-
BiFeO3 (BFO)作为反铁磁性和铁电性共存的多铁性材料, 其饱和极化强度理论值大于100 μC/cm2, 居里温度为830 ℃, 具有较强的电卡效应. 但是由于BFO高温烧结过程中Bi2O3易挥发, 铁离子易变价, 导致BFO中缺陷较多, 漏电流较大, 其铁电特性难以发挥出来. 虽然采用与BaTiO3 (BTO)等氧化物铁电体形成固溶体的方法可以减小漏电流, 但是漏电流和高介电损耗问题仍然存在. 本文试图通过添加锰离子到BFO-BTO固溶体的方法解决这一问题. 采用传统的高温固相反应法制备了0.7BiFeO3-0.3BaTiO3+x%MnO2 (BFO-BTO+x%MnO2, 其中x%为质量分数)陶瓷, 研究了MnO2 掺杂对BFO-BTO固溶体的微观结构、介电和铁电性能的影响. 值得注意的是, BFO-BTO+x%MnO2样品测试结果证明少量掺杂MnO2能降低BFO-BTO陶瓷的介电损耗和漏电流, 这是由于掺杂Mn4+补偿氧空位浓度所致. 另外, 0.7BFO-0.3BTO+0.5%MnO2 陶瓷在100 kV/cm时的最大极化强度达到50.53 μC/cm2. 最后利用热电偶直测法测试了BFO-BTO+x%MnO2陶瓷的电卡效应, 发现极化翻转方法能使BFO-BTO+x%MnO2陶瓷的电卡效应翻倍增大, 其中x = 0样品从0至–30 kV/cm的变化与30 kV/cm至0的电场变化相比, 增大近8倍, 并且证实该方法同样适用于多晶一级相变铁电体.As a kind of ferroelectric and antiferromagnetic coexistent multi-ferroic material, BiFeO3 (BFO) has a theoretical saturation polarization over 100 μC/cm2, and a Curie temperature of 830 ℃, which may offer a huge electrocaloric effect. However, owing to the evaporation of Bi2O3 in the sintering process at high temperatures and the variation of chemical valence of iron ions, there are lots of point defects and also a large leakage current existing in BFO, making the ferroelectricity of BFO hard to develop and measure. Although the forming of solid solution with BaTiO3 (BTO) or other oxide ferroelectrics may mitigate the leakage current, high loss tangent is still existent. This work tries to address this issue by adding manganese ions into the BFO-BTO solid solution. The 0.7(BFO)-0.3(BTO)+x%MnO2 ceramics are prepared through using the conventional solid-state reaction at high temperature. The microstructure, dielectric characteristic and ferroelectric characteristic are investigated by doping different Mn4+ ions. Results indicate that the crystallographic structure is of rhombohedral and pseudocubic phase coexistence. It is observed that a certain content of Mn4+ ions may lead both the loss tangent and the leakage current for BFO-BTO ceramic to decrease, which is due to the compensation of dopant Mn4+ ions for the oxygen vacancies. In addition, the 0.7BFO-0.3BTO+0.5%MnO2 ceramic arrives at a maximum polarization of 50.53 μC/cm2 at 100 kV/cm. Finally, a direct approach is used to measure the electrocaloric effect. It is found that using the polarization flip method, the ECE temperature change is observed to increase almost 8 times when the electric field changes from 0 to –30 kV/m with respect to that when the electric field decreases from 30 kV/cm to 0. This verifies that the Lu et al’s method is also applicable to polycrystalline first-order phase transition ferroelectrics.
-
Keywords:
- ferroelectric /
- dielectric loss /
- electrocaloric effect
[1] Nan C W 2015 Sci. Sin. Tech. 45 339
 Google Scholar
Google Scholar
[2] Meng K, Li W, Tang X G, Liu Q X, Jiang Y P 2021 ACS Appl. Electron. Mater. 4 9216
 Google Scholar
Google Scholar
[3] Khasbulatov S, Kallaev S, Gadjiev H, Omarov Z, Bakmaev A, Verbenko I, Pavelko A, Reznichenko L 2020 J. Adv. Dielectr. 10 2060019
 Google Scholar
Google Scholar
[4] Wang D W, Wang G, Murakami S, Fan Z, Feteira A, Zhou D, Sun S, Zhao Q, Reaney I M 2018 J. Adv. Dielectr. 8 1830004
 Google Scholar
Google Scholar
[5] Xun B, Song A, Yu J, Yin Y, Li J F, Zhang B P 2021 ACS Appl. Mater. Interfaces 13 4192
 Google Scholar
Google Scholar
[6] Kim A Y, Lee Y J, Kim J S, Han S H, Kang H W, Lee H G, Cheon C I 2012 J. Korean Phys. Soc. 60 83
 Google Scholar
Google Scholar
[7] Wang D, Wang M, Liu F, Cui Y, Zhao Q, Sun H, Jin H, Cao M 2015 Ceram. Int. 41 8768
 Google Scholar
Google Scholar
[8] Neaton J B, Ederer C, Waghmare U V, Spaldin N A, Rabe K M 2005 Phys. Rev. B 71 014113
 Google Scholar
Google Scholar
[9] Lebeugle D, Colson D, Forget A, Vire M 2007 Appl. Phys. Lett. 91 022907
 Google Scholar
Google Scholar
[10] Khesro A, Boston R, Sterianou I, Sinclair D C, Reaney I M 2016 J. Appl. Phys. 119 054101
 Google Scholar
Google Scholar
[11] Leontsev S O, Eitel R E 2009 J. Am. Ceram. Soc. 92 2957
 Google Scholar
Google Scholar
[12] Kumar M M, Srinivas A, Suryanarayana S V 2000 J. Appl. Phys. 87 855
 Google Scholar
Google Scholar
[13] Chaudhary P, Shukla R, Dabas S, Thakur O P 2021 J. Alloys Compd. 869 159228
 Google Scholar
Google Scholar
[14] Wan Y, Li Y, Li Q, Zhou W, Zheng Q, Wu X, Xu C, Zhu B, Lin D, Jones J 2014 J. Am. Ceram. Soc. 97 1809
 Google Scholar
Google Scholar
[15] Chen Z, Bai X, Wang H, Du J, Bai W, Li L, Wen F, Zheng P, Wu W, Zheng L, Zhang Y 2020 Ceram. Int. 46 11549
 Google Scholar
Google Scholar
[16] Lu Z, Wang G, Bao W, Li J, Li L, Mostaed A, Yang H, Ji H, Li D, Feteira A, Xu F, Sinclair D C, Wang D, Liu S Y, Reaney I M 2020 Energy Environ. Sci. 13 2938
 Google Scholar
Google Scholar
[17] Calisir I, Amirov A A, Kleppe A K, Hall D A 2018 J. Mater. Chem. A 6 5378
 Google Scholar
Google Scholar
[18] Liu X H, Xu Z, Qu S B, Wei X Y, Chen J L 2008 Ceram. Int. 34 797
 Google Scholar
Google Scholar
[19] Yang H, Zhou C, Liu X, Zhou Q, Chen G, Li W, Wang H 2013 J. Eur. Ceram. Soc. 33 1177
 Google Scholar
Google Scholar
[20] Li Q, Wei J X, Cheng J R, Chen J G 2017 J. Mater. Sci. 52 229
 Google Scholar
Google Scholar
[21] Li Q, Cheng J R, Chen J G 2017 J. Mater. Sci. :Mater. Electron. 28 1370
 Google Scholar
Google Scholar
[22] Alpay S P, Mantese J, Trolier-McKinstry S, Zhang Q, Whatmore R W 2014 MRS Bull. 39 1099
 Google Scholar
Google Scholar
[23] Jian X D, Lu B, Li D D, Yao Y B, Tao T, Liang B, Guo J H, Zeng Y J, Chen J L, Lu S G 2018 ACS Appl. Mater. Interfaces 10 4801
 Google Scholar
Google Scholar
[24] Neese B, Chu B, Lu S G, Wang Y, Furman E, Zhang Q M 2008 Science 321 821
 Google Scholar
Google Scholar
[25] 鲁圣国, 李丹丹, 林雄威, 简晓东, 赵小波, 姚英邦, 陶涛, 梁波 2020 69 127701
 Google Scholar
Google Scholar
Lu S G, Li D D, Lin X W, Jian X D, Zhao X B, Yao Y B, Tao T, Liang B 2020 Acta Phys. Sin. 69 127701
 Google Scholar
Google Scholar
[26] Larson A C, Von Dreele R B 2004 General Structure Analysis System (GSAS) Los Alamos: Los Alamos National Laboratory Report LAUR p86
[27] Toby H 2001 J. Appl. Crystallogr. 34 210
 Google Scholar
Google Scholar
[28] Niu X, Jian X, Chen X, Li H, Liang W, Liang B, Lu S G 2021 J. Adv. Ceram. 10 482
 Google Scholar
Google Scholar
[29] Dicastro V, Polzobetti G 1989 J. Electron Spectrosc. Relat. Phenom. 48 117
 Google Scholar
Google Scholar
[30] Allen G C, Harris S J, Jutson J A 1989 Appl. Surf. Sci. 37 111
 Google Scholar
Google Scholar
[31] Zhang X, Hu D, Pan Z, Lv X, He Z, Yang F, Li P, Liu J, Zhai J 2021 Chem. Eng. J. 406 126818
 Google Scholar
Google Scholar
[32] Basso V, Gerard J F, Pruvost S 2014 Appl. Phys. Lett. 105 052907
 Google Scholar
Google Scholar
[33] Lu B, Jian X, Lin X, Yao Y, Tao T, Liang B, Luo H, Lu S G 2020 Crystals 10 451
 Google Scholar
Google Scholar
-
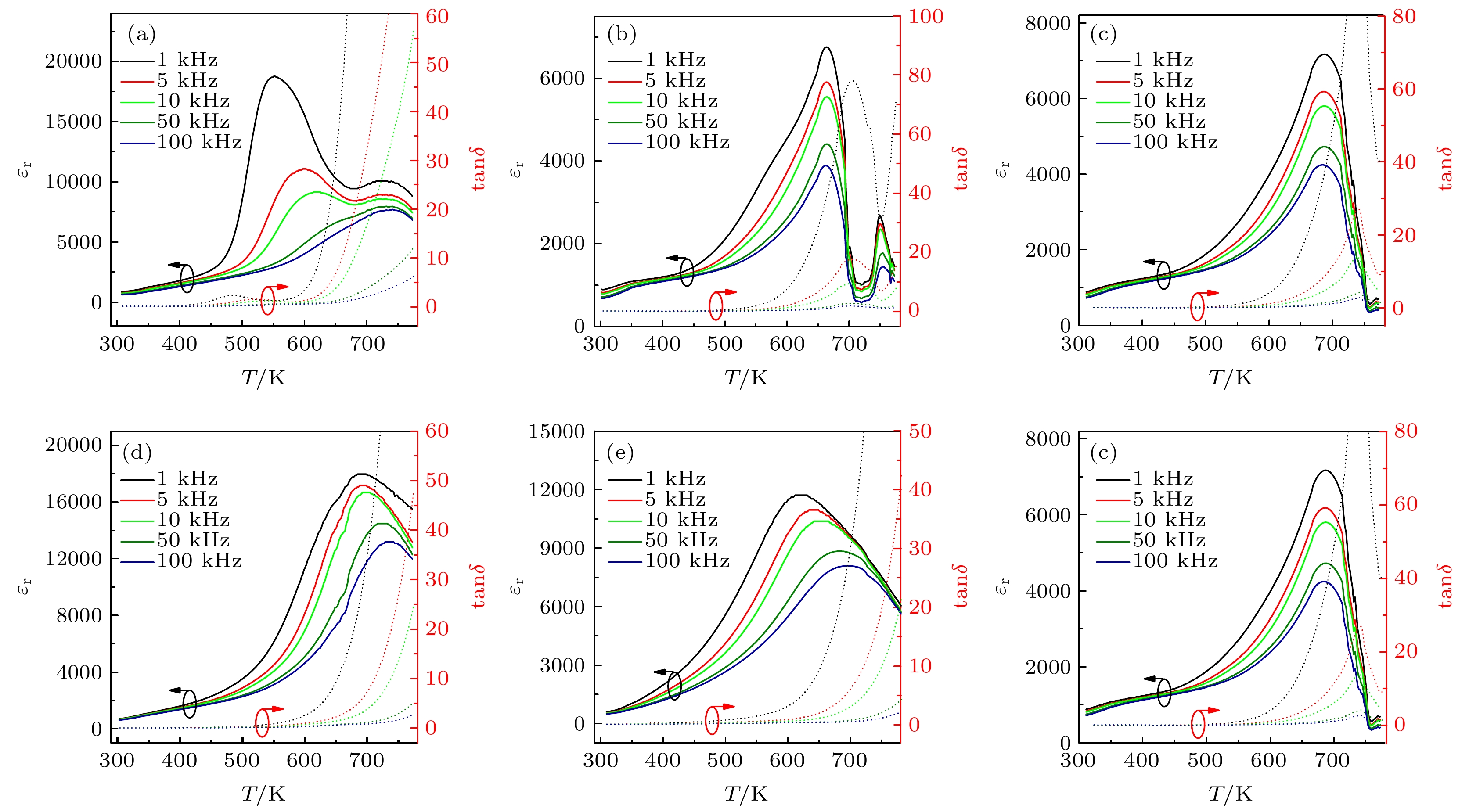
图 4 BFO-BTO+x%MnO2陶瓷在不同频率不同温度下的介电常数和介电损耗 (a) x = 0; (b) x = 0.05; (c) x = 0.10; (d) x = 0.20; (e) x = 0.50; (f) x = 1.00
Fig. 4. Permittivity and loss tangent as a function of temperature and frequency for BFO-BTO+x%MnO2 samples: (a) x = 0; (b) x = 0.05; (c) x = 0.10; (d) x = 0.20; (e) x = 0.50; (f) x = 1.00.
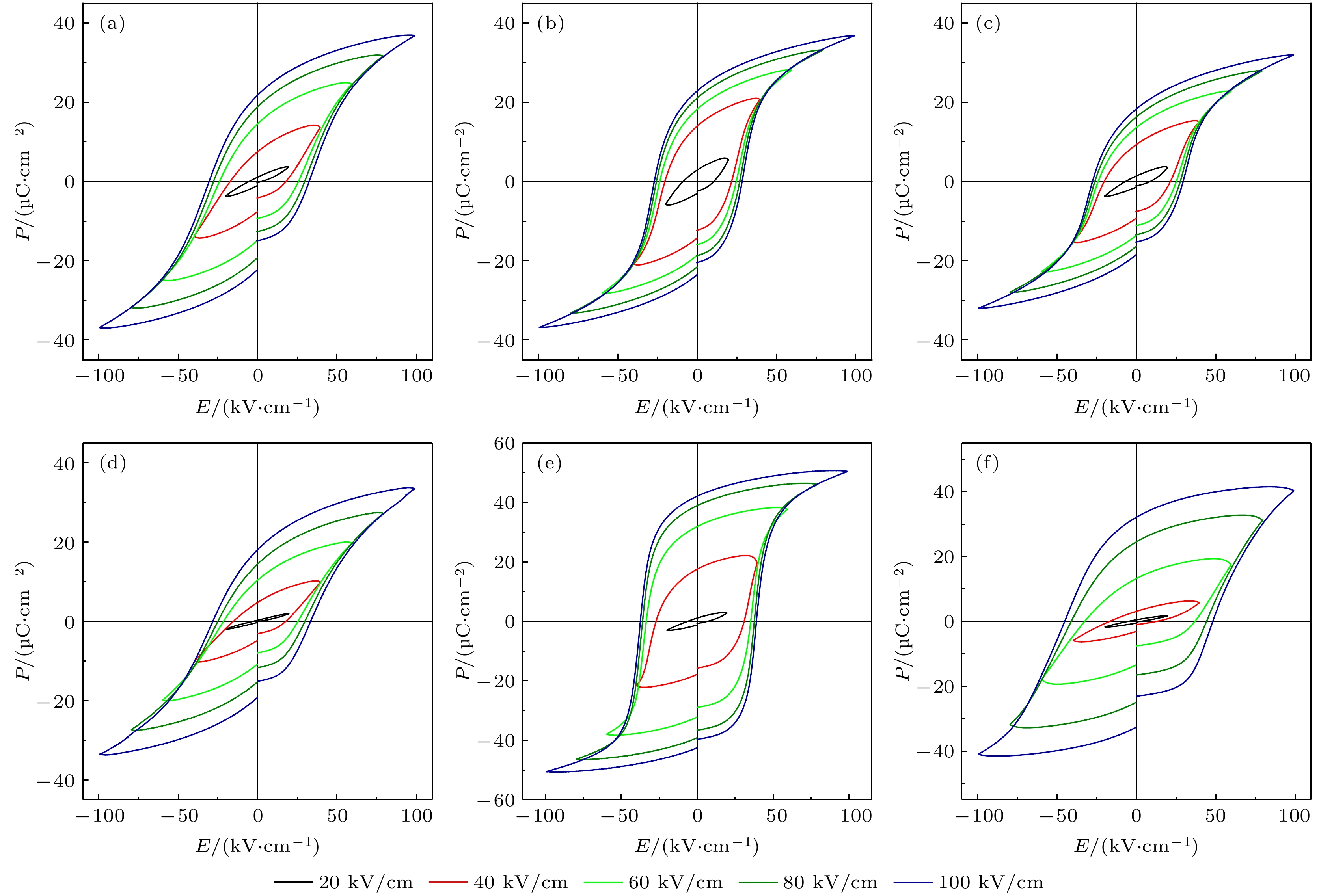
图 6 BFO-BTO+x%MnO2陶瓷在不同电场下的室温电滞回线 (a) x = 0; (b) x = 0.05; (c) x = 0.10; (d) x = 0.20; (e) x = 0.50; (f) x = 1.00
Fig. 6. The P-E hysteresis loops for BFO-BTO+x%MnO2 samples with different electric fields at room temperature: (a) x = 0; (b) x = 0.05; (c) x = 0.10; (d) x = 0.20; (e) x = 0.50; (f) x = 1.00.
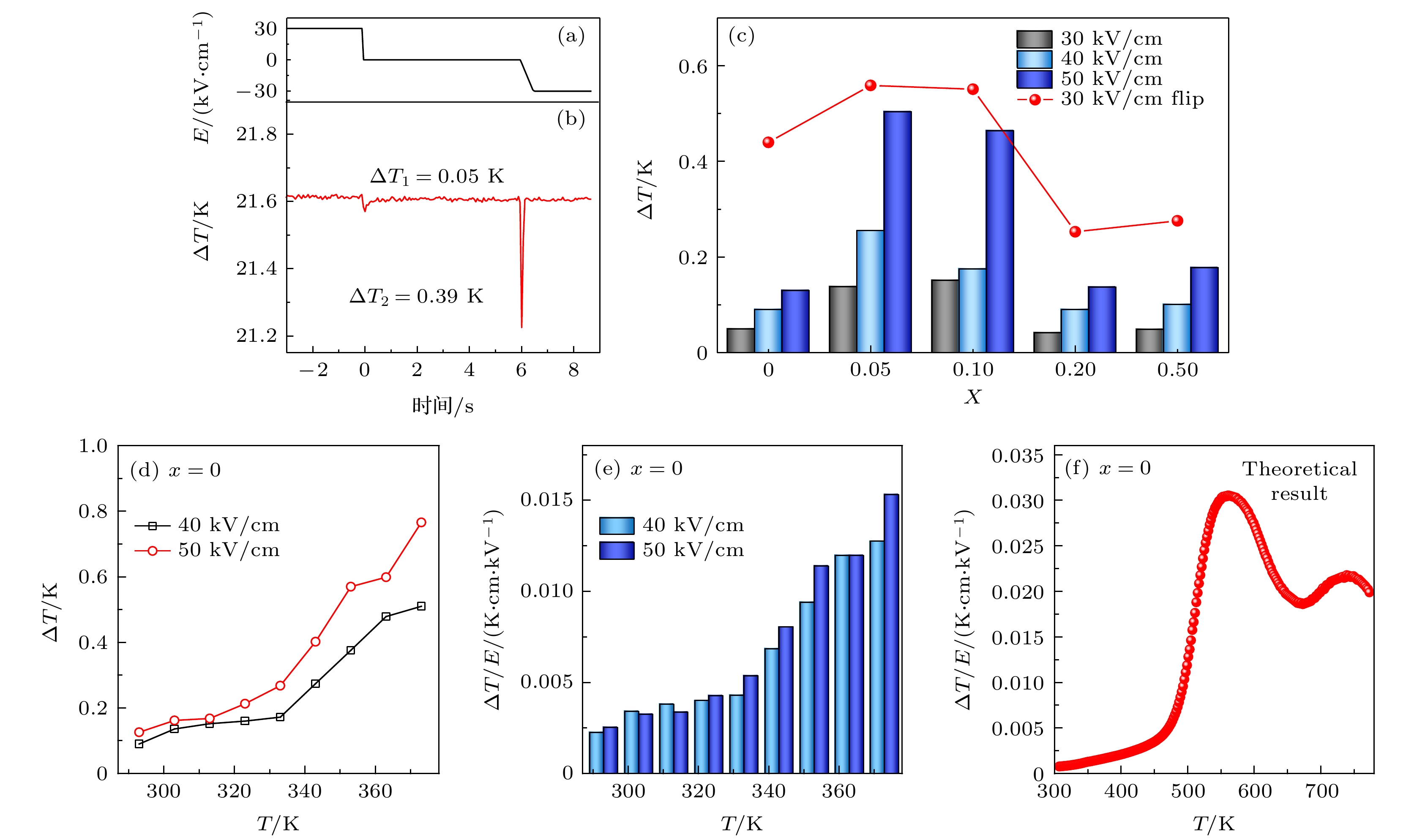
图 7 (a) Mn0陶瓷在直测电卡时的电压变化; (b) Mn0陶瓷在电场从+30 kV/cm至0 kV/cm和0 kV/cm至–30 kV/cm过程中的直测电卡; (c) BFO-BTO+x%MnO2陶瓷在不同电场直测电卡和电场转变从+30—–30 kV/cm时电畴翻转的直测电卡; (d) BFO-BTO陶瓷在电场40 kV/cm和50 kV/cm 时不同温度下直测电卡; (e) Mn0陶瓷在电场40 kV/cm和50 kV/cm 时不同温度下直测电卡电卡强度ΔT/E; (f) Mn0陶瓷理论计算的电卡强度ΔT/E
Fig. 7. (a) The change of electric field when the electrocaloric of Mn0 ceramics measured; (b) the direct measurement electrocaloric ΔT of Mn0 ceramics during the electric field changes from +30 kV/cm to 0 kV/cm and 0 kV/cm to –30 kV/cm; (c) the ΔT of BFO-BTO + x% MnO2 ceramics at different electric field and the ΔT of BFO-BTO + x% MnO2 ceramics with polarization flip during the electric field changes from +30 kV/cm to –30 kV/cm; (d) the ΔT of BFO-BTO ceramics at different temperatures under 40 kV/cm and 50 kV/cm; (e) the ΔT/E of Mn0 ceramics at different temperatures under 40 kV/cm and 50 kV/cm; (f) the theoretical ΔT/E of Mn0 ceramics.
表 1 BFO-BTO+x%MnO2样品的精修参数
Table 1. The refined retrieved lattice parameters, volumes and R factors for BFO-BTO+x%MnO2 ceramics.
x 相成分/% 晶格参数/Å 晶胞体积/Å3 R 因子 a c a R PC R PC R PC Rwp/% Rwp/% χ2 0 75.64 24.36 5.6400(2) 13.8964(0) 3.9893(6) 382.92 63.49 6.98 4.65 2.37 0.05 72.97 27.03 5.6411(7) 13.8983(8) 3.9902(5) 383.03 63.53 5.91 4.06 1.45 0.10 72.70 27.30 5.6390(5) 13.8909(8) 3.9938(5) 382.54 63.71 5.23 3.81 1.24 0.20 68.48 31.62 5.6398(2) 13.8962(4) 3.9911(4) 382.79 63.58 6.39 4.49 1.85 0.50 67.21 32.79 5.6458(1) 13.8768(6) 3.9898(0) 383.07 63.51 5.80 4.09 1.50 1.00 66.41 33.59 5.6487(1) 13.8299(8) 3.9914(5) 382.17 63.59 5.88 4.35 1.36 -
[1] Nan C W 2015 Sci. Sin. Tech. 45 339
 Google Scholar
Google Scholar
[2] Meng K, Li W, Tang X G, Liu Q X, Jiang Y P 2021 ACS Appl. Electron. Mater. 4 9216
 Google Scholar
Google Scholar
[3] Khasbulatov S, Kallaev S, Gadjiev H, Omarov Z, Bakmaev A, Verbenko I, Pavelko A, Reznichenko L 2020 J. Adv. Dielectr. 10 2060019
 Google Scholar
Google Scholar
[4] Wang D W, Wang G, Murakami S, Fan Z, Feteira A, Zhou D, Sun S, Zhao Q, Reaney I M 2018 J. Adv. Dielectr. 8 1830004
 Google Scholar
Google Scholar
[5] Xun B, Song A, Yu J, Yin Y, Li J F, Zhang B P 2021 ACS Appl. Mater. Interfaces 13 4192
 Google Scholar
Google Scholar
[6] Kim A Y, Lee Y J, Kim J S, Han S H, Kang H W, Lee H G, Cheon C I 2012 J. Korean Phys. Soc. 60 83
 Google Scholar
Google Scholar
[7] Wang D, Wang M, Liu F, Cui Y, Zhao Q, Sun H, Jin H, Cao M 2015 Ceram. Int. 41 8768
 Google Scholar
Google Scholar
[8] Neaton J B, Ederer C, Waghmare U V, Spaldin N A, Rabe K M 2005 Phys. Rev. B 71 014113
 Google Scholar
Google Scholar
[9] Lebeugle D, Colson D, Forget A, Vire M 2007 Appl. Phys. Lett. 91 022907
 Google Scholar
Google Scholar
[10] Khesro A, Boston R, Sterianou I, Sinclair D C, Reaney I M 2016 J. Appl. Phys. 119 054101
 Google Scholar
Google Scholar
[11] Leontsev S O, Eitel R E 2009 J. Am. Ceram. Soc. 92 2957
 Google Scholar
Google Scholar
[12] Kumar M M, Srinivas A, Suryanarayana S V 2000 J. Appl. Phys. 87 855
 Google Scholar
Google Scholar
[13] Chaudhary P, Shukla R, Dabas S, Thakur O P 2021 J. Alloys Compd. 869 159228
 Google Scholar
Google Scholar
[14] Wan Y, Li Y, Li Q, Zhou W, Zheng Q, Wu X, Xu C, Zhu B, Lin D, Jones J 2014 J. Am. Ceram. Soc. 97 1809
 Google Scholar
Google Scholar
[15] Chen Z, Bai X, Wang H, Du J, Bai W, Li L, Wen F, Zheng P, Wu W, Zheng L, Zhang Y 2020 Ceram. Int. 46 11549
 Google Scholar
Google Scholar
[16] Lu Z, Wang G, Bao W, Li J, Li L, Mostaed A, Yang H, Ji H, Li D, Feteira A, Xu F, Sinclair D C, Wang D, Liu S Y, Reaney I M 2020 Energy Environ. Sci. 13 2938
 Google Scholar
Google Scholar
[17] Calisir I, Amirov A A, Kleppe A K, Hall D A 2018 J. Mater. Chem. A 6 5378
 Google Scholar
Google Scholar
[18] Liu X H, Xu Z, Qu S B, Wei X Y, Chen J L 2008 Ceram. Int. 34 797
 Google Scholar
Google Scholar
[19] Yang H, Zhou C, Liu X, Zhou Q, Chen G, Li W, Wang H 2013 J. Eur. Ceram. Soc. 33 1177
 Google Scholar
Google Scholar
[20] Li Q, Wei J X, Cheng J R, Chen J G 2017 J. Mater. Sci. 52 229
 Google Scholar
Google Scholar
[21] Li Q, Cheng J R, Chen J G 2017 J. Mater. Sci. :Mater. Electron. 28 1370
 Google Scholar
Google Scholar
[22] Alpay S P, Mantese J, Trolier-McKinstry S, Zhang Q, Whatmore R W 2014 MRS Bull. 39 1099
 Google Scholar
Google Scholar
[23] Jian X D, Lu B, Li D D, Yao Y B, Tao T, Liang B, Guo J H, Zeng Y J, Chen J L, Lu S G 2018 ACS Appl. Mater. Interfaces 10 4801
 Google Scholar
Google Scholar
[24] Neese B, Chu B, Lu S G, Wang Y, Furman E, Zhang Q M 2008 Science 321 821
 Google Scholar
Google Scholar
[25] 鲁圣国, 李丹丹, 林雄威, 简晓东, 赵小波, 姚英邦, 陶涛, 梁波 2020 69 127701
 Google Scholar
Google Scholar
Lu S G, Li D D, Lin X W, Jian X D, Zhao X B, Yao Y B, Tao T, Liang B 2020 Acta Phys. Sin. 69 127701
 Google Scholar
Google Scholar
[26] Larson A C, Von Dreele R B 2004 General Structure Analysis System (GSAS) Los Alamos: Los Alamos National Laboratory Report LAUR p86
[27] Toby H 2001 J. Appl. Crystallogr. 34 210
 Google Scholar
Google Scholar
[28] Niu X, Jian X, Chen X, Li H, Liang W, Liang B, Lu S G 2021 J. Adv. Ceram. 10 482
 Google Scholar
Google Scholar
[29] Dicastro V, Polzobetti G 1989 J. Electron Spectrosc. Relat. Phenom. 48 117
 Google Scholar
Google Scholar
[30] Allen G C, Harris S J, Jutson J A 1989 Appl. Surf. Sci. 37 111
 Google Scholar
Google Scholar
[31] Zhang X, Hu D, Pan Z, Lv X, He Z, Yang F, Li P, Liu J, Zhai J 2021 Chem. Eng. J. 406 126818
 Google Scholar
Google Scholar
[32] Basso V, Gerard J F, Pruvost S 2014 Appl. Phys. Lett. 105 052907
 Google Scholar
Google Scholar
[33] Lu B, Jian X, Lin X, Yao Y, Tao T, Liang B, Luo H, Lu S G 2020 Crystals 10 451
 Google Scholar
Google Scholar
计量
- 文章访问数: 7424
- PDF下载量: 116
- 被引次数: 0














 下载:
下载: