-
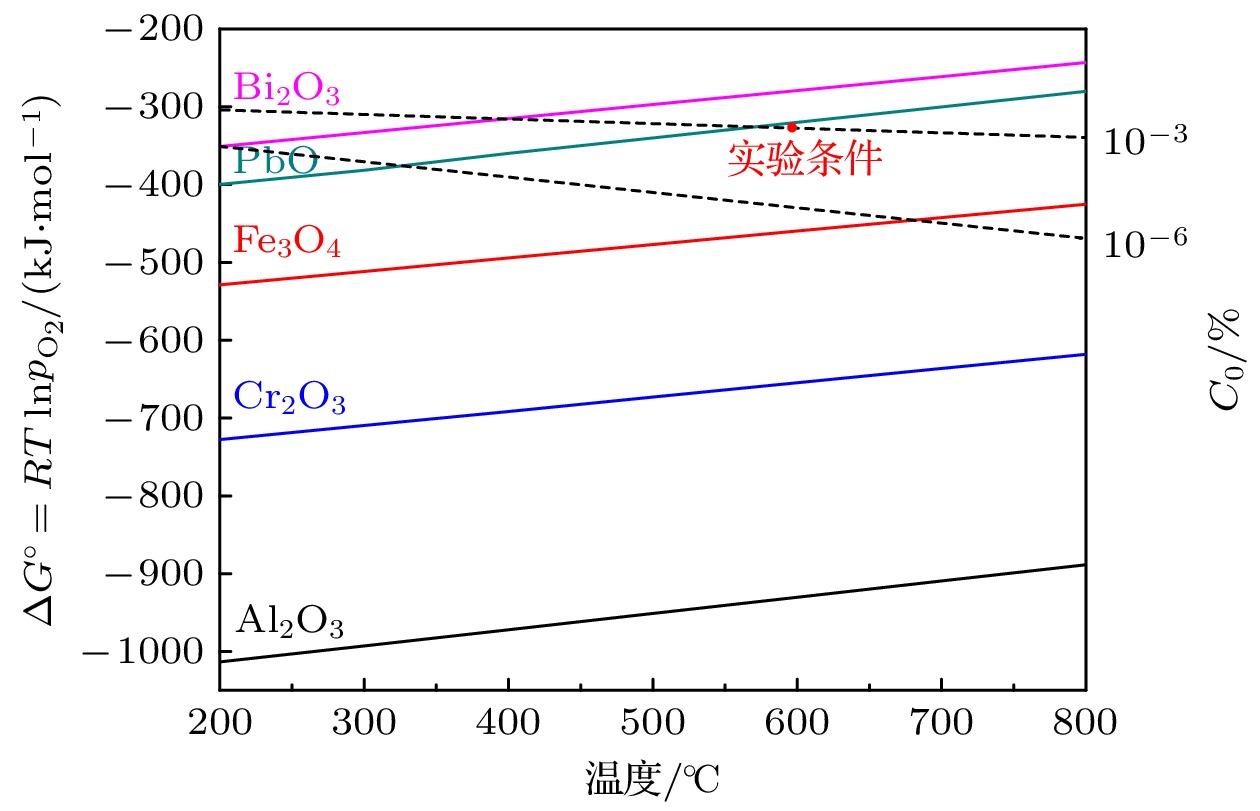
铁素体/马氏体钢, 如T91钢和SIMP钢, 被选为第4代铅冷快堆和加速器驱动系统(ADS)的主要候选结构材料. 但容器钢与液态铅铋共晶(LBE)在高温下的相容性限制了它们的应用. 铁素体/马氏体钢在600 ℃的LBE中腐蚀严重. 为了保护铁素体/马氏体钢免受高温LBE腐蚀, 在钢表面制备AlOx (x < 1.5)涂层. 本文采用磁控溅射法在T91钢和SIMP钢表面制备了AlOx涂层. 对表面有涂层的T91钢和SIMP钢以及表面无涂层的T91钢和SIMP钢在600 ℃的饱和氧浓度的LBE中腐蚀300 h和700 h的结果进行比较. 结果表明, 涂层钢表面的氧化层比无涂层钢表面的氧化层薄, 这表明AlOx涂层可以有效防止铁、铬和氧元素的快速扩散. 然而, 在LBE中腐蚀700 h后, AlOx涂层出现裂纹, 表面有涂层的T91钢和SIMP钢均遭受到明显的氧化腐蚀, 说明该涂层在600 ℃的LBE中可以在短时间内保护基体免受高温腐蚀. 但是涂层在600 ℃的LBE 中不能长时间保持稳定. 这可能是由于此次实验条件制备的AlOx涂层膜基结合力不强或制备的AlOx涂层里面存在大量的金属铝和结构缺陷. AlOx涂层在LBE中的高温稳定性有待进一步研究.Ferritic/martensitic steels, such as T91 steel and SIMP steel, are chosen as the main candidates of structural materials for the Generation IV lead-cooled fast reactors and accelerator driven system. However, the compatibility between container steel and liquid Pb-Bi eutectic (LBE) at high temperature limits their applications. The corrosion of ferritic/martensitic steels is serious in LBE at 600 ℃. In order to avoid corroding the ferritic/martensitic steels in LBE, it is proposed to coat AlOx (x < 1.5) on the steel surface. The AlOx coating is conducted on T91 steel and SIMP steel by magnetron sputtering. In this exploratory work, the corrosion results of AlOx coating steel are compared with the corrosion results of the uncoated steel in LBE with a saturated oxygen concentration at 600 ℃ for 300 h and 700 h. The results show that the AlOx coating can effectively prevent the iron chromium and oxygen from diffusing, so the oxide scale of the coated steel is thinner than that of the uncoated steel. However, the coating cracks after 700 h corrosion in LBE. Meanwhile, T91 steel and SIMP steel also suffer serious oxidative corrosion, indicating that the coating can protect the substrate from being corroded by 600 ℃ static LBE in a short time. However, the coating cannot keep stable for a long time in LBE at 600 ℃. This may be due to the weak film base bonding force of AlOx coating prepared under the experimental conditions, or a large number of metal aluminum and structural defects existing in AlOx coating. It is needed to further study the stability of AlOx coating in LBE at elevated temperature.
-
Keywords:
- T91 steel /
- SIMP steel /
- AlOx coating /
- liquid Pb-Bi eutectic /
- oxide layer /
- thermal expansion coefficient
[1] Sar F, Mhiaoui S, Gasser J G 2007 J. Non. Cryst. Solids. 353 3622
 Google Scholar
Google Scholar
[2] Sobolev V 2007 J. Nucl. Mater. 362 235
 Google Scholar
Google Scholar
[3] Zhang J 2014 Adv. Eng. Mater. 16 349
 Google Scholar
Google Scholar
[4] Zhang J, Ning L 2008 J. Nucl. Mater. 373 351
 Google Scholar
Google Scholar
[5] Xu Y C, Zhang Y G, Li X Y, Liu W, Li D D, Liu C S, Pan B C, Wang Z G 2017 Corros. Sci. 118 1
 Google Scholar
Google Scholar
[6] Barbier F, Rusanov A 2001 J. Nucl. Mater. 296 231
 Google Scholar
Google Scholar
[7] Martinelli L, Jean-Louis C, Fanny B C 2011 Nucl. Eng. Des. 241 1288
 Google Scholar
Google Scholar
[8] Concetta F 2015 Handbook on Lead-bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-hydraulics and Technologies (2015 Edition-Introduction) (OECD Nuclear Energy Agency)
[9] Zhang J 2009 Corros. Sci. 51 1207
 Google Scholar
Google Scholar
[10] Takaya S, Furukawa T, Müller G, Heinzel A, Jianu A, Weisenburger A, Aoto K, Inoue M, Okuda T, Abe F, Ohnuki S, Fujisawa T, Kimura A 2012 J. Nucl. Mater. 428 125
 Google Scholar
Google Scholar
[11] Srinivasan P B, Kumar M 2009 Mater. Chem. Phys. 115 179
 Google Scholar
Google Scholar
[12] Müller G, Schumacher G, Zimmermann F 2000 J. Nucl. Mater. 278 85
 Google Scholar
Google Scholar
[13] Deloffre P, Balbaud-Célérier F, Terlain A 2004 J. Nucl. Mater. 335 180
 Google Scholar
Google Scholar
[14] Weisenburger A, Heinzel A, Müller G, Muscher H, Rousanov A 2008 J. Nucl. Mater. 376 274
 Google Scholar
Google Scholar
[15] Fetzer R, Weisenburger A, Jianu A, Müller G 2012 Corros. Sci. 55 213
 Google Scholar
Google Scholar
[16] Short M P, Ballinger R G, Hänninen H E 2013 J. Nucl. Mater. 434 259
 Google Scholar
Google Scholar
[17] Hosemann P, Thau H T, Johnson A L, Maloy S A, Li N 2008 J. Nucl. Mater. 373 246
 Google Scholar
Google Scholar
[18] Takaya S, Furukawa T, Aoto K, Müller G, Weisenburger A, Heinzel A, Inoue M, Okuda T, Abe F, Ohnuki S, Fujisawa T and Kimura A 2009 J. Nucl. Mater. 386–388 507
 Google Scholar
Google Scholar
[19] Takaya S, Furukawa T, Inoue M, Fujisawa T, Okuda T, Abe F, Ohnuki S, Kimura A 2010 J. Nucl. Mater. 398 132
 Google Scholar
Google Scholar
[20] Heinzel A, Kondo M, Takahashi M 2006 J. Nucl. Mater. 350 264
 Google Scholar
Google Scholar
[21] Kurata Y, Futakawa M, Saito S 2004 J. Nucl. Mater. 335 501
 Google Scholar
Google Scholar
[22] Ferré F G, Mairov A, Iadicicco D, Vanazzi M, Bassini S, Utili M, Tarantino M, Bragaglia M, Lamastra F R, Nanni F, Ceseracciu L, Serruys Y, Trocellier P, Beck L, Sridharan K, Beghi M G , Di Fonzo F 2017 Corros. Sci. 124 80
 Google Scholar
Google Scholar
[23] Glasbrenner H, Gröschel F 2006 J. Nucl. Mater. 356 213
 Google Scholar
Google Scholar
[24] Weisenburger A, Jianu A, Doyle S, Bruns M, Fetzer R, Heinzel A, Del Giacco M, An W, Müller G 2013 J. Nucl. Mater 437 282
 Google Scholar
Google Scholar
[25] Ferré G, Ormellese M, Fonzo F D, Beghi M G 2013 Corros. Sci. 77 375
 Google Scholar
Google Scholar
[26] Sordo F, Abánades A, Lafuente A, Martínez-Val J M, Perlado M 2009 Nucl. Eng. Des. 239 2573
 Google Scholar
Google Scholar
[27] Borgstedt H U, Frees G 1995 Liquid Metal Systems. (New York: Springer) p339
[28] Ellingham H J T 1994 J. Soc. Chem. Ind. 63 125
[29] Yeliseyeva O, Tsisar V, Zhou Z 2013 J. Nucl. Mater. 442 434
 Google Scholar
Google Scholar
[30] Weisenburger A, Schroer C, Jianu A, Heinzel A, Konys J, Steiner H, Müller G, Fazio C, Gessi A, Babayan S, Kobzova A, Martinelli L, Ginestar K, Balbaud-Célerier F, Martín-Muñoz F J, Soler Crespo L 2011 J. Nucl. Mater. 415 260
 Google Scholar
Google Scholar
[31] Weisenburger A, Mansani L, Schumacher G, Müller G 2014 Nucl. Eng. Des. 273 584
 Google Scholar
Google Scholar
[32] Martinelli L, Balbaud-Célérier F, Terlain A, Bosonnet S, Picard G, Santarini G 2008 Corros. Sci. 50 2537
 Google Scholar
Google Scholar
[33] Martinelli L, Balbaud-Célérier F, Picard G, Santarini G 2008 Corros. Sci. 50 2549
 Google Scholar
Google Scholar
[34] Miorin E, Montagner F, Zin V, Giuranno D, Deambrosis S M 2019 Surf. Coat. Technol. 377 124890
 Google Scholar
Google Scholar
[35] Comstock M 2009 J. Nucl. Mater. 382 272
 Google Scholar
Google Scholar
[36] Tan L, Machut M T, Sridharan K 2007 J. Nucl. Mater. 371 161
 Google Scholar
Google Scholar
[37] Li B S, Liao Q, Zhang H P, Shen T L, Ge F F, Nabil D 2021 Corros. Sci. 187 109477
 Google Scholar
Google Scholar
[38] Zhang L L, Yan W, Shi Q Q, Li Y F, Shen Y Y, Yang K 2020 Corros. Sci. 167 108519
 Google Scholar
Google Scholar
[39] Liu J, Yan W, Sha W, Wang W, Shan Y Y, Yang K 2016 J. Nucl. Mater. 473 189
 Google Scholar
Google Scholar
[40] Li Y, Wang S, Sun P, Xu D, Ren M, Guo Y, Lin G 2017 Corros. Sci. 128 241
 Google Scholar
Google Scholar
[41] Shi Q, Liu J, Luan H, Yang Z, Wang W, Yan W, Shan Y, Yang K 2015 J. Nucl. Mater. 457 135
 Google Scholar
Google Scholar
[42] Behnamian Y, Mostafaei A, Kohandehghan A, Amirkhiz B S, Serate D, Sun Y, Liu S, Aghaie E, Zeng Y, Chmielus M, Zheng W, Guzonas D 2016 Corros. Sci. 106 188
 Google Scholar
Google Scholar
[43] Martinelli L, Balbaud-Célérier F, Terlian A, Delpech S, Santarini G, Favergeon J, Moulin G, Tabarant M, Picard G 2008 Corros. Sci. 50 2523
 Google Scholar
Google Scholar
[44] Bian L Z, Chen Z Y, Wang L J, Li F S, Chou K C 2017 J. Iron. Steel. Res. Int. 24 77
 Google Scholar
Google Scholar
[45] Huntz A M, Maréchal L, Lesage B, Molins R 2006 Appl. Surf. Sci. 252 7781
 Google Scholar
Google Scholar
[46] Melander A 1997 Int. J. Fatigue 19 13
 Google Scholar
Google Scholar
[47] Wang Q S, Wang W Q, Shi Z M 2018 E. Science. 113 012146
 Google Scholar
Google Scholar
[48] Echsler H, Martinez E A, Singheiser L, Quadakkers W J 2004 Mater. Sci. Eng. A 384 1
 Google Scholar
Google Scholar
[49] Hayashi H, Watanabe M, Inaba H 2000 Thermochim Acta. 359 77
 Google Scholar
Google Scholar
[50] Mavko G, Mukerji T, Dvorkin J 2009 The Rock Physics Handbook: Elasticity and Hooke's law 2 21
 Google Scholar
Google Scholar
-
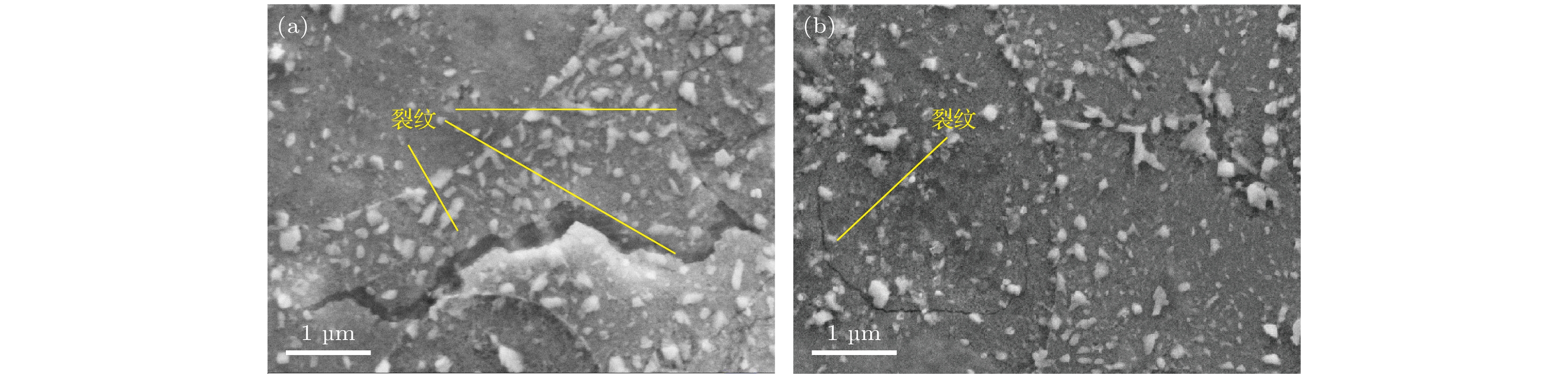
图 4 T91钢和SIMP钢在600 ℃的LBE中腐蚀300 h和700 h后的表面SEM图 (a) LBE中腐蚀300 h后无涂层的T91钢表面; (b) LBE中腐蚀300 h后有涂层的T91钢表面; (c) LBE中腐蚀300 h后无涂层的SIMP钢表面; (d) LBE中腐蚀300 h后有涂层的SIMP钢表面; (e) LBE中腐蚀700 h后无涂层的T91钢表面; (f) LBE中腐蚀700 h后有涂层的T91钢表面; (g) LBE中腐蚀700 h后无涂层的SIMP钢表面; (h) LBE中腐蚀700 h后无涂层的SIMP钢表面
Fig. 4. SEM images showing the surface morphology of SIMP and T91 steels after 300 h and 700 h corrosion in LBE at 600 ℃: (a) The uncoated surface of T91 steel in LBE for 300 h; (b) the coated surface of T91 steel in LBE for 300 h; (c) the uncoated surface of SIMP steel in LBE for 300 h; (d) the coated surface of SIMP steel in LBE for 300 h; (e) the uncoated surface of T91 steel in LBE for 700 h; (f) the coated surface of T91 steel in LBE for 700 h; (g) the uncoated surface of SIMP steel in LBE for 700 h; (h) the coated surface of SIMP steel in LBE for 700 h.
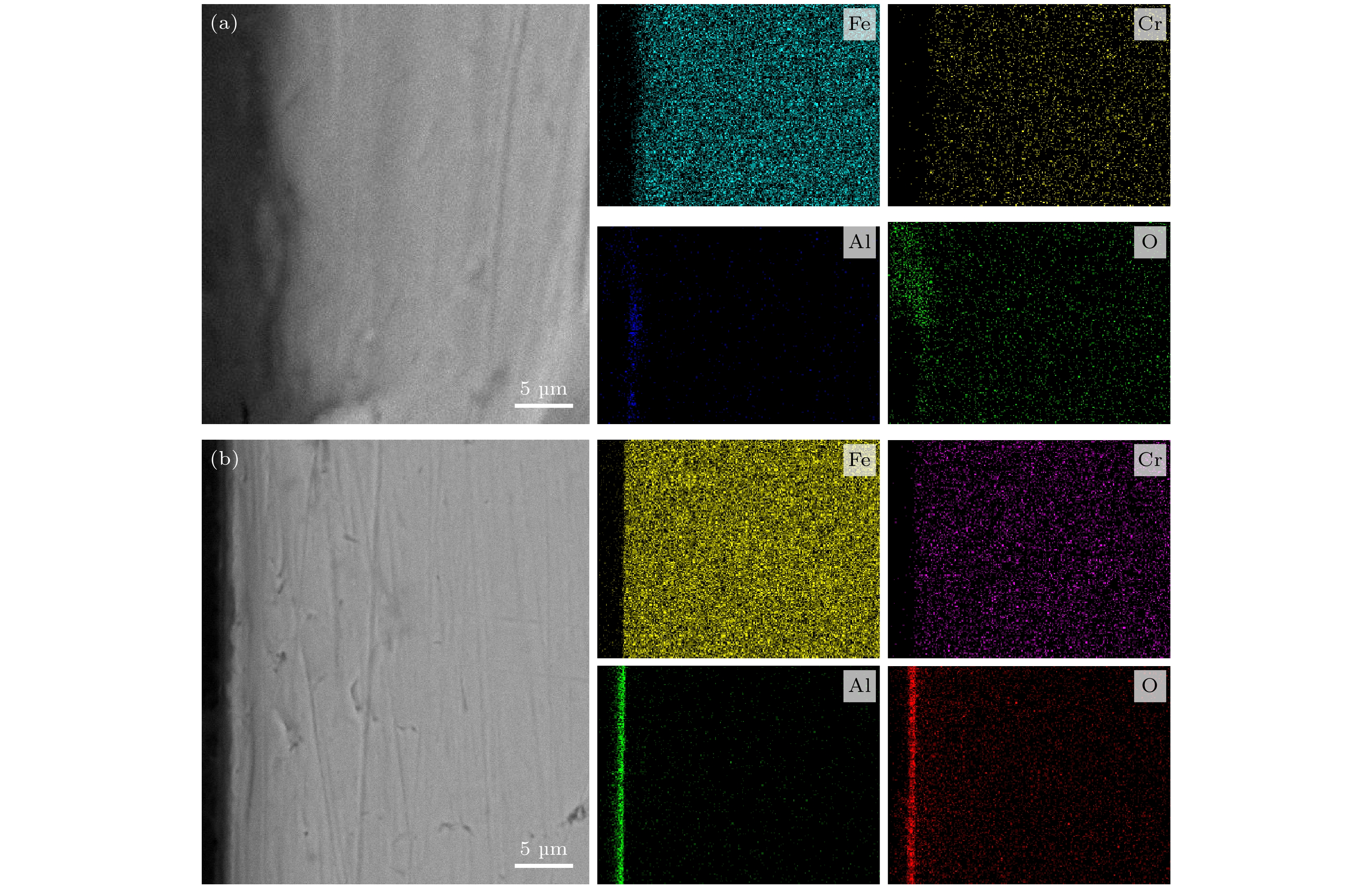
图 7 在600 ℃的LBE中腐蚀300 h后T91钢和SIMP钢的横截面SEM图像和EDS线性分析(扫描方向从左到右) (a), (b)涂层T91钢; (c), (d)无涂层T91钢; (e), (f)涂层SIMP钢; (g), (h)无涂层SIMP钢
Fig. 7. Cross-sectional SEM images and EDS linear analysis of T91 and SIMP steels after 300 h corrosion in LBE at 600 ℃: (a), (b) The coated T91; (c), (d) the uncoated T91; (e), (f) the coated SIMP; (g), (h) the uncoated SIMP.
图 9 T91钢和SIMP钢在600℃的LBE中腐蚀700 h后的横截面SEM图像和EDS线性分析(扫描方向从左到右) (a), (b)涂层T91钢; (c), (d)无涂层T91钢; (e), (f)涂层SIMP钢; (g), (h)无涂层SIMP钢.
Fig. 9. Cross-sectional SEM images and EDS linear analysis of T91 and SIMP steels after 700 h corrosion in LBE at 600 ℃: (a), (b) The coated T91; (c), (d) the uncoated T91; (e), (f) the coated SIMP; (g), (h) the uncoated SIMP.
表 1 研究钢材的化学成分(质量分数)
Table 1. Chemical compositions of the studied steels (mass fraction%)
元素 Fe Cr Ni Mo V Si C Nb N Ta W T91 Bal 8.50 0.25 0.95 0.19 0.20 0.10 0.067 0.05 — — SIMP Bal 10.50 — — 0.20 1.40 0.20 0.01 — 0.15 1.50 表 2 磁控溅射制备AlOx薄膜的典型工艺参数(1 sccm = 1 mL/min)
Table 2. Typical process parameters of the AlOx films prepared by magnetron sputtering
靶功率/W 频率 /kHz O2 流量/sccm 氩气流量/sccm 靶温度/℃ 沉积速率/(nm·min–1) 400 350 8.6 32 25 9 表 3 T91钢和SIMP钢在600 ℃静态LBE下表面氧化物在图4标记位置的EDS点分析
Table 3. EDS analyses of the surface oxides of T91 and SIMP steel exposed to static LBE at 600 ℃ in Fig. 4.
原子分数/% 元素 Fe Cr O Al Pb Si Point A 43.89 — 55.12 — 0.34 0.65 Point B 0.89 — 61.9 36.4 — 0.81 Point C 42.7 — 54.37 — 0.38 2.55 Point D 3.01 — 58.63 34.6 — 3.76 Point E 44.21 — 54.30 — 0.88 0.61 Point F 41.17 — 58.26 — — 0.57 Point G 40.59 — 55.23 — 0.60 3.58 Point H 32.51 — 63.80 — — 3.69 -
[1] Sar F, Mhiaoui S, Gasser J G 2007 J. Non. Cryst. Solids. 353 3622
 Google Scholar
Google Scholar
[2] Sobolev V 2007 J. Nucl. Mater. 362 235
 Google Scholar
Google Scholar
[3] Zhang J 2014 Adv. Eng. Mater. 16 349
 Google Scholar
Google Scholar
[4] Zhang J, Ning L 2008 J. Nucl. Mater. 373 351
 Google Scholar
Google Scholar
[5] Xu Y C, Zhang Y G, Li X Y, Liu W, Li D D, Liu C S, Pan B C, Wang Z G 2017 Corros. Sci. 118 1
 Google Scholar
Google Scholar
[6] Barbier F, Rusanov A 2001 J. Nucl. Mater. 296 231
 Google Scholar
Google Scholar
[7] Martinelli L, Jean-Louis C, Fanny B C 2011 Nucl. Eng. Des. 241 1288
 Google Scholar
Google Scholar
[8] Concetta F 2015 Handbook on Lead-bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-hydraulics and Technologies (2015 Edition-Introduction) (OECD Nuclear Energy Agency)
[9] Zhang J 2009 Corros. Sci. 51 1207
 Google Scholar
Google Scholar
[10] Takaya S, Furukawa T, Müller G, Heinzel A, Jianu A, Weisenburger A, Aoto K, Inoue M, Okuda T, Abe F, Ohnuki S, Fujisawa T, Kimura A 2012 J. Nucl. Mater. 428 125
 Google Scholar
Google Scholar
[11] Srinivasan P B, Kumar M 2009 Mater. Chem. Phys. 115 179
 Google Scholar
Google Scholar
[12] Müller G, Schumacher G, Zimmermann F 2000 J. Nucl. Mater. 278 85
 Google Scholar
Google Scholar
[13] Deloffre P, Balbaud-Célérier F, Terlain A 2004 J. Nucl. Mater. 335 180
 Google Scholar
Google Scholar
[14] Weisenburger A, Heinzel A, Müller G, Muscher H, Rousanov A 2008 J. Nucl. Mater. 376 274
 Google Scholar
Google Scholar
[15] Fetzer R, Weisenburger A, Jianu A, Müller G 2012 Corros. Sci. 55 213
 Google Scholar
Google Scholar
[16] Short M P, Ballinger R G, Hänninen H E 2013 J. Nucl. Mater. 434 259
 Google Scholar
Google Scholar
[17] Hosemann P, Thau H T, Johnson A L, Maloy S A, Li N 2008 J. Nucl. Mater. 373 246
 Google Scholar
Google Scholar
[18] Takaya S, Furukawa T, Aoto K, Müller G, Weisenburger A, Heinzel A, Inoue M, Okuda T, Abe F, Ohnuki S, Fujisawa T and Kimura A 2009 J. Nucl. Mater. 386–388 507
 Google Scholar
Google Scholar
[19] Takaya S, Furukawa T, Inoue M, Fujisawa T, Okuda T, Abe F, Ohnuki S, Kimura A 2010 J. Nucl. Mater. 398 132
 Google Scholar
Google Scholar
[20] Heinzel A, Kondo M, Takahashi M 2006 J. Nucl. Mater. 350 264
 Google Scholar
Google Scholar
[21] Kurata Y, Futakawa M, Saito S 2004 J. Nucl. Mater. 335 501
 Google Scholar
Google Scholar
[22] Ferré F G, Mairov A, Iadicicco D, Vanazzi M, Bassini S, Utili M, Tarantino M, Bragaglia M, Lamastra F R, Nanni F, Ceseracciu L, Serruys Y, Trocellier P, Beck L, Sridharan K, Beghi M G , Di Fonzo F 2017 Corros. Sci. 124 80
 Google Scholar
Google Scholar
[23] Glasbrenner H, Gröschel F 2006 J. Nucl. Mater. 356 213
 Google Scholar
Google Scholar
[24] Weisenburger A, Jianu A, Doyle S, Bruns M, Fetzer R, Heinzel A, Del Giacco M, An W, Müller G 2013 J. Nucl. Mater 437 282
 Google Scholar
Google Scholar
[25] Ferré G, Ormellese M, Fonzo F D, Beghi M G 2013 Corros. Sci. 77 375
 Google Scholar
Google Scholar
[26] Sordo F, Abánades A, Lafuente A, Martínez-Val J M, Perlado M 2009 Nucl. Eng. Des. 239 2573
 Google Scholar
Google Scholar
[27] Borgstedt H U, Frees G 1995 Liquid Metal Systems. (New York: Springer) p339
[28] Ellingham H J T 1994 J. Soc. Chem. Ind. 63 125
[29] Yeliseyeva O, Tsisar V, Zhou Z 2013 J. Nucl. Mater. 442 434
 Google Scholar
Google Scholar
[30] Weisenburger A, Schroer C, Jianu A, Heinzel A, Konys J, Steiner H, Müller G, Fazio C, Gessi A, Babayan S, Kobzova A, Martinelli L, Ginestar K, Balbaud-Célerier F, Martín-Muñoz F J, Soler Crespo L 2011 J. Nucl. Mater. 415 260
 Google Scholar
Google Scholar
[31] Weisenburger A, Mansani L, Schumacher G, Müller G 2014 Nucl. Eng. Des. 273 584
 Google Scholar
Google Scholar
[32] Martinelli L, Balbaud-Célérier F, Terlain A, Bosonnet S, Picard G, Santarini G 2008 Corros. Sci. 50 2537
 Google Scholar
Google Scholar
[33] Martinelli L, Balbaud-Célérier F, Picard G, Santarini G 2008 Corros. Sci. 50 2549
 Google Scholar
Google Scholar
[34] Miorin E, Montagner F, Zin V, Giuranno D, Deambrosis S M 2019 Surf. Coat. Technol. 377 124890
 Google Scholar
Google Scholar
[35] Comstock M 2009 J. Nucl. Mater. 382 272
 Google Scholar
Google Scholar
[36] Tan L, Machut M T, Sridharan K 2007 J. Nucl. Mater. 371 161
 Google Scholar
Google Scholar
[37] Li B S, Liao Q, Zhang H P, Shen T L, Ge F F, Nabil D 2021 Corros. Sci. 187 109477
 Google Scholar
Google Scholar
[38] Zhang L L, Yan W, Shi Q Q, Li Y F, Shen Y Y, Yang K 2020 Corros. Sci. 167 108519
 Google Scholar
Google Scholar
[39] Liu J, Yan W, Sha W, Wang W, Shan Y Y, Yang K 2016 J. Nucl. Mater. 473 189
 Google Scholar
Google Scholar
[40] Li Y, Wang S, Sun P, Xu D, Ren M, Guo Y, Lin G 2017 Corros. Sci. 128 241
 Google Scholar
Google Scholar
[41] Shi Q, Liu J, Luan H, Yang Z, Wang W, Yan W, Shan Y, Yang K 2015 J. Nucl. Mater. 457 135
 Google Scholar
Google Scholar
[42] Behnamian Y, Mostafaei A, Kohandehghan A, Amirkhiz B S, Serate D, Sun Y, Liu S, Aghaie E, Zeng Y, Chmielus M, Zheng W, Guzonas D 2016 Corros. Sci. 106 188
 Google Scholar
Google Scholar
[43] Martinelli L, Balbaud-Célérier F, Terlian A, Delpech S, Santarini G, Favergeon J, Moulin G, Tabarant M, Picard G 2008 Corros. Sci. 50 2523
 Google Scholar
Google Scholar
[44] Bian L Z, Chen Z Y, Wang L J, Li F S, Chou K C 2017 J. Iron. Steel. Res. Int. 24 77
 Google Scholar
Google Scholar
[45] Huntz A M, Maréchal L, Lesage B, Molins R 2006 Appl. Surf. Sci. 252 7781
 Google Scholar
Google Scholar
[46] Melander A 1997 Int. J. Fatigue 19 13
 Google Scholar
Google Scholar
[47] Wang Q S, Wang W Q, Shi Z M 2018 E. Science. 113 012146
 Google Scholar
Google Scholar
[48] Echsler H, Martinez E A, Singheiser L, Quadakkers W J 2004 Mater. Sci. Eng. A 384 1
 Google Scholar
Google Scholar
[49] Hayashi H, Watanabe M, Inaba H 2000 Thermochim Acta. 359 77
 Google Scholar
Google Scholar
[50] Mavko G, Mukerji T, Dvorkin J 2009 The Rock Physics Handbook: Elasticity and Hooke's law 2 21
 Google Scholar
Google Scholar
计量
- 文章访问数: 9324
- PDF下载量: 127
- 被引次数: 0














 下载:
下载: