-
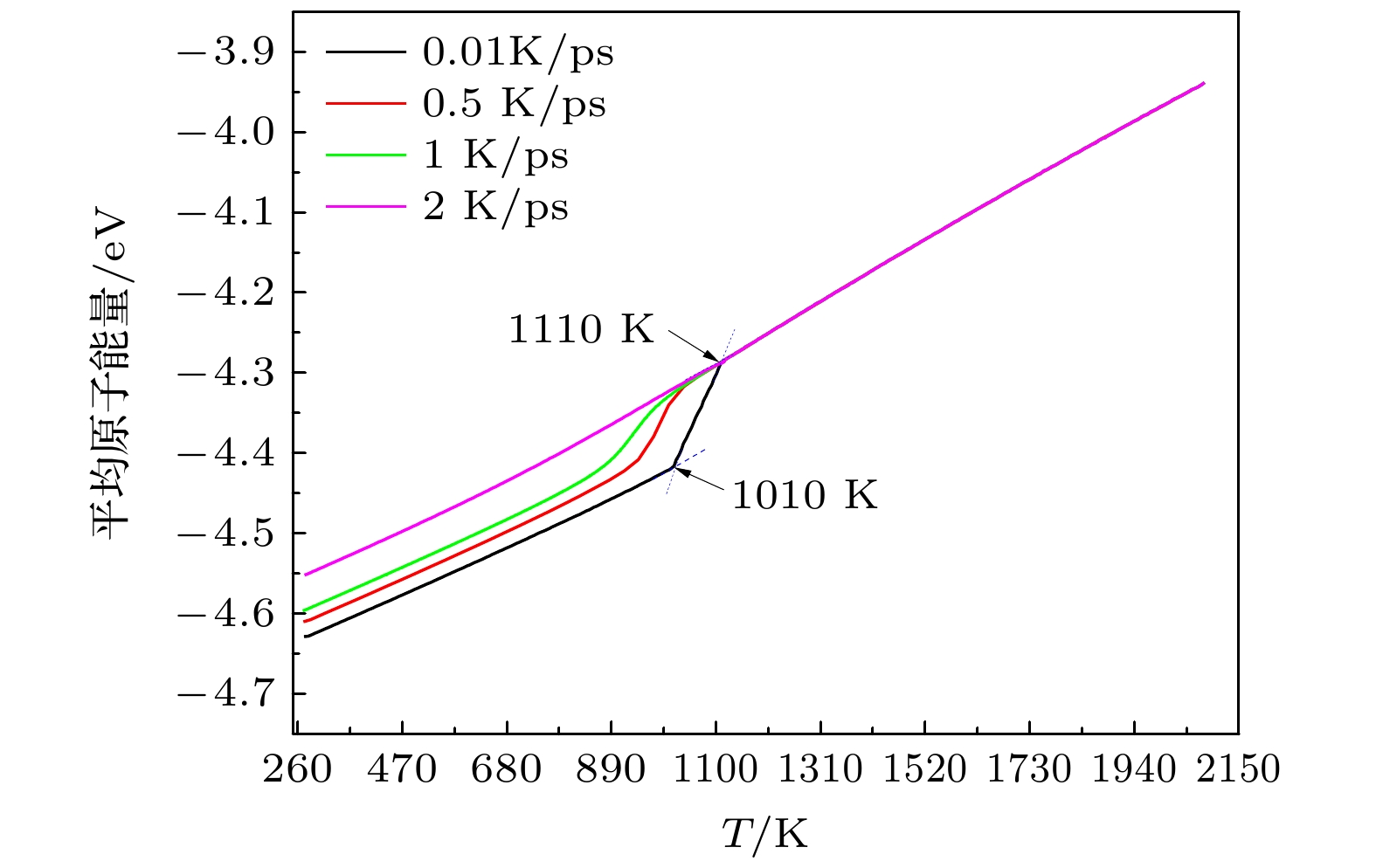
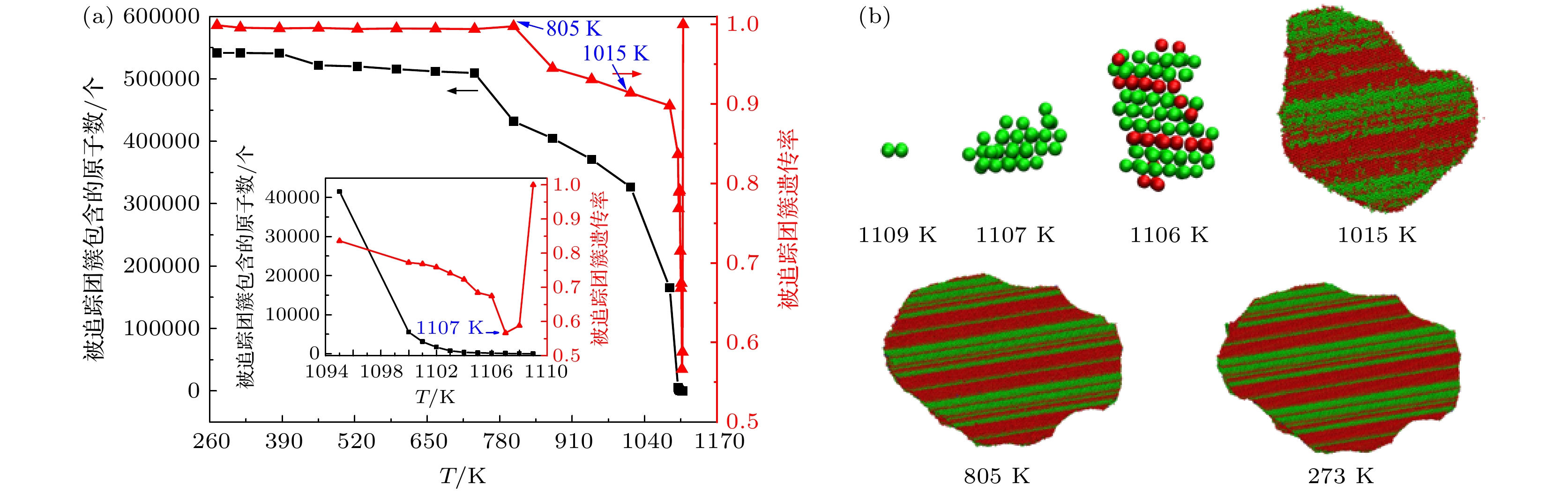
采用分子动力学方法对Ti3Al合金的形核机理进行了模拟研究, 采用团簇类型指数法(CTIM), 对凝固过程不同尺度的原子团簇结构进行了识别和表征, 深入研究了临界晶核的形成和长大过程. 结果表明, 凝固过程体系包含了数万种不同类型的原子团簇结构, 但其中22种团簇结构类型对结晶形核过程起关键性作用. 在晶核的形成和长大过程, 类二十面体(ICO)原子团簇、类BCC原子团簇和缺陷FCC及缺陷HCP原子团簇在3个特征温度点T1 (1110 K), T2 (1085 K)和T3 (1010 K)时达到数量上的饱和, 并根据数量和空间分布随温度的变化, 得到了它们在形核和长大过程相互竞争的关系. 跟踪平行孪生晶粒形成和长大的过程发现, 临界晶核是由FCC原子构成的单相结构, 并未观察到亚稳BCC相优先形核的过程; 平行孪生结构是由FCC单相晶核在沿密排面逐层生长过程中形成的. 结果还表明, CTIM相比于其他微观结构表示方法, 能更为准确地揭示凝固过程微观结构的转变特征.The nucleation mechanism of Ti3Al alloy is simulated by the molecular dynamics method in this work. The atomic clusters on different spatial scales are identified in the solidification process by the cluster-type index method (CTIM), and the formation process and the growth process of critical nucleus are studied in depth. It is found that the solidification system contains ten thousands of different types of atomic cluster structures, but only 22 types play a key role in the nucleation process. In the nucleation and growth process of nuclei, the ICO-like cluster, the BCC-like cluster, and the defective FCC cluster and the defective HCP cluster respectively reach their saturation points at the characteristic temperature T1 (1110 K), T2 (1085 K) and T3 (1010 K). And the competition processes of these clusters are revealed according to the changes of their number and spatial distribution with temperature. By tracing the nucleation and growth process of the grain with parallel twin, it is found that the critical crystal nucleus is composed of single-phase FCC structures, and the preferent nucleation of metastable bcc structure is not observed. The twinned structure is formed by the layer-by-layer growth along the close-packed plane. It is also found that the CTIM is more accurate than other methods in revealing the microstructural characteristics during the solidification.
-
Keywords:
- TiAl alloy /
- nucleation mechanism /
- molecular dynamics simulation /
- atomic cluster
[1] Mayer S, Erdely P, Fischer F D, Holec D, Kastenhuber M, Klein T, Clemens H 2017 Adv. Eng. Mater. 19 1600735
 Google Scholar
Google Scholar
[2] Chen G, Peng Y, Zheng G, Qi Z, Wand M, Yu H, Dong C, Liu C T 2016 Nat. Mater. 15 876
 Google Scholar
Google Scholar
[3] 杨锐 2015 金属学报 51 129
 Google Scholar
Google Scholar
Yang R. 2015 Acta Metall. Sin. 51 129
 Google Scholar
Google Scholar
[4] Hao Y, Liu J, Li J, Liu X, Feng X 2017 Mater. Sci. Eng., A 705 210
 Google Scholar
Google Scholar
[5] Edwards J E T, Gioacchino F D, Clegg W J 2019 Int. J. Plast. 118 291
 Google Scholar
Google Scholar
[6] Christoph K, Christian L 2015 J. Alloys. Compd. 637 242
 Google Scholar
Google Scholar
[7] Ilyas M U, Kabir M R 2021 Intermetallics 132 107129
 Google Scholar
Google Scholar
[8] Pei Q X, Lu C, Fu M W 2004 J. Phys. Condens. Matter 16 4203
 Google Scholar
Google Scholar
[9] Xie Z C, Gao T H, Guo X T, Qin X M, Xie Q 2014 J. Non-Cryst. Solids 394–395 16
[10] Xie Z C, Gao T H, Guo X T, Xie Q 2014 J. Non-Cryst. Solids 406 95
 Google Scholar
Google Scholar
[11] Li P T, Yang Y Q, Zhang W, Luo X, Jin N, Liu G 2016 RSC Adv. 6 54763
 Google Scholar
Google Scholar
[12] Li P T, Yang Y Q, Zhang W, Luo X, Jin N, Liu G 2017 RSC Adv. 7 48315
 Google Scholar
Google Scholar
[13] 刘永利, 赵星, 张宗宁, 张林, 王绍青, 叶恒强 2009 58 246
 Google Scholar
Google Scholar
Liu Y L, Zhao X, Zhang Z N, Zhang L, Wang S Q, Ye H Q 2009 Acta Phys. Sin. 58 246
 Google Scholar
Google Scholar
[14] 宋成粉, 樊沁娜, 李蔚, 刘永利, 张林 2011 60 063104
 Google Scholar
Google Scholar
Song C F, Fan Q N, Li W, Liu Y L, Zhang L 2011 Acta Phys. Sin. 60 063104
 Google Scholar
Google Scholar
[15] Honeycutt J D, Andersen H C 1987 J. Phys. Chem. 91 4950
 Google Scholar
Google Scholar
[16] Finney J L 1970 Proc. R. Soc. London, Ser. A 319 479
 Google Scholar
Google Scholar
[17] Liu R S, Dong K J, Tian Z A, Liu H R, Peng P, Yu A B 2007 J. Phys. Condens. Matter 19 751
 Google Scholar
Google Scholar
[18] Hou Z Y, Li C, Liu L X, Gao Q H, Dong K J 2021 Comput. Mater. Sci. 197 110639
 Google Scholar
Google Scholar
[19] 大东, 彭平, 蒋元祺, 田泽安, 刘让苏 2013 62 196101
 Google Scholar
Google Scholar
Da D, Peng P, Jiang Y Q, Tian Z A, Liu R S 2013 Acta Phys. Sin. 62 196101
 Google Scholar
Google Scholar
[20] Plimpton S J 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[21] Zope R R, Mishin Y 2003 Phys. Rev. B 68 366
 Google Scholar
Google Scholar
[22] Fu R, Rui Z, Dong Y, Luo D, Yan C 2021 Comput. Mater. Sci. 194 110428
 Google Scholar
Google Scholar
[23] Bizot Q, Politano O, Nepapushev A A, Vadchenko S G, Baras F 2020 J. Appl. Phys. 127 145304
 Google Scholar
Google Scholar
[24] Wang J S, Horsfield A, Schwingenschlögl U, Lee P D 2010 Phys. Rev. B 82 184203
 Google Scholar
Google Scholar
[25] Gasser U, Weeks ER, Schofield A, Pusey P N, Weitz D A 2001 Science 292 258
 Google Scholar
Google Scholar
[26] Wang Z, Wang F, Peng Y, Zheng Z, Han Y 2012 Science 338 87
 Google Scholar
Google Scholar
[27] E J C, Wang L, Cai Y, Wu H A, Luo S N 2015 J. Chem. Phys. 142 064704
 Google Scholar
Google Scholar
-
图 2 CTIM表征基本原子团簇结构方法示意图 (a) FCC基本原子团簇(12, 12/1421); (b)缺陷FCC基本原子团簇(12, 2/1311 1/1411 9/1421)
Fig. 2. Schematic diagram of topological structure of basic atomic cluster characterized by CTIM: (a) FCC basic atomic cluster (12, 12/1421); (b) defective FCC basic atomic cluster (12, 2/1311, 1/1411, 9/1421).
图 3 CTIM表征的较大尺寸原子团簇结构 (a)由1个HCP基本原子团簇(12, 6/1421 6/1422)和1个FCC基本原子团簇(12, 12/1421)构成的包含20个原子的团簇结构; (b)由6个FCC基本原子团簇(12, 12/1421)构成的包含38个原子的纳米级团簇结构. 灰色原子为团簇的中心原子
Fig. 3. Topological structure of larger atomic clusters characterized by CTIM: (a) Cluster with 220 atoms consisting of one HCP basic atomic cluster (12, 6/1421, 6/1422) and one FCC basic atomic cluster (12, 12/1421); (b) nano-cluster with 38 atoms consisting of six FCC basic atomic clusters (12, 12/1421). The gray atoms are central atoms of basic atomic clusters.
图 5 不同冷却速率下Ti3Al合金的凝固结构(273 K) (a) 2 K/ps; (b) 1 K/ps; (c) 0.5 K/ps; (d) 0.01 K/ps. 其中绿色、红色和蓝色小球分别代表FCC, HCP和BCC晶态结构原子; 其他类型结构原子用灰色小球表示
Fig. 5. Microstructures of solidification solids (273 K) under different cooling rates: (a) 2 K/ps; (b) 1 K/ps; (c) 0.5 K/ps; (d) 0.01 K/ps. The crystal atoms with FCC, HCP and BCC structures are shown in green, red and blue, other atoms are shown in gray.
图 8 Ti3Al合金凝固过程体系内22种主要基本原子团簇的数目随温度的变化 (a1)类FCC基本原子团簇; (a2)类HCP基本原子团簇; (a3)类BCC基本原子团簇; (a4)类ICO基本原子团簇. 为了清晰起见, (b1)−(b4)分别给出了图(a1)−(a4)在(1110−814 K)温度区间的局部图. 类ICO、类BCC和缺陷FCC、缺陷HCP基本原子团簇分别在温度T1 = 1110 K, T2 = 1085 K和T3 = 1010 K达到饱和
Fig. 8. Relationship of the number of 22 major basic atomic clusters with temperature during the solidification process of Ti3Al alloy: (a1) FCC-like basic atomic cluster; (a2) HCP-like basic atomic cluster; (a3) BCC-like basic atomic cluster; (a4) ICO-like basic atomic cluster. For clarity, (b1)−(b4) show the enlarged views of (a1)−(a4) in the temperature range (1110−814 K), respectively. The numbers of ICO-like, BCC-like and defective FCC, defective HCP basic atomic clusters reach saturation point at T1 = 1110 K, T2 = 1085 K and T3 = 1010 K.
图 9 Ti3Al合金凝固过程中类FCC、类HCP、类BCC和类ICO原子结构空间分布的演化过程 (a) 2073 K; (b) 1110 K; (c) 1085 K; (d) 1010 K; (e) 273 K. 其中, 绿色、红色、蓝色和黄色小球分别代表类FCC、类HCP、类BCC和类ICO原子. 其中G1和G2分别为选定的两个平行孪生晶粒和五重孪生晶粒.
Fig. 9. Evolution of spatial distribution of FCC-like, HCP-like, BCC-like and ICO-like atoms during the solidification process of Ti3Al alloy: (a) 2073 K; (b) 1110 K; (c) 1085 K; (d) 1010 K; (e) 273 K. The FCC-like, HCP-like, BCC-like and ICO-like atoms are shown in green, red, blue and yellow color, respectively. The parallel and fivefold twin grains are labelled in G1 and G2, respectively.
图 10 图9(e)中标记为G1的平行孪生晶粒的形成过程 (a) 团簇遗传率和尺寸(包含的中心原子数)与温度的变化关系; (b) 原子团簇结构演变过程. 其中绿色和红色小球分表代表类FCC和类HCP原子
Fig. 10. Formation process of parallel twin grains labeled G1 in Fig. 9(e): (a) Relationship of heritability and size (number of central atoms) of tracing clusters with temperature; (b) evolution process of the structure of atomic clusters. The FCC-like and HCP-like atoms are shown in green and red color, respectively.
图 11 图10(b)中临界晶核形成过程不同局域结构的竞争过程 (a)不同结构类型原子数目占比的变化; (b)不同结构原子的空间分布. 类FCC、类HCP、类BCC、类ICO和无序结构(其他)原子分别用绿色、红色、橘黄色和白色表示
Fig. 11. Competition process of different local structures in the formation process critical nucleus shown in Fig. 10(b): (a) Change of the proportion of the atoms with different local structures; (b) spatial distribution of the atoms with different local structures. The FCC-like, HCP-like, BCC-like and ICO-like atoms are shown in green, red, blue and yellow color, respectively. Others with disordered structure are shown in white color.
-
[1] Mayer S, Erdely P, Fischer F D, Holec D, Kastenhuber M, Klein T, Clemens H 2017 Adv. Eng. Mater. 19 1600735
 Google Scholar
Google Scholar
[2] Chen G, Peng Y, Zheng G, Qi Z, Wand M, Yu H, Dong C, Liu C T 2016 Nat. Mater. 15 876
 Google Scholar
Google Scholar
[3] 杨锐 2015 金属学报 51 129
 Google Scholar
Google Scholar
Yang R. 2015 Acta Metall. Sin. 51 129
 Google Scholar
Google Scholar
[4] Hao Y, Liu J, Li J, Liu X, Feng X 2017 Mater. Sci. Eng., A 705 210
 Google Scholar
Google Scholar
[5] Edwards J E T, Gioacchino F D, Clegg W J 2019 Int. J. Plast. 118 291
 Google Scholar
Google Scholar
[6] Christoph K, Christian L 2015 J. Alloys. Compd. 637 242
 Google Scholar
Google Scholar
[7] Ilyas M U, Kabir M R 2021 Intermetallics 132 107129
 Google Scholar
Google Scholar
[8] Pei Q X, Lu C, Fu M W 2004 J. Phys. Condens. Matter 16 4203
 Google Scholar
Google Scholar
[9] Xie Z C, Gao T H, Guo X T, Qin X M, Xie Q 2014 J. Non-Cryst. Solids 394–395 16
[10] Xie Z C, Gao T H, Guo X T, Xie Q 2014 J. Non-Cryst. Solids 406 95
 Google Scholar
Google Scholar
[11] Li P T, Yang Y Q, Zhang W, Luo X, Jin N, Liu G 2016 RSC Adv. 6 54763
 Google Scholar
Google Scholar
[12] Li P T, Yang Y Q, Zhang W, Luo X, Jin N, Liu G 2017 RSC Adv. 7 48315
 Google Scholar
Google Scholar
[13] 刘永利, 赵星, 张宗宁, 张林, 王绍青, 叶恒强 2009 58 246
 Google Scholar
Google Scholar
Liu Y L, Zhao X, Zhang Z N, Zhang L, Wang S Q, Ye H Q 2009 Acta Phys. Sin. 58 246
 Google Scholar
Google Scholar
[14] 宋成粉, 樊沁娜, 李蔚, 刘永利, 张林 2011 60 063104
 Google Scholar
Google Scholar
Song C F, Fan Q N, Li W, Liu Y L, Zhang L 2011 Acta Phys. Sin. 60 063104
 Google Scholar
Google Scholar
[15] Honeycutt J D, Andersen H C 1987 J. Phys. Chem. 91 4950
 Google Scholar
Google Scholar
[16] Finney J L 1970 Proc. R. Soc. London, Ser. A 319 479
 Google Scholar
Google Scholar
[17] Liu R S, Dong K J, Tian Z A, Liu H R, Peng P, Yu A B 2007 J. Phys. Condens. Matter 19 751
 Google Scholar
Google Scholar
[18] Hou Z Y, Li C, Liu L X, Gao Q H, Dong K J 2021 Comput. Mater. Sci. 197 110639
 Google Scholar
Google Scholar
[19] 大东, 彭平, 蒋元祺, 田泽安, 刘让苏 2013 62 196101
 Google Scholar
Google Scholar
Da D, Peng P, Jiang Y Q, Tian Z A, Liu R S 2013 Acta Phys. Sin. 62 196101
 Google Scholar
Google Scholar
[20] Plimpton S J 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[21] Zope R R, Mishin Y 2003 Phys. Rev. B 68 366
 Google Scholar
Google Scholar
[22] Fu R, Rui Z, Dong Y, Luo D, Yan C 2021 Comput. Mater. Sci. 194 110428
 Google Scholar
Google Scholar
[23] Bizot Q, Politano O, Nepapushev A A, Vadchenko S G, Baras F 2020 J. Appl. Phys. 127 145304
 Google Scholar
Google Scholar
[24] Wang J S, Horsfield A, Schwingenschlögl U, Lee P D 2010 Phys. Rev. B 82 184203
 Google Scholar
Google Scholar
[25] Gasser U, Weeks ER, Schofield A, Pusey P N, Weitz D A 2001 Science 292 258
 Google Scholar
Google Scholar
[26] Wang Z, Wang F, Peng Y, Zheng Z, Han Y 2012 Science 338 87
 Google Scholar
Google Scholar
[27] E J C, Wang L, Cai Y, Wu H A, Luo S N 2015 J. Chem. Phys. 142 064704
 Google Scholar
Google Scholar
计量
- 文章访问数: 8341
- PDF下载量: 185
- 被引次数: 0














 下载:
下载: