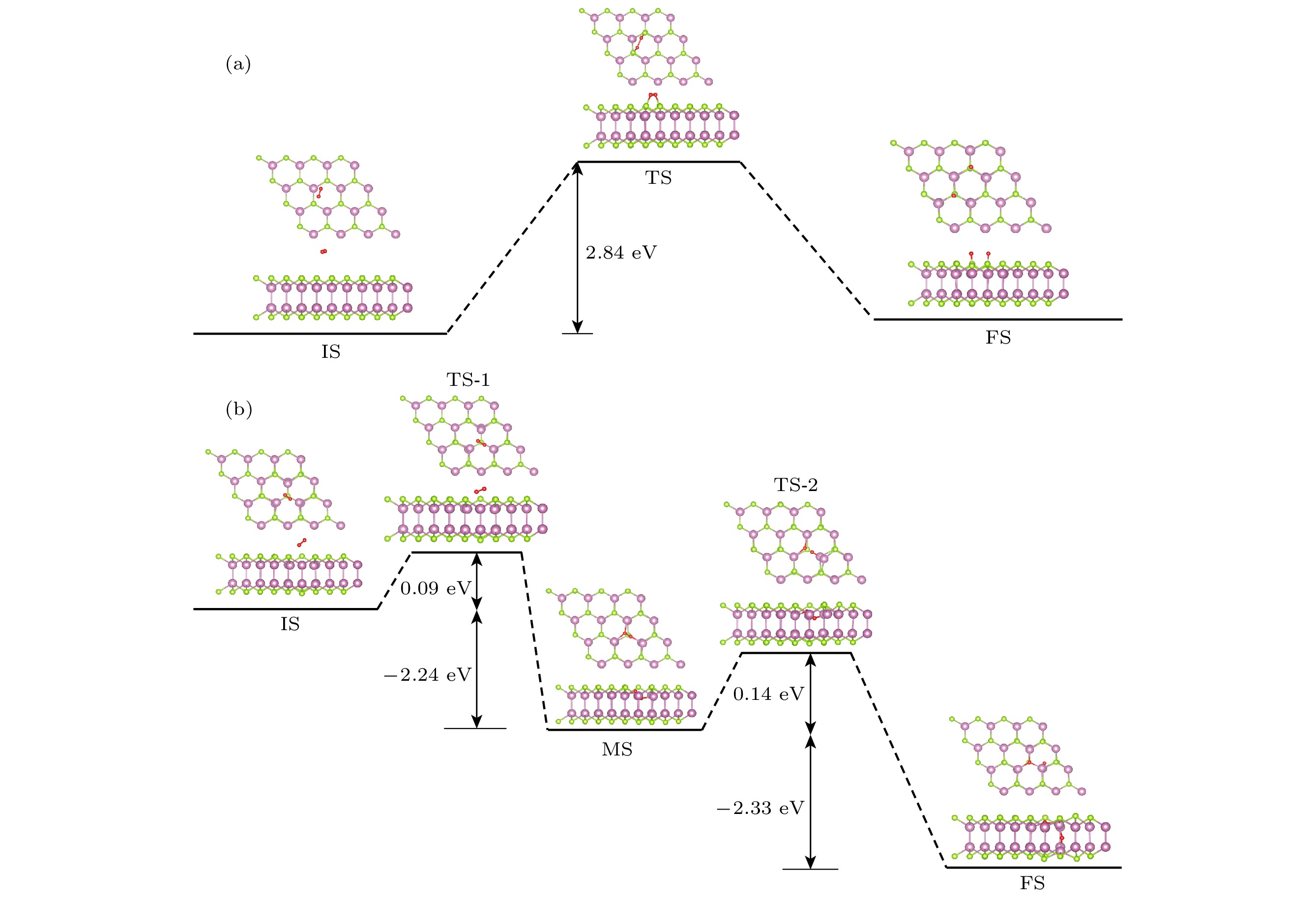
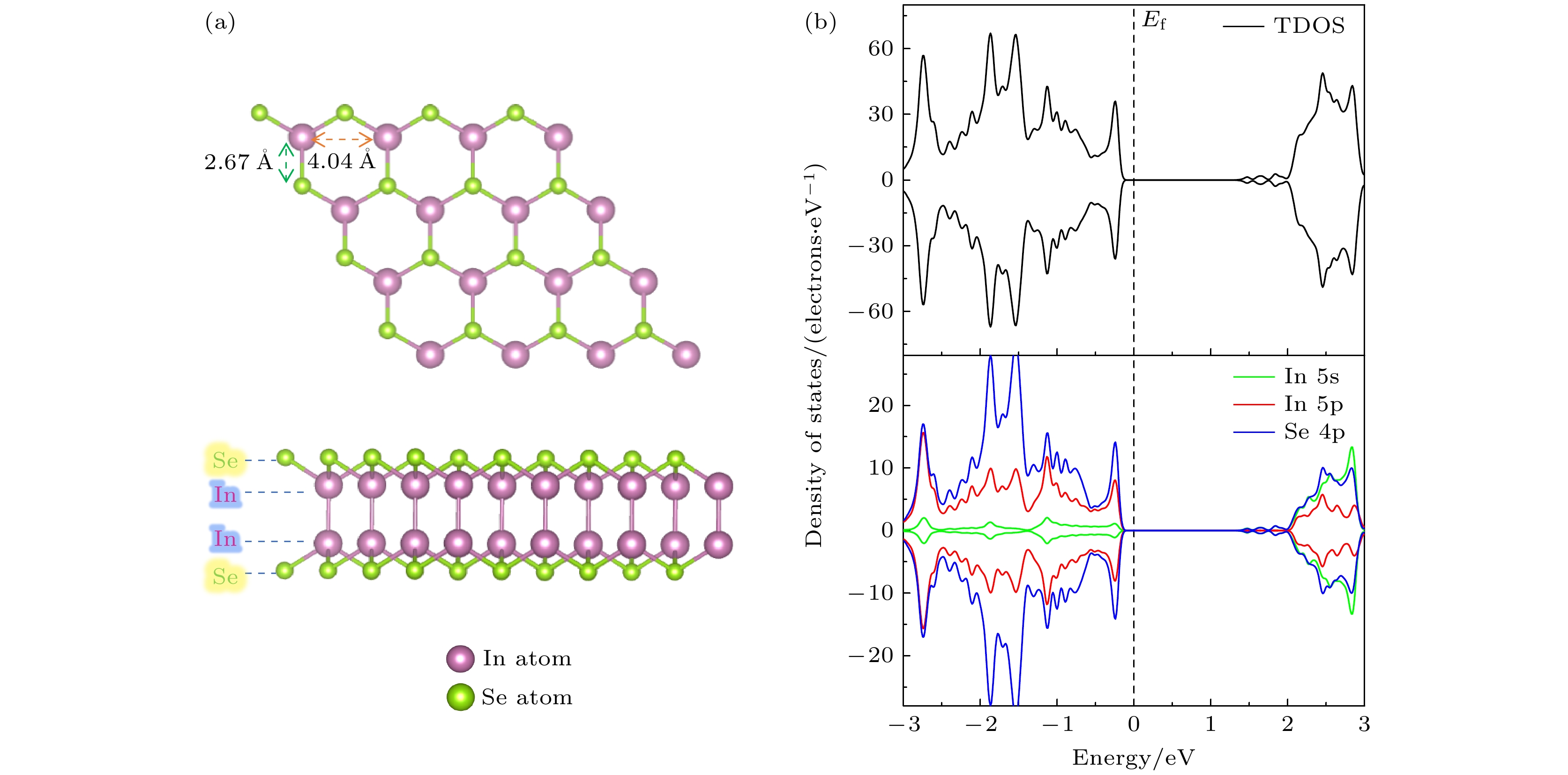
InSe作为一种典型的二维层状半导体材料, 具有优异的电学性能以及适中可调的带隙, 在光电器件中表现出诱人的应用前景. 然而有研究表明, 单硒空位(Vse)体系的InSe易受O2分子影响, 造成InSe材料降解, 严重影响其在电子器件领域的应用. 本文基于InSe降解机理, 提出了碲(Te)替位掺杂的方法, 用于提升该材料的环境稳定性. 利用密度泛函理论对不同体系电子结构、吸附能、能量反应路径等进行分析, 发现Te掺杂不仅显著改善缺陷引起的InSe降解问题, 同时可消除Vse产生的缺陷态, 起到缺陷补偿作用. 具体研究结果如下: 1) O2分子在Te掺杂InSe表面(InSe-Te)的解离能垒高达2.67 eV, 说明其具有较强的抗氧化能力; 2) O2分子在InSe-Te表面保持3.87 Å的距离, 吸附能仅有–0.03 eV, 表明O2分子物理吸附在其单层表面; 3) Te掺杂不仅提升材料抗氧化能力, 同时还消除了Vse产生的缺陷态. 该研究结果将有助于进一步提升InSe二维材料器件的环境稳定性, 推动InSe二维器件研究和发展.InSe is a typical two-dimensional (2D) layered semiconductor material, which has excellent electrical properties and moderate adjustable band gap. It is found that InSe has an attractive application prospect in optoelectronic devices. However, some studies have shown that InSe in a single selenium vacancy (Vse) system is easily degraded when exposed to the environment of O2 molecule, which seriously affects the application of InSe in the field of electronic devices. In order to improve the environmental stability of the material, the substitution doping method of Te is proposed in this work. Density functional theory (DFT) is used to analyze the electronic structure, adsorption energy, Bader charge and energy reaction paths of the different systems. It is found that Te substitution doping can significantly improve the stability of InSe. At the same time, the defect state produced by Vse can be eliminated. The specific research results are as follows. First, the dissociation barrier of O2 molecule on Te doped InSe surface (InSe—Te) is as high as 2.67 eV, indicating that Te-doped InSe has a strong antioxidant capacity. Second, the distance between O2 molecule and the surface of InSe—Te is 3.87 Å, and the adsorption energy is only –0.03 eV, indicating that O2 molecules are physically adsorbed on the monolayer surface. Third, Te doping not only improves the antioxidant capacity of the InSe, but also eliminates the defect state produced by Vse. Fourth, the Te-doping obviously eliminates the original Vse defect state or impurity band. The density of states and band structure of Te-doped InSe are almost the same as those of perfect InSe, which can maintain the stability of InSe structure and effectively reduce the damage of oxidation environment to defective InSe monolayer. The results of this study will be helpful in improving the environmental stability of InSe 2D material devices and promoting the research and development of InSe 2D devices.
-
Keywords:
- InSe /
- oxidation resistance /
- doping /
- electronic structure
[1] Ang Y S, Cao L M, Ang L K 2021 InfoMat 3 502
 Google Scholar
Google Scholar
[2] Xu K, Yin L, Huang Y, Shifa T A, Chu J W, Wang F, Cheng R Q, Wang Z X, He J 2016 Nanoscale 8 16802
 Google Scholar
Google Scholar
[3] Huang W J, Gan L, Li H Q, Ma Y, Zhai T Y 2016 CrystEngComm 18 3968
 Google Scholar
Google Scholar
[4] Sun Y H, Li Y W, Li T S, Biswas K, Patan A, Zhang L J 2020 Adv. Funct. Mater. 30 2001920
 Google Scholar
Google Scholar
[5] Ma D W, Ju W W, Tang Y N, Chen Y 2017 Appl. Surf. Sci. 426 244
 Google Scholar
Google Scholar
[6] Sun C, Xiang H, Xu B, Xia Y D, Yin J, Liu Z G 2016 Appl. Phys. Express 9 035203
 Google Scholar
Google Scholar
[7] Bandurin D A, Tyurnina A V, Yu G L, Mishchenko A, Zolyomi V, Morozov S V, Kumar R K, Gorbachev R V, Kudrynskyi Z R, Pezzini S, Kovalyuk Z D, Zeitler U, Novoselov K S, Patane A, Eaves L, Grigorieva I V, Fal'ko V I, Geim A K, Cao Y 2017 Nat. Nanotechnol. 12 223
 Google Scholar
Google Scholar
[8] Dai M J, Gao C F, Nie Q F, Wang Q J, Lin Y F, Chu J H, Li W W 2022 Adv. Mater. Technol. 7 2200321
 Google Scholar
Google Scholar
[9] Tamalampudi S R, Lu Y Y, Kumar U R, Sankar R, Liao C D, Moorthy B K, Cheng C H, Chou F C, Chen Y T 2014 Nano Lett. 14 2800
 Google Scholar
Google Scholar
[10] Balakrishnan N, Kudrynskyi Z R, Smith E F, Fay M W, Makarovsky O, Kovalyuk Z D, Eaves L, Beton P H, Patanè A 2017 2D Mater. 4 025043
 Google Scholar
Google Scholar
[11] Shi L, Zhou Q H, Zhao Y H, Ouyang Y X, Ling C Y, Li Q, Wang J L 2017 J. Phys. Chem. C 8 4368
 Google Scholar
Google Scholar
[12] Nan H Y, Guo S J, Cai S, Chen Z R, Zafar A, Zhang X M, Gu X F, Xiao S Q, Ni Z H 2018 Semicond. Sci. Tech. 33 074002
 Google Scholar
Google Scholar
[13] Wang X Y, Nan H Y, Dai W, Lin Q, Liu Z, Gu X F, Ni Z H, Xiao S Q 2019 Appl. Surf. Sci. 467 860
 Google Scholar
Google Scholar
[14] Yang B C, Wan B S, Zhou Q H, Wang Y, Hu W T, Lyu W M, Chen Q, Zeng Z M, Wen F S, Xiang J Y, Yuan S J, Wang J L, Zhang B S, Wang W H, Zhang J Y, Xu B, Zhao Z S, Tian Y J, Liu Z Y 2016 Adv. Mater. 28 9408
 Google Scholar
Google Scholar
[15] Rahman R S, Asokan K, Zulfequar M 2022 J. Phys. Chem. C 126 6065
 Google Scholar
Google Scholar
[16] Li Q, Zheng S X, Pu J B, Wang W Z, Li L, Wang L P 2019 Appl. Surf. Sci. 487 1121
 Google Scholar
Google Scholar
[17] Ding Y, Wang Y L 2015 J. Phys. Chem. C 119 27848
 Google Scholar
Google Scholar
[18] Ma D W, Li T X, Yuan D, He C Z, Lu Z, Lu Z S, Yang Z X, Wang Y X 2018 Appl. Surf. Sci. 434 215
 Google Scholar
Google Scholar
[19] Li X P, Xia C X, Song X H, Du J, Xiong W Q 2017 J. Mater. Sci. 52 7207
 Google Scholar
Google Scholar
[20] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[21] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[22] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[23] Wei X, Dong C F, Xu A N, Li X G, MacDonald D D 2018 Phys. Chem. Chem. Phys. 20 2238
 Google Scholar
Google Scholar
[24] Wu X, Vargas M C, Nayak S, Lotrich V, Scoles G 2001 J. Phys. Chem. C 115 8748
 Google Scholar
Google Scholar
[25] 刘子媛, 潘金波, 张余洋, 杜世萱 2021 70 027301
 Google Scholar
Google Scholar
Liu Z Y, Pan J B, Zhang Y Y, Du S X 2021 Acta Phys. Sin. 70 027301
 Google Scholar
Google Scholar
[26] Mortensen J J, Hansen L B, Jacobsen K W 2005 Phys. Rev. B 71 035109
 Google Scholar
Google Scholar
[27] Moellmann J, Grimme S 2014 J. Phys. Chem. C 118 7615
 Google Scholar
Google Scholar
[28] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[29] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Phys. Chem. C 113 9901
 Google Scholar
Google Scholar
[30] Kistanov A A, Cai Y Q, Kripalani D R, Zhou K, Dmitriev S V, Zhang Y W 2018 J. Mater. Chem. C 6 4308
 Google Scholar
Google Scholar
[31] 孙建平, 缪应蒙, 曹相春 2013 62 036301
 Google Scholar
Google Scholar
Sun J P, Liao Y M, Cao X C 2013 Acta Phys. Sin. 62 036301
 Google Scholar
Google Scholar
[32] 林雪玲, 潘凤春 2013 62 166102
 Google Scholar
Google Scholar
Lin X L, Pan F C 2013 Acta Phys. Sin. 62 166102
 Google Scholar
Google Scholar
[33] 王应, 李勇, 李宗宝 2016 65 037101
 Google Scholar
Google Scholar
Wang Y, Li Y, Li Z B 2016 Acta Phys. Sin. 65 037101
 Google Scholar
Google Scholar
[34] Guo Y, Zhou S, Bai Y Z, Zhao J J 2017 J. Phys. Chem. C 147 104709
 Google Scholar
Google Scholar
[35] Qiu H, Xu T, Wang Z L, Ren W, Nan H Y, Ni Z H, Chen Q, Yuan S J, Miao F, Song F Q, Long G, Shi Y, Sun L T, Wang J L, Wang X R 2013 Nat. Commun. 4 2642
 Google Scholar
Google Scholar
[36] Meng Y Z, Ling C Y, Xin R, Wang P, Song Y, Bu H J, Gao S, Wang X F, Song F Q, Wang J L, Wang X R, Wang B G, Wang G H 2017 npj Quantum Mater. 2 16
 Google Scholar
Google Scholar
[37] Wang D, Li X B, Sun H B 2017 Nanoscale 9 11619
 Google Scholar
Google Scholar
-
图 7 O2吸附于InSe-Vse和InSe-Te的差分电荷密度 (a), (d) InSe-Vse/O2; (b), (e) InSe-Vse@O2; (c), (f) InSe-Te/O2. 分子-表面的差分电荷密度(
$ \Delta \rho $ , 等值面设为0.001e/bohr3), 黄色代表电子积累区域($ \Delta \rho > 0 $ ), 蓝色代表电子缺失区域($ \Delta \rho < 0 $ )Fig. 7. Differential charge density of O2 adsorbed on InSe-Vse and InSe-Te: (a), (d) InSe-Vse/O2; (b), (e) InSe-Vse@O2; (c), (f) InSe-Te. Differential charge density of molecular-surface (
$ \Delta \rho $ , the equivalent surface is set to 0.001e/bohr3), yellow represents areas where electrons accumulate ($ \Delta \rho > 0 $ ), blue is the electron missing region ($ \Delta \rho < 0 $ ).表 1 O2分子在InSe-Te表面不同位点的吸附能
Table 1. Adsorption energy of O2 molecule at different sites on InSe-Te surface.
吸附能 吸附位点 $ {T}_{{\rm{T}}{\rm{e}}} $ $ {T}_{{\rm{h}}{\rm{o}}{\rm{l}}{\rm{l}}{\rm{o}}{\rm{w}}} $ $ {T}_{{\rm{I}}{\rm{n}}} $ $ {T}_{{\rm{S}}{\rm{e}}} $ $ {T}_{{\rm{S}}{\rm{e}}-{\rm{T}}{\rm{e}}} $ $ {T}_{{\rm{I}}{\rm{n}}-{\rm{T}}{\rm{e}}} $ $ {E}_{{\rm{a}}{\rm{d}}} $/eV –0.03 –0.07 –0.08 –0.05 –0.07 –0.05 表 2 O2在完美InSe, InSe-Te, InSe-Vse表面的吸附能(
$ {{E}}_{\rm{ad}} $ )、电荷转移量($ {\Delta {n}}_{\rm{e}} $ )、O—O键长(d)以及原子距离高度(h)Table 2. Adsorption energy (
$ {{E}}_{\rm{ad}} $ ), charge transfer ($ {\Delta {n}}_{\rm{e}} $ ), O—O bond length (d) and atomic distance height (h) of O2 on perfect InSe, InSe-Te and InSe-Vse surfaces, respectively. -
[1] Ang Y S, Cao L M, Ang L K 2021 InfoMat 3 502
 Google Scholar
Google Scholar
[2] Xu K, Yin L, Huang Y, Shifa T A, Chu J W, Wang F, Cheng R Q, Wang Z X, He J 2016 Nanoscale 8 16802
 Google Scholar
Google Scholar
[3] Huang W J, Gan L, Li H Q, Ma Y, Zhai T Y 2016 CrystEngComm 18 3968
 Google Scholar
Google Scholar
[4] Sun Y H, Li Y W, Li T S, Biswas K, Patan A, Zhang L J 2020 Adv. Funct. Mater. 30 2001920
 Google Scholar
Google Scholar
[5] Ma D W, Ju W W, Tang Y N, Chen Y 2017 Appl. Surf. Sci. 426 244
 Google Scholar
Google Scholar
[6] Sun C, Xiang H, Xu B, Xia Y D, Yin J, Liu Z G 2016 Appl. Phys. Express 9 035203
 Google Scholar
Google Scholar
[7] Bandurin D A, Tyurnina A V, Yu G L, Mishchenko A, Zolyomi V, Morozov S V, Kumar R K, Gorbachev R V, Kudrynskyi Z R, Pezzini S, Kovalyuk Z D, Zeitler U, Novoselov K S, Patane A, Eaves L, Grigorieva I V, Fal'ko V I, Geim A K, Cao Y 2017 Nat. Nanotechnol. 12 223
 Google Scholar
Google Scholar
[8] Dai M J, Gao C F, Nie Q F, Wang Q J, Lin Y F, Chu J H, Li W W 2022 Adv. Mater. Technol. 7 2200321
 Google Scholar
Google Scholar
[9] Tamalampudi S R, Lu Y Y, Kumar U R, Sankar R, Liao C D, Moorthy B K, Cheng C H, Chou F C, Chen Y T 2014 Nano Lett. 14 2800
 Google Scholar
Google Scholar
[10] Balakrishnan N, Kudrynskyi Z R, Smith E F, Fay M W, Makarovsky O, Kovalyuk Z D, Eaves L, Beton P H, Patanè A 2017 2D Mater. 4 025043
 Google Scholar
Google Scholar
[11] Shi L, Zhou Q H, Zhao Y H, Ouyang Y X, Ling C Y, Li Q, Wang J L 2017 J. Phys. Chem. C 8 4368
 Google Scholar
Google Scholar
[12] Nan H Y, Guo S J, Cai S, Chen Z R, Zafar A, Zhang X M, Gu X F, Xiao S Q, Ni Z H 2018 Semicond. Sci. Tech. 33 074002
 Google Scholar
Google Scholar
[13] Wang X Y, Nan H Y, Dai W, Lin Q, Liu Z, Gu X F, Ni Z H, Xiao S Q 2019 Appl. Surf. Sci. 467 860
 Google Scholar
Google Scholar
[14] Yang B C, Wan B S, Zhou Q H, Wang Y, Hu W T, Lyu W M, Chen Q, Zeng Z M, Wen F S, Xiang J Y, Yuan S J, Wang J L, Zhang B S, Wang W H, Zhang J Y, Xu B, Zhao Z S, Tian Y J, Liu Z Y 2016 Adv. Mater. 28 9408
 Google Scholar
Google Scholar
[15] Rahman R S, Asokan K, Zulfequar M 2022 J. Phys. Chem. C 126 6065
 Google Scholar
Google Scholar
[16] Li Q, Zheng S X, Pu J B, Wang W Z, Li L, Wang L P 2019 Appl. Surf. Sci. 487 1121
 Google Scholar
Google Scholar
[17] Ding Y, Wang Y L 2015 J. Phys. Chem. C 119 27848
 Google Scholar
Google Scholar
[18] Ma D W, Li T X, Yuan D, He C Z, Lu Z, Lu Z S, Yang Z X, Wang Y X 2018 Appl. Surf. Sci. 434 215
 Google Scholar
Google Scholar
[19] Li X P, Xia C X, Song X H, Du J, Xiong W Q 2017 J. Mater. Sci. 52 7207
 Google Scholar
Google Scholar
[20] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[21] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[22] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[23] Wei X, Dong C F, Xu A N, Li X G, MacDonald D D 2018 Phys. Chem. Chem. Phys. 20 2238
 Google Scholar
Google Scholar
[24] Wu X, Vargas M C, Nayak S, Lotrich V, Scoles G 2001 J. Phys. Chem. C 115 8748
 Google Scholar
Google Scholar
[25] 刘子媛, 潘金波, 张余洋, 杜世萱 2021 70 027301
 Google Scholar
Google Scholar
Liu Z Y, Pan J B, Zhang Y Y, Du S X 2021 Acta Phys. Sin. 70 027301
 Google Scholar
Google Scholar
[26] Mortensen J J, Hansen L B, Jacobsen K W 2005 Phys. Rev. B 71 035109
 Google Scholar
Google Scholar
[27] Moellmann J, Grimme S 2014 J. Phys. Chem. C 118 7615
 Google Scholar
Google Scholar
[28] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[29] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Phys. Chem. C 113 9901
 Google Scholar
Google Scholar
[30] Kistanov A A, Cai Y Q, Kripalani D R, Zhou K, Dmitriev S V, Zhang Y W 2018 J. Mater. Chem. C 6 4308
 Google Scholar
Google Scholar
[31] 孙建平, 缪应蒙, 曹相春 2013 62 036301
 Google Scholar
Google Scholar
Sun J P, Liao Y M, Cao X C 2013 Acta Phys. Sin. 62 036301
 Google Scholar
Google Scholar
[32] 林雪玲, 潘凤春 2013 62 166102
 Google Scholar
Google Scholar
Lin X L, Pan F C 2013 Acta Phys. Sin. 62 166102
 Google Scholar
Google Scholar
[33] 王应, 李勇, 李宗宝 2016 65 037101
 Google Scholar
Google Scholar
Wang Y, Li Y, Li Z B 2016 Acta Phys. Sin. 65 037101
 Google Scholar
Google Scholar
[34] Guo Y, Zhou S, Bai Y Z, Zhao J J 2017 J. Phys. Chem. C 147 104709
 Google Scholar
Google Scholar
[35] Qiu H, Xu T, Wang Z L, Ren W, Nan H Y, Ni Z H, Chen Q, Yuan S J, Miao F, Song F Q, Long G, Shi Y, Sun L T, Wang J L, Wang X R 2013 Nat. Commun. 4 2642
 Google Scholar
Google Scholar
[36] Meng Y Z, Ling C Y, Xin R, Wang P, Song Y, Bu H J, Gao S, Wang X F, Song F Q, Wang J L, Wang X R, Wang B G, Wang G H 2017 npj Quantum Mater. 2 16
 Google Scholar
Google Scholar
[37] Wang D, Li X B, Sun H B 2017 Nanoscale 9 11619
 Google Scholar
Google Scholar
计量
- 文章访问数: 5439
- PDF下载量: 117
- 被引次数: 0













 下载:
下载: