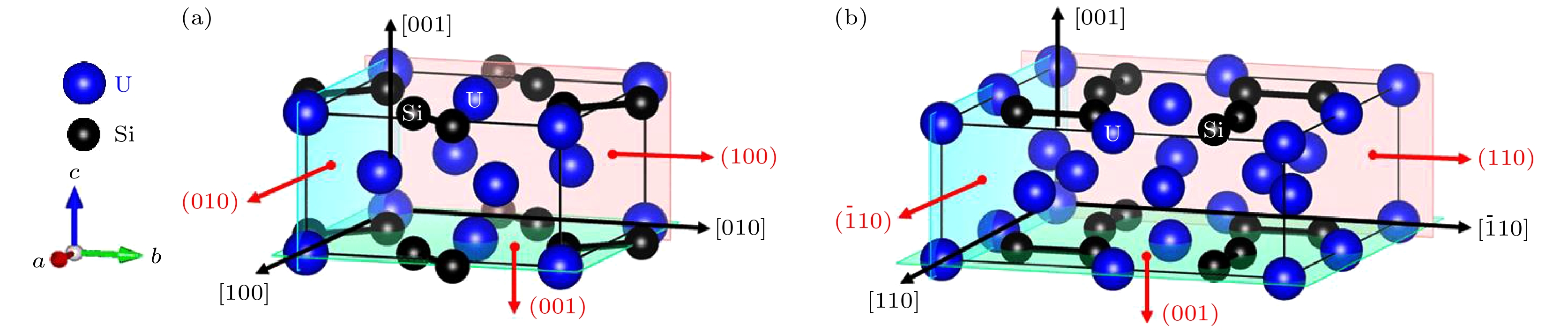
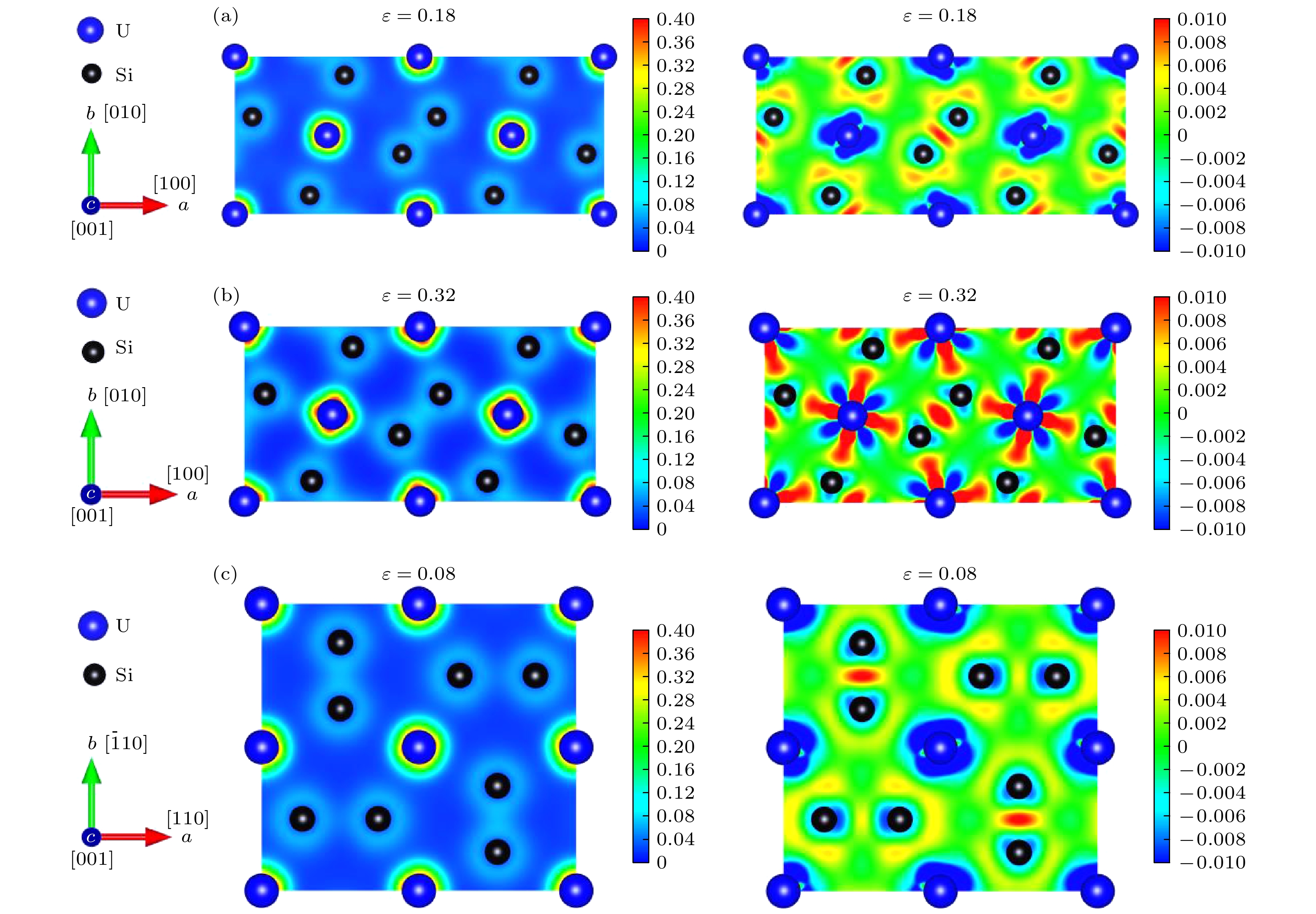
-
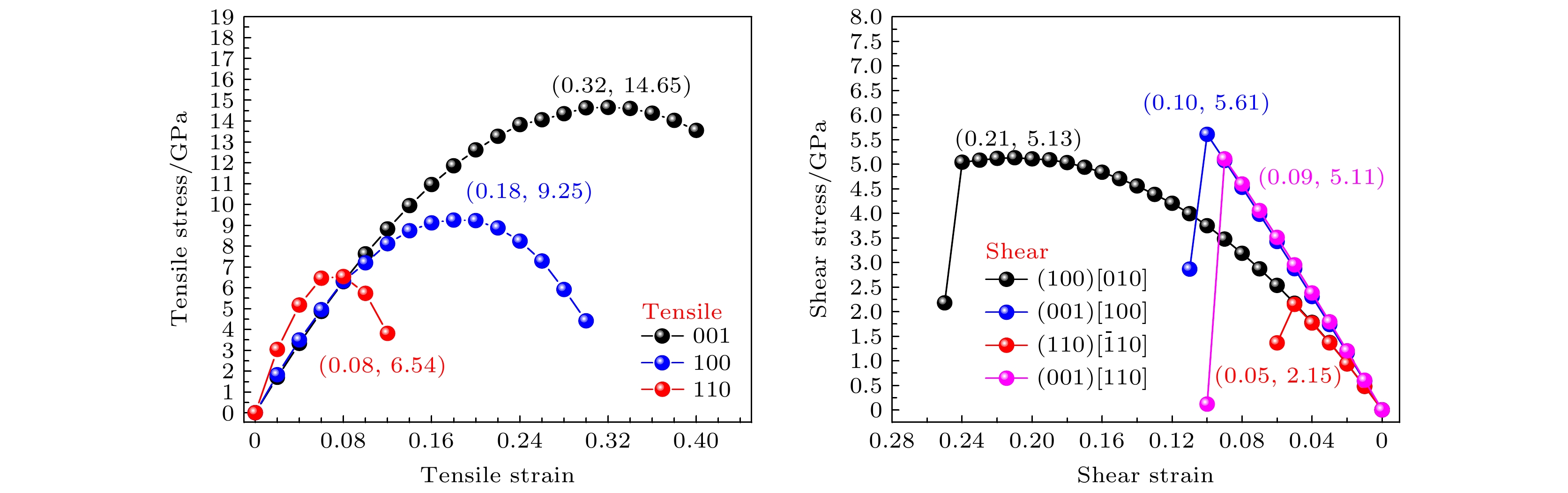
2011年福岛核事故之后, U3Si2作为可代替UO2的核燃料被预测为重要的耐事故燃料. 近年来的研究结果表明, U3Si2作为耐事故燃料的候选材料, 其在微观尺度上进行的模拟还不够深入. 在宏观尺度上不足以建立燃料数据库和模型来有效预测U3Si2的一些性能. 因此, 采用第一性原理计算U3Si2核燃料的一些物理化学数据受到了广泛关注. 在之前的工作中, 我们采用第一性原理计算拉伸/剪切实验 (FPCTT/FPCST) 的方法预测了U3Si2在几个低指数晶面/晶向上的理想强度. 然而, 并未对U3Si2的断裂行为进行过多的解释. 因此, 本论文通过论述理想拉伸/剪切应变对U3Si2化学键键长及电荷密度分布影响, 分析了U3Si2在这几个低指数晶面/晶向上的断裂行为. 结果表明: U3Si2在理想拉伸应变的作用下, 晶体的破坏主要受化学键变化的影响, 而在剪切应变的作用下应变能或应力的突然下降, 可能与U3Si2的应变诱导结构相变有关.After the Fukushima nuclear accident in 2011, U3Si2 was predicted to be an important accident tolerant fuel that can replace UO2. The results of recent studies have shown that the simulation at the micro-scale of U3Si2 serving as a candidate for accident tolerant fuel is not deep enough. It is not sufficient to build fuel databases and models at a macro-scale to effectively predict some properties of U3Si2. Therefore, employing the first principles to calculate some physicochemical data of U3Si2 nuclear fuel has received extensive attention. In previous work, we predicted the ideal strength of U3Si2 in several low-index crystal planes/directions by the first-principles computational tensile/shear test (FPCTT/FPCST) approach. However, the fracture behavior of U3Si2 has not been explained much. Therefore, in this work, the effects of ideal tensile/shear strain on the chemical bond length and charge density distribution of U3Si2 are discussed to analyze the fracture behaviors of U3Si2 in these low-index crystal planes/directions. The effect of strain is achieved by using the incremental simulation elements in the specified crystal plane/direction. The crystal structures of U3Si2 under different strains are optimized by using the first principles based on density functional theory. The variation ranges of chemical bond length and the charge density distributions of U3Si2 under different ultimate strains are summarized and calculated respectively. The results show that the elongation of the U—U bond is the main contributor to the tensile deformation of U3Si2 in the [100] crystal direction under tensile load. The toughness of U3Si2 in the [001] crystal direction is mainly due to the elongation of the U—Si bond and U—U bond. However, the tensile deformation produced in the [110] crystal direction of U3Si2 is mainly related to the elongation of the Si—Si bond. In the (100)[010] slip system, U3Si2 has great deformation and the crystal breaks when the Si—Si bond length reaches a limit of 3.038 Å. For the (001)[100], (110)[
$ \bar 1 $ 10] and (001)[110] slip systems of U3Si2, the crystal is broken under small shear deformation, and the change of its bond length is not obvious, reflecting that the sudden decrease of the strain energy or stress in these several slip systems may be related to the strain-induced structural phase transition of U3Si2.-
Keywords:
- first-principles /
- U3Si2 nuclear fuel /
- chemical bonds /
- charge density
[1] Miao Y B, Harp J, Mo K, Kim Y S, Zhu S, Yacout A M 2018 J. Nucl. Mater. 503 314
 Google Scholar
Google Scholar
[2] Srinivasu K, Modak B, Ghanty T K 2018 J. Nucl. Mater. 510 360
 Google Scholar
Google Scholar
[3] Zhang Y F, Andersson A D R 2017 A Thermal Conductivity Model for USi Compounds. United States: N.p.2017
[4] Liu R, Zhou W Z, Cai J J 2018 Nucl. Eng. Des. 330 106
 Google Scholar
Google Scholar
[5] Beeler B, Baskes M, Andersson D, Cooper M W D, Zhang Y F 2017 J. Nucl. Mater. 495 267
 Google Scholar
Google Scholar
[6] Kim Y S 2012 Comprehensive Nuclear Materials (Oxford: Elsevier) pp391–422
[7] Birtcher R C, Wang L M 2011 MRS Proceedings 235 467
 Google Scholar
Google Scholar
[8] Rest J 1997 J. Nucl. Mater. 240 205
 Google Scholar
Google Scholar
[9] Yao T K, Gong B W, He L F, Harp J, Tonks M, Lian J 2018 J. Nucl. Mater. 498 169
 Google Scholar
Google Scholar
[10] Carvajal-Nunez U, Saleh T A, White J T, Maiorov B, Nelson A T 2018 J. Nucl. Mater. 498 438
 Google Scholar
Google Scholar
[11] Jossou E, Eduok U, Dzade N Y, Szpunar B, Szpunar J A 2018 Phys. Chem. Chem. Phys. 20 4708
 Google Scholar
Google Scholar
[12] Wang T, Qiu N X, Wen X D, Tian Y H, He J, Luo K, Zha X H, Zhou Y H, Huang Q, Lang J J, Du S Y 2016 J. Nucl. Mater. 469 194
 Google Scholar
Google Scholar
[13] Noordhoek M J, Besmann T M, Andersson D, Middleburgh S C, Chernatynskiy A 2016 J. Nucl. Mater. 479 216
 Google Scholar
Google Scholar
[14] Chattaraj D, Majumder C 2018 J. Alloy. Compd. 732 160
 Google Scholar
Google Scholar
[15] Liu H, Claisse A, Middleburgh S C, Olsson P 2019 J. Nucl. Mater. 527 151828
 Google Scholar
Google Scholar
[16] Remschnig K, Le Bihan T, Noël H, Rogl P 1992 J. Solid State Chem. 97 391
 Google Scholar
Google Scholar
[17] Miyadai T, Mori H, Oguchi T, Tazuke Y, Amitsuka H, Kuwai T, Miyako Y 1992 J. Magn. Magn. Mater. 104–107 47
 Google Scholar
Google Scholar
[18] Wang K, Qiao Y J, Zhang X H, Wang X D, Zhang Y M, Wang P, Du S Y 2021 Eur. Phys. J. Plus 136 409
 Google Scholar
Google Scholar
[19] Roundy D, Krenn C R, Cohen M L, Morris J W 1999 Phys. Rev. Lett. 82 2713
 Google Scholar
Google Scholar
[20] Roundy D, Krenn C R, Cohen M L, Morris J W 2001 Philos. Mag. A 81 1725
 Google Scholar
Google Scholar
[21] Ogata S, Li J, Hirosaki N, Shibutani Y, Yip S 2004 Phys. Rev. B 70 104104
 Google Scholar
Google Scholar
[22] Li X Q, Schönecker S, Zhao J J, Johansson B, Vitos L 2014 Phys. Rev. B 87 291
[23] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[24] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[25] Kresse G G, Furthmüller J J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[26] Kresse G, Hafner J 1993 Phys. Rev. B Condens. Matter. 47 558
 Google Scholar
Google Scholar
[27] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[28] Liechtenstein A I, Anisimov V V, Zaanen J 1995 Phys. Rev. B 52 R5467
 Google Scholar
Google Scholar
[29] Sarma D D, Krummacher S, Hillebrecht F U, Koelling D D 1988 Phys. Rev. B: Condens. Matter. 38 1
 Google Scholar
Google Scholar
[30] Shih B C, Zhang Y B, Zhang W Q, Zhang P H 2012 Phys. Rev. B 85 045132
 Google Scholar
Google Scholar
[31] Szpunar B 2012 J. Phys. Chem. Solids 73 1003
 Google Scholar
Google Scholar
[32] Setyawan W, Curtarolo S 2010 Comput. Mater. Sci. 49 299
 Google Scholar
Google Scholar
[33] Mei Z G, Miao Y B, Liang L Y, Yacout A M 2019 J. Nucl. Mater. 513 192
 Google Scholar
Google Scholar
[34] Yang X Y, Korzhavyi P A, Liu Y, Wei Q L, Arslanov T R, Wärnå J P A, Yang Y, Zhang P 2022 Prog. Nucl. Energy 148 104229
 Google Scholar
Google Scholar
[35] Zachariasen W H 1949 Acta Crystallogr. 2 94
 Google Scholar
Google Scholar
[36] Smirnov M B, Kazimirov V Y, Rita B H, Smirnov K S, Pereira-Ramos J P 2014 J. Phys. Chem. Solids 75 115
 Google Scholar
Google Scholar
[37] Manikandan M, Rajeswarapalanichamy R, Iyakutti K 2017 Philos. Mag. 98 1
 Google Scholar
Google Scholar
[38] M I, T L, Bihan, S H, J R 2004 Phys. Rev. B 70 014113
 Google Scholar
Google Scholar
[39] Dubois S M M, Rignanese G M, Pardoen T, Charlier J C 2006 Phys. Rev. B 74 235203
 Google Scholar
Google Scholar
-
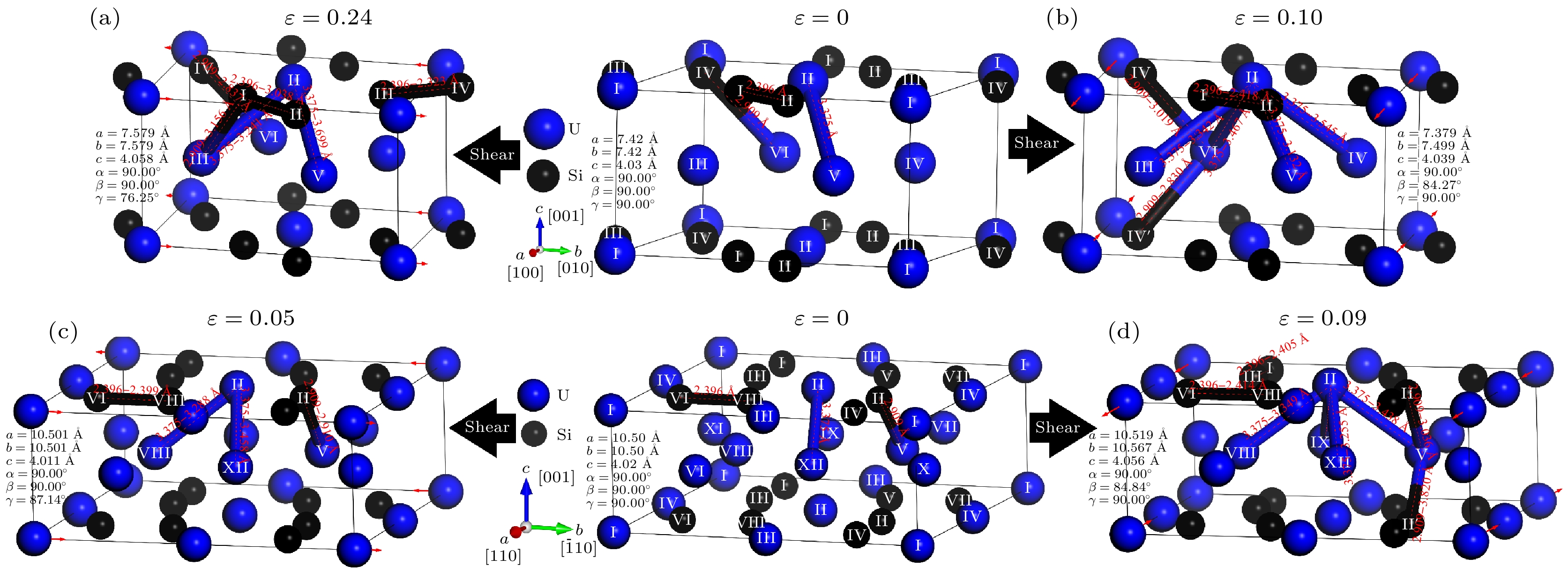
图 7 在剪切应变的作用下U3Si2键长的变化: (a) (100)[010]滑移系; (b) (001)[100]滑移系; (c) (110)[
$\bar 1$ 10]滑移系; (d) (001)[110]滑移系Fig. 7. Variation of the chemical bond length of U3Si2 under shear strain: (a) (100)[010] slip system; (b) (001)[100] slip system; (c) (110)[
$\bar 1$ 10] slip system; (d) (001)[110] slip system.表 1 U3Si2的弹性常数Cij, 弹性模量E, B和G (单位: GPa), B/G以及泊松比υ
Table 1. The elastic constants Cij, elastic moduli E, B and G (unit: GPa), B/G and Poisson’s ratio υ of U3Si2.
表 2 铁磁性条件下采用DFT+U计算得到U3Si2晶格常数及化学键长信息
Table 2. The lattice constants and chemical bond length of U3Si2 obtained by DFT+U calculation under ferromagnetic conditions.
-
[1] Miao Y B, Harp J, Mo K, Kim Y S, Zhu S, Yacout A M 2018 J. Nucl. Mater. 503 314
 Google Scholar
Google Scholar
[2] Srinivasu K, Modak B, Ghanty T K 2018 J. Nucl. Mater. 510 360
 Google Scholar
Google Scholar
[3] Zhang Y F, Andersson A D R 2017 A Thermal Conductivity Model for USi Compounds. United States: N.p.2017
[4] Liu R, Zhou W Z, Cai J J 2018 Nucl. Eng. Des. 330 106
 Google Scholar
Google Scholar
[5] Beeler B, Baskes M, Andersson D, Cooper M W D, Zhang Y F 2017 J. Nucl. Mater. 495 267
 Google Scholar
Google Scholar
[6] Kim Y S 2012 Comprehensive Nuclear Materials (Oxford: Elsevier) pp391–422
[7] Birtcher R C, Wang L M 2011 MRS Proceedings 235 467
 Google Scholar
Google Scholar
[8] Rest J 1997 J. Nucl. Mater. 240 205
 Google Scholar
Google Scholar
[9] Yao T K, Gong B W, He L F, Harp J, Tonks M, Lian J 2018 J. Nucl. Mater. 498 169
 Google Scholar
Google Scholar
[10] Carvajal-Nunez U, Saleh T A, White J T, Maiorov B, Nelson A T 2018 J. Nucl. Mater. 498 438
 Google Scholar
Google Scholar
[11] Jossou E, Eduok U, Dzade N Y, Szpunar B, Szpunar J A 2018 Phys. Chem. Chem. Phys. 20 4708
 Google Scholar
Google Scholar
[12] Wang T, Qiu N X, Wen X D, Tian Y H, He J, Luo K, Zha X H, Zhou Y H, Huang Q, Lang J J, Du S Y 2016 J. Nucl. Mater. 469 194
 Google Scholar
Google Scholar
[13] Noordhoek M J, Besmann T M, Andersson D, Middleburgh S C, Chernatynskiy A 2016 J. Nucl. Mater. 479 216
 Google Scholar
Google Scholar
[14] Chattaraj D, Majumder C 2018 J. Alloy. Compd. 732 160
 Google Scholar
Google Scholar
[15] Liu H, Claisse A, Middleburgh S C, Olsson P 2019 J. Nucl. Mater. 527 151828
 Google Scholar
Google Scholar
[16] Remschnig K, Le Bihan T, Noël H, Rogl P 1992 J. Solid State Chem. 97 391
 Google Scholar
Google Scholar
[17] Miyadai T, Mori H, Oguchi T, Tazuke Y, Amitsuka H, Kuwai T, Miyako Y 1992 J. Magn. Magn. Mater. 104–107 47
 Google Scholar
Google Scholar
[18] Wang K, Qiao Y J, Zhang X H, Wang X D, Zhang Y M, Wang P, Du S Y 2021 Eur. Phys. J. Plus 136 409
 Google Scholar
Google Scholar
[19] Roundy D, Krenn C R, Cohen M L, Morris J W 1999 Phys. Rev. Lett. 82 2713
 Google Scholar
Google Scholar
[20] Roundy D, Krenn C R, Cohen M L, Morris J W 2001 Philos. Mag. A 81 1725
 Google Scholar
Google Scholar
[21] Ogata S, Li J, Hirosaki N, Shibutani Y, Yip S 2004 Phys. Rev. B 70 104104
 Google Scholar
Google Scholar
[22] Li X Q, Schönecker S, Zhao J J, Johansson B, Vitos L 2014 Phys. Rev. B 87 291
[23] Hohenberg P, Kohn W 1964 Phys. Rev. 136 B864
 Google Scholar
Google Scholar
[24] Kohn W, Sham L J 1965 Phys. Rev. 140 A1133
 Google Scholar
Google Scholar
[25] Kresse G G, Furthmüller J J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[26] Kresse G, Hafner J 1993 Phys. Rev. B Condens. Matter. 47 558
 Google Scholar
Google Scholar
[27] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[28] Liechtenstein A I, Anisimov V V, Zaanen J 1995 Phys. Rev. B 52 R5467
 Google Scholar
Google Scholar
[29] Sarma D D, Krummacher S, Hillebrecht F U, Koelling D D 1988 Phys. Rev. B: Condens. Matter. 38 1
 Google Scholar
Google Scholar
[30] Shih B C, Zhang Y B, Zhang W Q, Zhang P H 2012 Phys. Rev. B 85 045132
 Google Scholar
Google Scholar
[31] Szpunar B 2012 J. Phys. Chem. Solids 73 1003
 Google Scholar
Google Scholar
[32] Setyawan W, Curtarolo S 2010 Comput. Mater. Sci. 49 299
 Google Scholar
Google Scholar
[33] Mei Z G, Miao Y B, Liang L Y, Yacout A M 2019 J. Nucl. Mater. 513 192
 Google Scholar
Google Scholar
[34] Yang X Y, Korzhavyi P A, Liu Y, Wei Q L, Arslanov T R, Wärnå J P A, Yang Y, Zhang P 2022 Prog. Nucl. Energy 148 104229
 Google Scholar
Google Scholar
[35] Zachariasen W H 1949 Acta Crystallogr. 2 94
 Google Scholar
Google Scholar
[36] Smirnov M B, Kazimirov V Y, Rita B H, Smirnov K S, Pereira-Ramos J P 2014 J. Phys. Chem. Solids 75 115
 Google Scholar
Google Scholar
[37] Manikandan M, Rajeswarapalanichamy R, Iyakutti K 2017 Philos. Mag. 98 1
 Google Scholar
Google Scholar
[38] M I, T L, Bihan, S H, J R 2004 Phys. Rev. B 70 014113
 Google Scholar
Google Scholar
[39] Dubois S M M, Rignanese G M, Pardoen T, Charlier J C 2006 Phys. Rev. B 74 235203
 Google Scholar
Google Scholar
计量
- 文章访问数: 9972
- PDF下载量: 173
- 被引次数: 0














 下载:
下载: