-
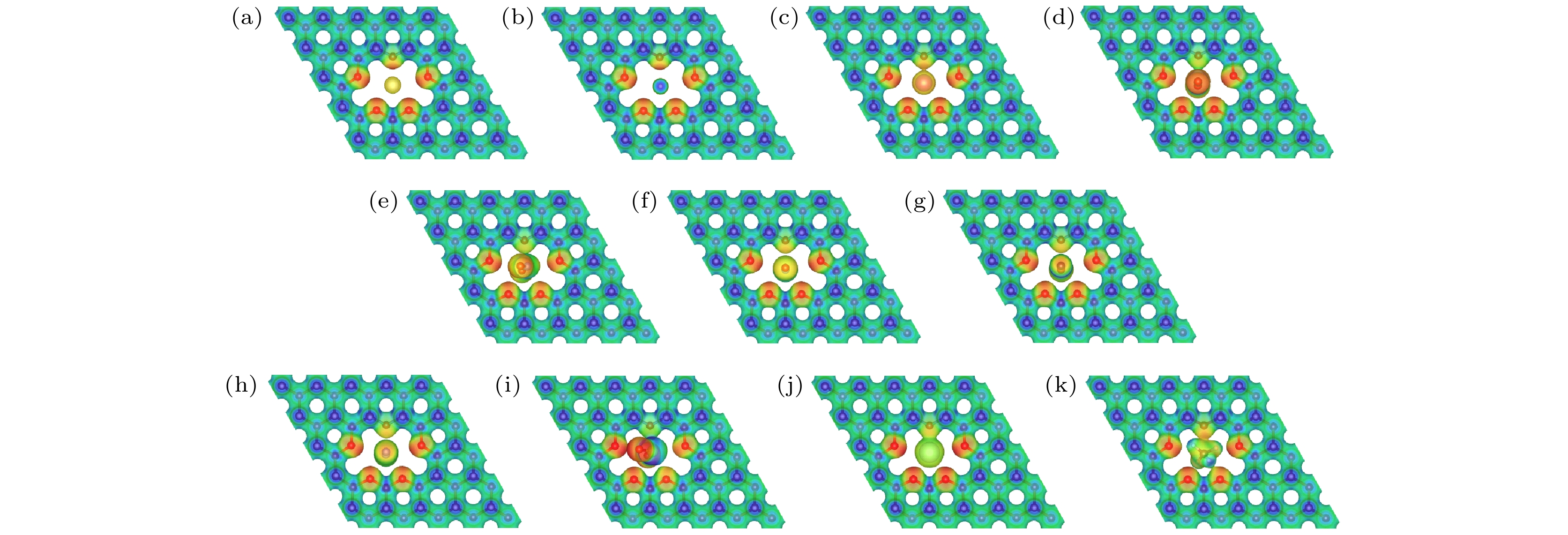
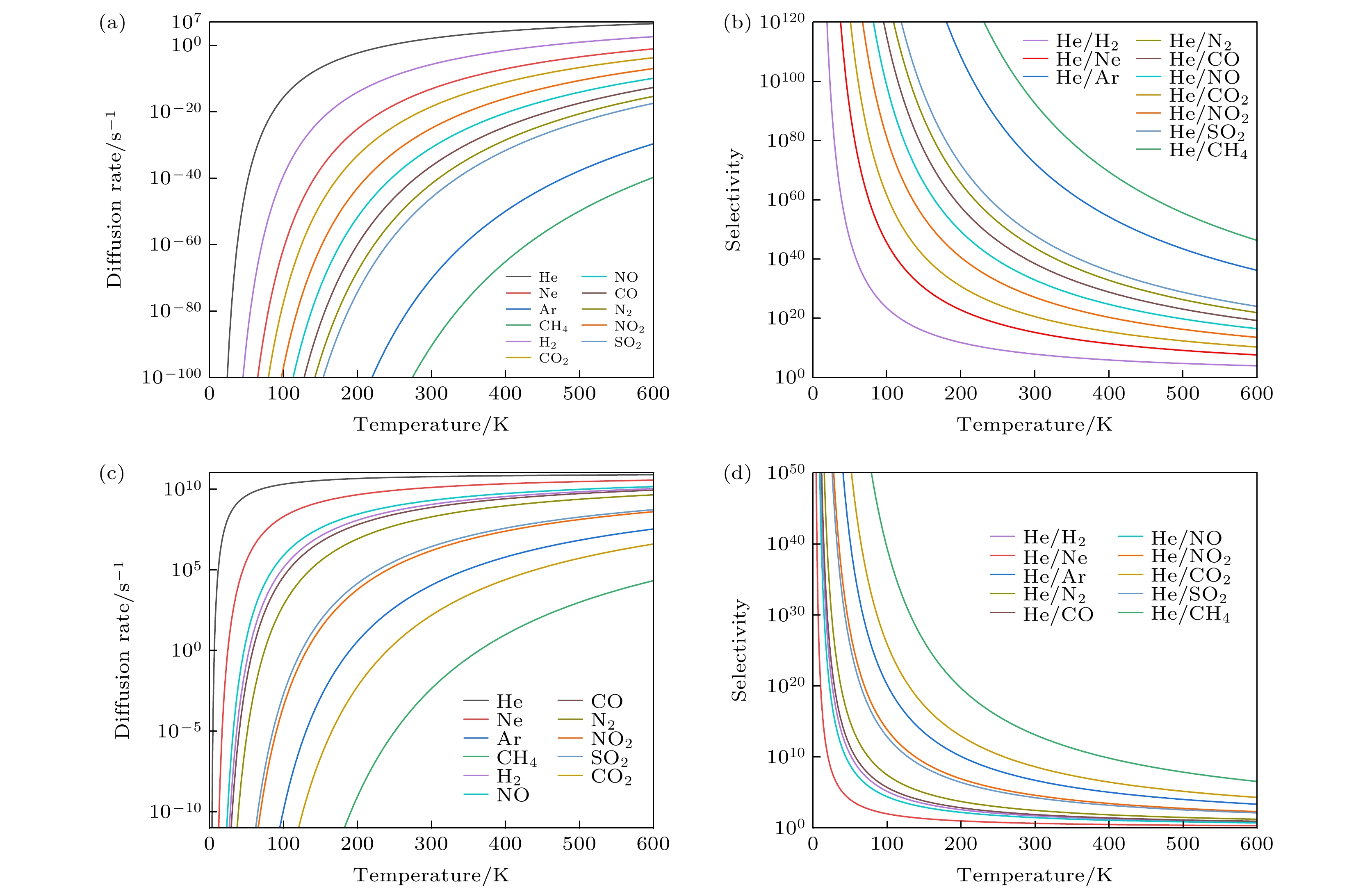
氦气(He)在众多科学和工业领域中都具有非常广泛的应用, He资源的短缺和需求的不断增长使得He分离具有极其重要的意义. 石墨烷合成简单、晶体结构稳定, 是一种用于构建气体分离膜的潜在理想二维材料. 本文通过第一性原理计算, 对四种具有不同尺寸冠醚孔的石墨烷膜(crown ether graphane-n, CG-n, n = 3, 4, 5, 6)的He分离性能进行了研究. 计算结果表明, 四种冠醚石墨烷结构都具有较高的热力学、化学稳定性, 并且CG-5和CG-6具有合适的孔径, 可用于He的有效筛分. 在11种气体分子(He, Ne, Ar, H2, CO, NO, NO2, N2, CO2, SO2和CH4)中, He最容易通过CG-n膜, 其能垒分别为4.55, 1.05, 0.53和0.01 eV. 据我们所知, He通过CG-6的能垒是迄今为止报道的最低值, 将可显著地提升He的分离效率. 基于阿伦尼乌斯方程的选择性计算结果表明, CG-5在较宽的温度范围内(0—600 K)都表现出优异的He选择性(相对于其他10种气体), 而CG-6由于冠醚环孔径较大, 仅相对于部分气体分子具有较好的He选择性. 本研究同时分析了膜孔径大小、气体分子动力学直径和气体分子类型对冠醚石墨烷膜的He分离性能影响的协同机制. 因此, 孔径合适的冠醚石墨烷膜(CG-5和CG-6)是一类潜在的选择性高、性能优异的 He分离膜.Helium (He) is widely used in many scientific and industrial fields, and the shortage of He resources and the growing demand make He separation extremely important. In this work, the He separation performances of a series of graphanes containing crown ether nanopores (crown ether graphane, CG-n, n = 3, 4, 5, 6) are studied by first-principles calculations. At first, the minimum energy paths of He and other 10 gas molecules (Ne, Ar, H2, CO, NO, NO2, N2, CO2, SO2 and CH4) passing through CG-n membranes are calculated, and the factors affecting the energy barriers are also investigated. The calculated results show that He is the easiest to pass through all the four CG-n membranes with energy barriers of 4.55, 1.05, 0.53 and 0.01 eV, respectively. He can be separated by CG-5 and CG-6 with very low energy barriers, and the energy barrier of He passing through CG-6 is the lowest, so far as we know. Moreover, all gas molecules can pass through CG-6 with low energy barriers, including many molecules with large kinetic diameters, such as CO (0.13 eV) and N2 (0.16 eV). Therefore, CG-6 is also expected to be used in the screening field of other gas molecules. In addition, it is found that the energy barriers of gas molecules passing through CG-n are synergistically affected by the size of the crown ether nanopore, the kinetic diameter and the type of the gas molecules. Secondly, the diffusion rates of gas molecules passing through CG-5 and CG-6 and the He selectivity towards other 10 gases of CG-5 and CG-6 at different temperatures are calculated. It is found that CG-5 exhibits extremely high He selectivity in a wide temperature range (0–600 K). In summary, the crown ether graphanes CG-5 and CG-6 can serve as excellent He separation membranes with high He selectivity. This work is expected to inspire one to develop other graphene-based two-dimensional separation membranes for separating He and other gas molecules.
-
Keywords:
- crown ether /
- hydrogenated graphene /
- membrane separation /
- density functional theory calculation /
- helium
[1] Cho A 2009 Science 326 778
 Google Scholar
Google Scholar
[2] 杨初平, 耿屹南, 王捷, 刘兴南, 时振刚 2021 70 135102
 Google Scholar
Google Scholar
Yang C P, Geng Y N, Wang J, Liu X N, Shi Z G 2021 Acta Phys. Sin. 70 135102
 Google Scholar
Google Scholar
[3] Fatemi S M, Abbasi Z, Rajabzadeh H, Hashemizadeh S A, Deldar A N 2017 Eur. Phys. J. D 71 194
 Google Scholar
Google Scholar
[4] Dai Z, Deng J, He X, Scholes C A, Jiang X, Wang B, Guo H, Ma Y, Deng L 2021 Sep. Purif. Technol. 274 119044
 Google Scholar
Google Scholar
[5] 王倩, 赵江山, 范元媛, 郭馨, 周翊 2020 69 174207
 Google Scholar
Google Scholar
Wang Q, Zhao J S, Fan Y Y, Guo X, Zhou Y 2020 Acta Phys. Sin. 69 174207
 Google Scholar
Google Scholar
[6] Wei S, Zhou S, Wu Z, Wang M, Wang Z, Guo W, Lu X 2018 Appl. Surf. Sci. 441 631
 Google Scholar
Google Scholar
[7] Rufford T E, Chan K I, Huang S H, May E F 2014 Adsorpt. Sci. Technol. 32 49
 Google Scholar
Google Scholar
[8] Stern S A, Sinclair T F, Gareis P J, Vahldieck N P, Mohr P H 1965 Ind. Eng. Chem. 57 49
[9] Yao B, Mandrà S, Curry J O, Shaikhutdinov S, Freund H J, Schrier J 2017 ACS Appl. Mater. Interfaces 9 43061
 Google Scholar
Google Scholar
[10] Pakdel S, Erfan-Niya H, Azamat J 2022 J. Mol. Graphics Modell. 115 108211
 Google Scholar
Google Scholar
[11] Mirzaei M, Karimi-Sabet J, Nikkho S, Towfighi-Darian J 2022 ACS Appl. Nano Mater. 5 1745
 Google Scholar
Google Scholar
[12] Schrier J 2010 J. Phys. Chem. Lett. 1 2284
 Google Scholar
Google Scholar
[13] Andrews N L P, Fan J Z, Forward R L, Chen M C, Loock H P 2017 Phys. Chem. Chem. Phys. 19 73
 Google Scholar
Google Scholar
[14] Malekian F, Ghafourian H, Zare K, Sharif A A, Zamani Y 2019 Eur. Phys. J. Plus 134 212
 Google Scholar
Google Scholar
[15] Liu M, Gurr P A, Fu Q, Webley P A, Qiao G G 2018 J. Mater. Chem. A 6 23169
 Google Scholar
Google Scholar
[16] Koenig S P, Wang L, Pellegrino J, Bunch J S 2012 Nat. Nanotechnol. 7 728
 Google Scholar
Google Scholar
[17] Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, Yang W 2014 Science 346 1356
 Google Scholar
Google Scholar
[18] Oyama S, Lee D, Hacarlioglu P, Saraf R 2004 J. Membr. Sci. 244 45
 Google Scholar
Google Scholar
[19] Kim H W, Yoon H W, Yoon S M, Yoo B M, Ahn B K, Cho Y H, Shin H J, Yang H, Paik U, Kwon S, Choi J Y, Park H B 2013 Science 342 91
 Google Scholar
Google Scholar
[20] Sun W 2021 Nat. Nanotechnol. 16 1054
 Google Scholar
Google Scholar
[21] Liu X, Chang X, Zhu L, Li X 2019 Comput. Mater. Sci. 157 1
 Google Scholar
Google Scholar
[22] Chen X, Zhang S, Hou D, Duan H, Deng B, Zeng Z, Liu B, Sun L, Song R, Du J, Gao P, Peng H, Liu Z, Wang L 2021 ACS Appl. Mater. Interfaces 13 29926
 Google Scholar
Google Scholar
[23] Wang Y, Li J, Yang Q, Zhong C 2016 ACS Appl. Mater. Interfaces 8 8694
 Google Scholar
Google Scholar
[24] Boutilier M S H, Sun C, O’Hern S C, Au H, Hadjiconstantinou N G, Karnik R 2014 ACS Nano 8 841
 Google Scholar
Google Scholar
[25] Hu W, Wu X, Li Z, Yang J 2013 Nanoscale 5 9062
 Google Scholar
Google Scholar
[26] Sluiter M H F, Kawazoe Y 2003 Phys. Rev. B 68 085410
 Google Scholar
Google Scholar
[27] Elias D C, Nair R R, Mohiuddin T M G, Morozov S V, Blake P, Halsall M P, Ferrari A C, Boukhvalov D W, Katsnelson M I, Geim A K, Novoselov K S 2009 Science 323 610
 Google Scholar
Google Scholar
[28] Pumera M, Wong C H A 2013 Chem. Soc. Rev. 42 5987
 Google Scholar
Google Scholar
[29] Guo K, Liu S, Tu H, Wang Z, Chen L, Lin H, Miao M, Xu J, Liu W 2021 Phys. Chem. Chem. Phys. 23 18983
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[34] Chadi D J 1977 Phys. Rev. B 16 1746
 Google Scholar
Google Scholar
[35] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[36] Li X, Guo T, Zhu L, Ling C, Xue Q, Xing W 2018 Chem. Eng. J. 338 92
 Google Scholar
Google Scholar
[37] Li J R, Kuppler R J, Zhou H C 2009 Chem. Soc. Rev. 38 1477
 Google Scholar
Google Scholar
[38] Li F, Qu Y, Zhao M 2015 Carbon 95 51
 Google Scholar
Google Scholar
[39] Zhu L, Jin Y, Xue Q, Li X, Zheng H, Wu T, Ling C 2016 J. Mater. Chem. A 4 15015
 Google Scholar
Google Scholar
[40] Blankenburg S, Bieri M, Fasel R, Müllen K, Pignedoli C A, Passerone D 2010 Small 6 2266
 Google Scholar
Google Scholar
[41] Zhu L, Xue Q, Li X, Wu T, Jin Y, Xing W 2015 J. Mater. Chem. A 3 21351
 Google Scholar
Google Scholar
[42] Zhu Z 2006 J. Membr. Sci. 281 754
 Google Scholar
Google Scholar
-
表 1 气体分子在CG-n上稳定吸附时的吸附能Ead 和吸附高度H
Table 1. Adsorption energies Ead and the adsorption heights H of gas molecules adsorbed stably on CG-n.
CG-3 CG-4 CG-5 CG-6 Ead/eV H/Å Ead/eV H/Å Ead/eV H/Å Ead/eV H/Å He –0.15 2.89 –0.10 2.00 –0.12 2.40 –0.11 2.00 Ne –0.22 3.11 –0.17 2.82 –0.11 2.00 –0.14 2.00 Ar –0.18 4.00 –0.16 4.00 –0.15 4.00 –0.22 2.00 CH4 — — –0.37 2.40 –0.36 2.30 –0.29 1.79 H2 –0.24 2.70 –0.23 2.50 –0.14 2.00 –0.18 2.00 CO2 — — –0.15 3.70 –0.15 3.70 –0.53 0.00 NO — — –0.49 2.90 –0.22 3.10 –0.27 1.80 CO — — –0.25 3.00 –0.23 2.90 –0.22 2.00 N2 –0.33 3.10 –0.24 3.20 –0.22 3.10 –0.24 1.90 NO2 — — –0.40 3.10 –0.11 3.60 –0.29 0.00 SO2 — — — — –0.20 3.60 –0.27 0.00 表 2 气体分子的动力学直径 (D) 和通过CG-n膜时的能垒 Ebarrier. D值来自文献[37]
Table 2. Kinetic diameters (D) of the gas molecules, and energy barriers Ebarrier for gas molecules passing through each CG-n membrane. D values from literature [37].
D/Å Ebarrier/eV CG-3 CG-4 CG-5 CG-6 He 2.60 4.55 1.05 0.53 0.01 Ne 2.82 12.07 2.80 1.44 0.05 Ar 3.54 22.80 8.90 4.86 0.42 CH4 3.80 — 10.81 6.07 0.80 H2 2.89 6.23 1.91 1.00 0.12 CO2 3.30 — 3.45 1.76 0.53 NO 3.17 — 5.12 2.50 0.10 CO 3.69 — 5.48 2.83 0.13 N2 3.64 15.56 5.95 3.15 0.16 NO2 — — 5.42 2.15 0.29 SO2 4.12 — — 3.40 0.27 表 3 室温(300 K) 下, 多孔膜材料对He (相对于其他气体)的选择性 (S)
Table 3. Selectivity (S) of porous membrane materials for He (over other gases) at room temperature (300 K).
Type CG-5a CG-6a IGPb CTF-0c C2Nd g-C3N4e g-C2Of PGg S(He/Ne) 1.63×1015 4.66 1×106 4×106 3×103 1×1010 30 2×107 S(He/CH4) 4.03×1092 1.32×1013 7×1031 6×1038 7×1031 1×1065 1.15×1028 8×1037 S(He/Ar) 2.39×1072 5.24×106 6×1021 5×1035 4×1018 1×1051 1.68×1014 6×1036 S(He/N2) 6.24×1043 3.09×102 1×1012 2×1027 3×1012 1×1034 1.54×106 6×1027 S(He/CO) 2.79×1038 80.5 1×1011 5×1024 — 1×1030 6.72×104 6×1024 S(He/CO2) 3.63×1020 4.22×108 3×108 4×1016 8×1018 — 5.82×102 — S(He/H2) 7.18×107 52.7 — — — — — — S(He/NO) 8.51×1032 29.6 — — — — — — S(He/NO2) 1.20×1027 4.11×104 — — — — — — S(He/SO2) 9.42×1047 1.90×104 — — — — — — 注: a本工作, b文献[13], c文献[23], d文献[41], e文献[38], f文献[21], g文献[6]. -
[1] Cho A 2009 Science 326 778
 Google Scholar
Google Scholar
[2] 杨初平, 耿屹南, 王捷, 刘兴南, 时振刚 2021 70 135102
 Google Scholar
Google Scholar
Yang C P, Geng Y N, Wang J, Liu X N, Shi Z G 2021 Acta Phys. Sin. 70 135102
 Google Scholar
Google Scholar
[3] Fatemi S M, Abbasi Z, Rajabzadeh H, Hashemizadeh S A, Deldar A N 2017 Eur. Phys. J. D 71 194
 Google Scholar
Google Scholar
[4] Dai Z, Deng J, He X, Scholes C A, Jiang X, Wang B, Guo H, Ma Y, Deng L 2021 Sep. Purif. Technol. 274 119044
 Google Scholar
Google Scholar
[5] 王倩, 赵江山, 范元媛, 郭馨, 周翊 2020 69 174207
 Google Scholar
Google Scholar
Wang Q, Zhao J S, Fan Y Y, Guo X, Zhou Y 2020 Acta Phys. Sin. 69 174207
 Google Scholar
Google Scholar
[6] Wei S, Zhou S, Wu Z, Wang M, Wang Z, Guo W, Lu X 2018 Appl. Surf. Sci. 441 631
 Google Scholar
Google Scholar
[7] Rufford T E, Chan K I, Huang S H, May E F 2014 Adsorpt. Sci. Technol. 32 49
 Google Scholar
Google Scholar
[8] Stern S A, Sinclair T F, Gareis P J, Vahldieck N P, Mohr P H 1965 Ind. Eng. Chem. 57 49
[9] Yao B, Mandrà S, Curry J O, Shaikhutdinov S, Freund H J, Schrier J 2017 ACS Appl. Mater. Interfaces 9 43061
 Google Scholar
Google Scholar
[10] Pakdel S, Erfan-Niya H, Azamat J 2022 J. Mol. Graphics Modell. 115 108211
 Google Scholar
Google Scholar
[11] Mirzaei M, Karimi-Sabet J, Nikkho S, Towfighi-Darian J 2022 ACS Appl. Nano Mater. 5 1745
 Google Scholar
Google Scholar
[12] Schrier J 2010 J. Phys. Chem. Lett. 1 2284
 Google Scholar
Google Scholar
[13] Andrews N L P, Fan J Z, Forward R L, Chen M C, Loock H P 2017 Phys. Chem. Chem. Phys. 19 73
 Google Scholar
Google Scholar
[14] Malekian F, Ghafourian H, Zare K, Sharif A A, Zamani Y 2019 Eur. Phys. J. Plus 134 212
 Google Scholar
Google Scholar
[15] Liu M, Gurr P A, Fu Q, Webley P A, Qiao G G 2018 J. Mater. Chem. A 6 23169
 Google Scholar
Google Scholar
[16] Koenig S P, Wang L, Pellegrino J, Bunch J S 2012 Nat. Nanotechnol. 7 728
 Google Scholar
Google Scholar
[17] Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, Yang W 2014 Science 346 1356
 Google Scholar
Google Scholar
[18] Oyama S, Lee D, Hacarlioglu P, Saraf R 2004 J. Membr. Sci. 244 45
 Google Scholar
Google Scholar
[19] Kim H W, Yoon H W, Yoon S M, Yoo B M, Ahn B K, Cho Y H, Shin H J, Yang H, Paik U, Kwon S, Choi J Y, Park H B 2013 Science 342 91
 Google Scholar
Google Scholar
[20] Sun W 2021 Nat. Nanotechnol. 16 1054
 Google Scholar
Google Scholar
[21] Liu X, Chang X, Zhu L, Li X 2019 Comput. Mater. Sci. 157 1
 Google Scholar
Google Scholar
[22] Chen X, Zhang S, Hou D, Duan H, Deng B, Zeng Z, Liu B, Sun L, Song R, Du J, Gao P, Peng H, Liu Z, Wang L 2021 ACS Appl. Mater. Interfaces 13 29926
 Google Scholar
Google Scholar
[23] Wang Y, Li J, Yang Q, Zhong C 2016 ACS Appl. Mater. Interfaces 8 8694
 Google Scholar
Google Scholar
[24] Boutilier M S H, Sun C, O’Hern S C, Au H, Hadjiconstantinou N G, Karnik R 2014 ACS Nano 8 841
 Google Scholar
Google Scholar
[25] Hu W, Wu X, Li Z, Yang J 2013 Nanoscale 5 9062
 Google Scholar
Google Scholar
[26] Sluiter M H F, Kawazoe Y 2003 Phys. Rev. B 68 085410
 Google Scholar
Google Scholar
[27] Elias D C, Nair R R, Mohiuddin T M G, Morozov S V, Blake P, Halsall M P, Ferrari A C, Boukhvalov D W, Katsnelson M I, Geim A K, Novoselov K S 2009 Science 323 610
 Google Scholar
Google Scholar
[28] Pumera M, Wong C H A 2013 Chem. Soc. Rev. 42 5987
 Google Scholar
Google Scholar
[29] Guo K, Liu S, Tu H, Wang Z, Chen L, Lin H, Miao M, Xu J, Liu W 2021 Phys. Chem. Chem. Phys. 23 18983
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[34] Chadi D J 1977 Phys. Rev. B 16 1746
 Google Scholar
Google Scholar
[35] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[36] Li X, Guo T, Zhu L, Ling C, Xue Q, Xing W 2018 Chem. Eng. J. 338 92
 Google Scholar
Google Scholar
[37] Li J R, Kuppler R J, Zhou H C 2009 Chem. Soc. Rev. 38 1477
 Google Scholar
Google Scholar
[38] Li F, Qu Y, Zhao M 2015 Carbon 95 51
 Google Scholar
Google Scholar
[39] Zhu L, Jin Y, Xue Q, Li X, Zheng H, Wu T, Ling C 2016 J. Mater. Chem. A 4 15015
 Google Scholar
Google Scholar
[40] Blankenburg S, Bieri M, Fasel R, Müllen K, Pignedoli C A, Passerone D 2010 Small 6 2266
 Google Scholar
Google Scholar
[41] Zhu L, Xue Q, Li X, Wu T, Jin Y, Xing W 2015 J. Mater. Chem. A 3 21351
 Google Scholar
Google Scholar
[42] Zhu Z 2006 J. Membr. Sci. 281 754
 Google Scholar
Google Scholar
计量
- 文章访问数: 7313
- PDF下载量: 85
- 被引次数: 0















 下载:
下载: