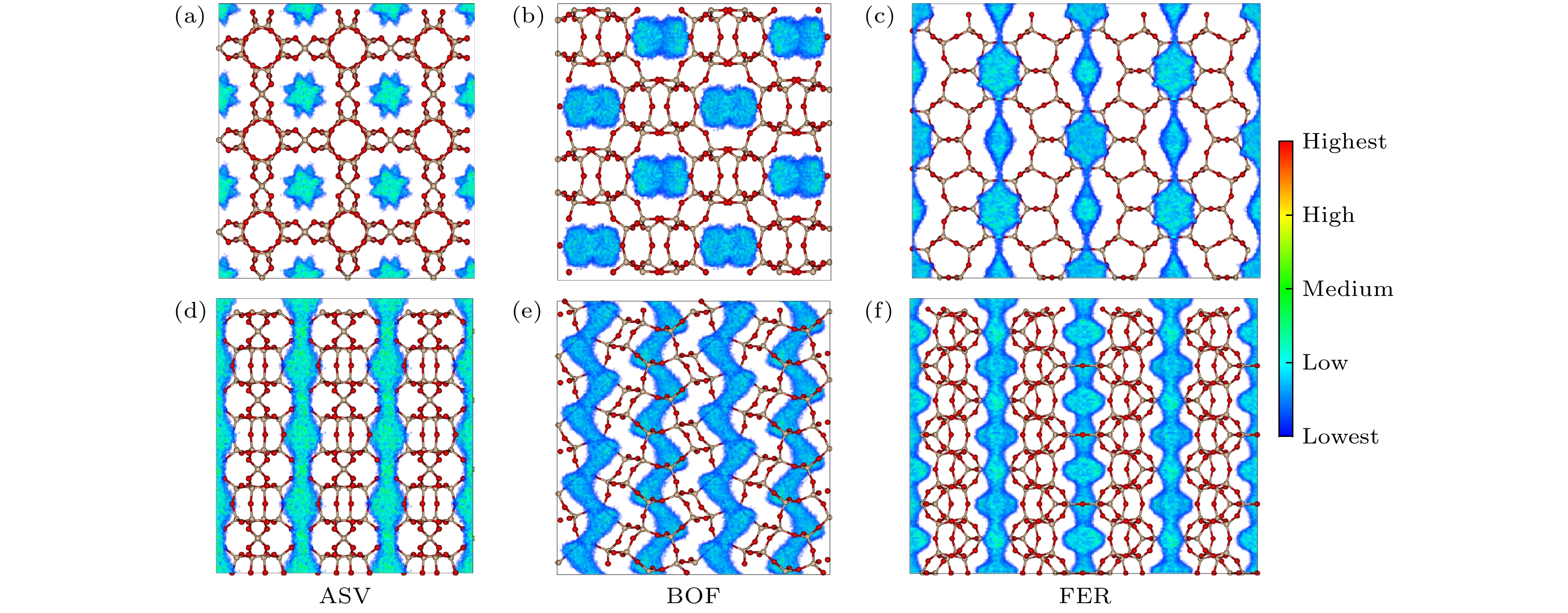
-
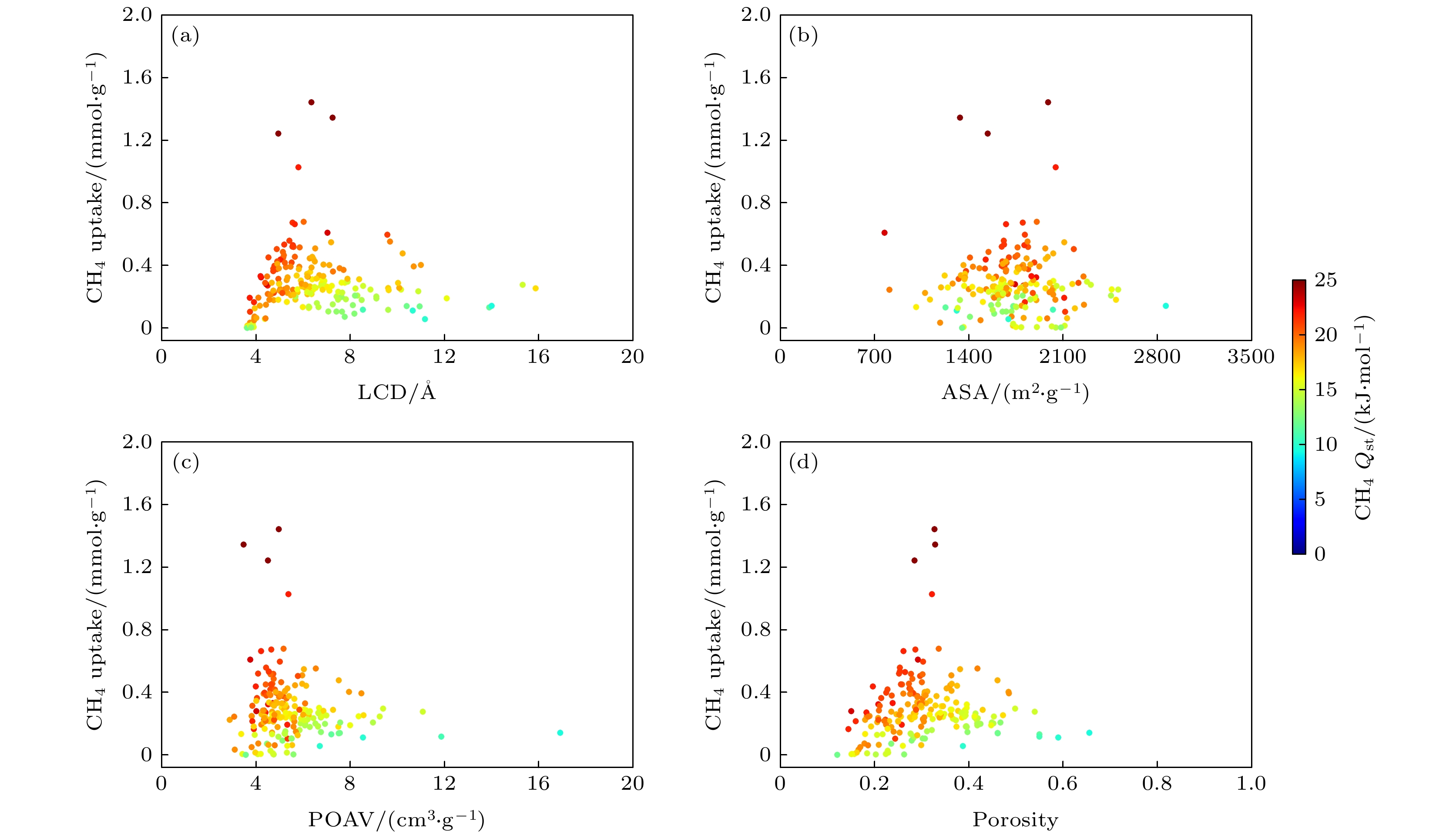
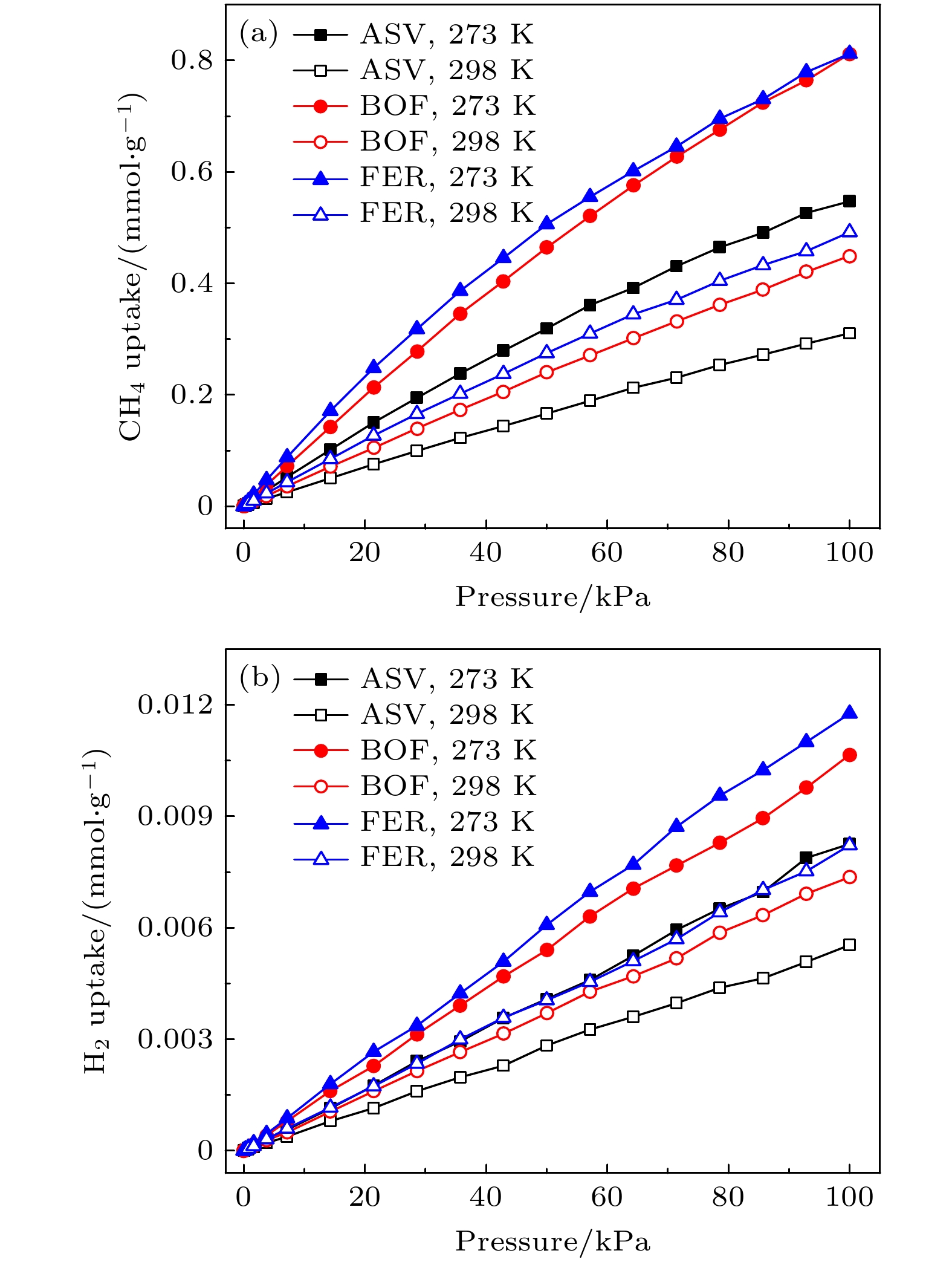
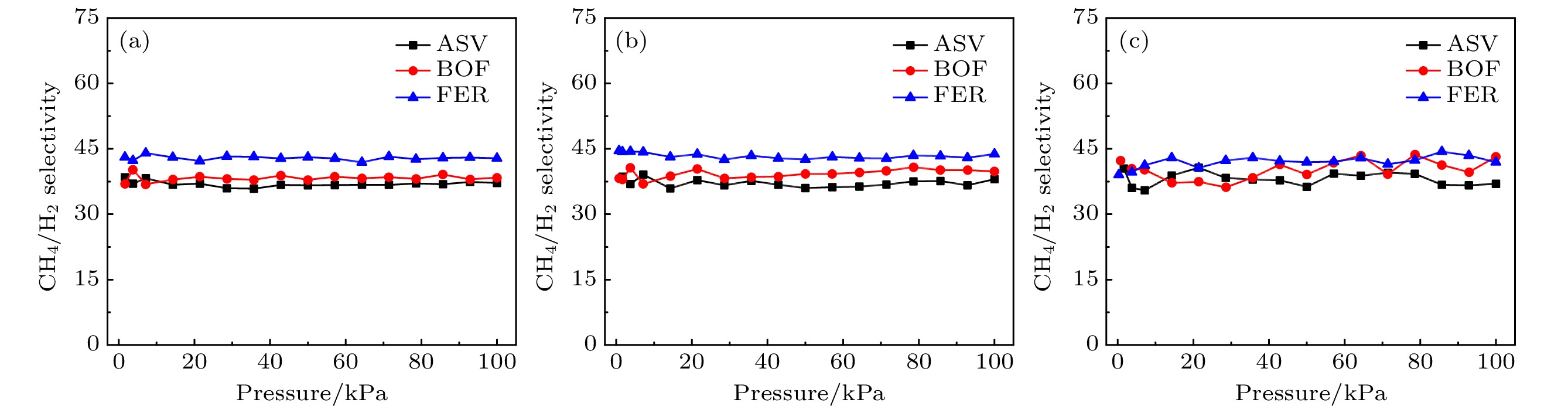
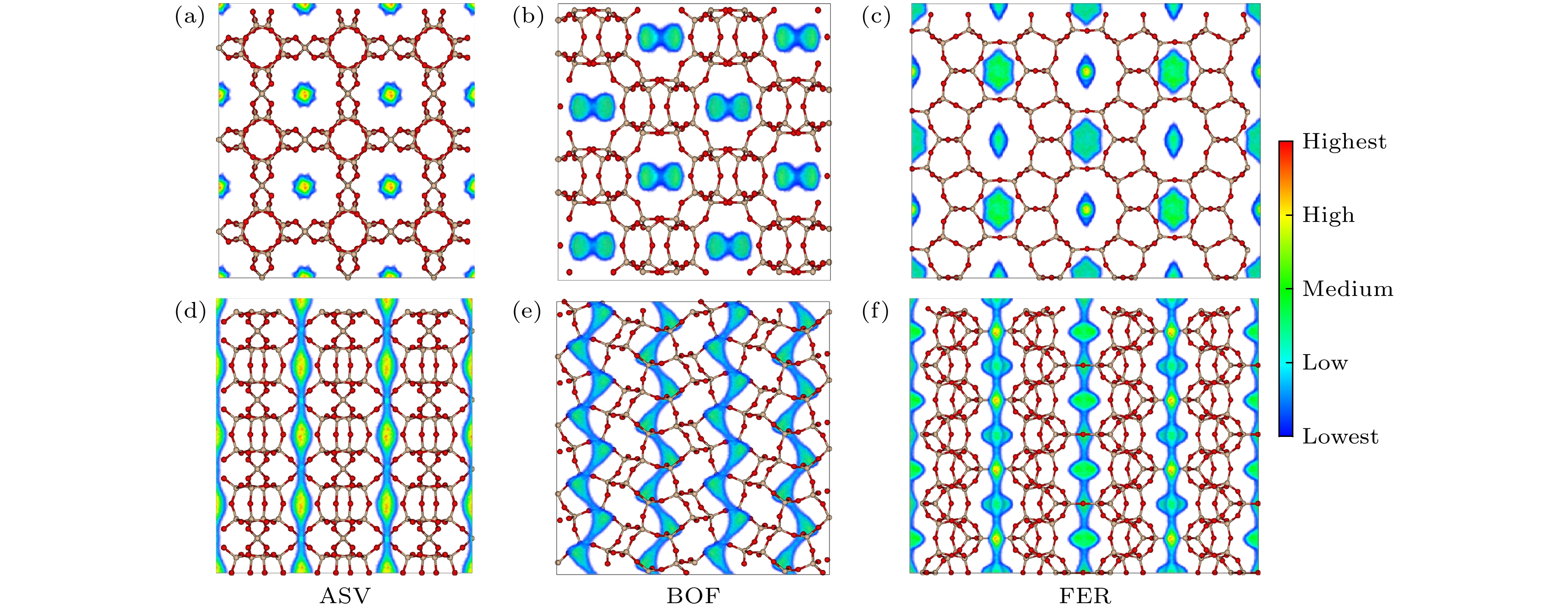
基于分子模拟的高通量计算方法, 通过对199个沸石结构特征和吸附分离性能之间的关联性研究, 发现具有超微孔结构的沸石材料对CH4分子有良好的吸附分离性能, 等摩尔CH4/H2混合组份的CH4吸附选择性和单组份的CH4吸附量之间存在明显的线性关系. 通过使用巨正则蒙特卡罗模拟方法, 获得了3个孔道形沸石对CH4和H2的吸附等温线和等量吸附热等物理量. 结果表明在相同外界环境下, 孔道形沸石的孔结构(表面积和孔体积)对CH4吸附量的影响高于能量效应(吸附热). 结合甲烷蒸汽重整制氢的工业背景, 进一步研究了CH4/H2混合体系在不同组份下的分离选择性能, 结果表明, 超微孔沸石材料对CH4的吸附选择性与体相压力和进料比无关. 通过气体分子的质心分布密度发现, CH4在孔道形沸石中优先占据更小孔窗的空间, 而H2的分布范围更大但是未存在明显的优先吸附位点.Based on the high-throughput calculation method of molecular simulation, except the structures with zero surface area and less than zero adsorption capacity, four geometric descriptors (largest cavity diameter, specific surface area, pore volume and porosity), an energy descriptor (heat of adsorption), adsorption capacity, and adsorption selectivity coefficient of 199 zeolites are obtained. By studying the correlation between structural characteristics and adsorption separation performance, the result shows that when the largest cavity diameter is 6 Å, the surface area is 1400–2100 m2·g–1, and the pore volume is in a range of 0.2–0.3 cm3·g–1, the zeolite has the greatest influence on the adsorption capacity and adsorption selectivity for methane molecules. At the same time, it is found that the largest cavity diameter and porosity of zeolite molecular sieves have a positive correlation, and there is also an obvious linear relationship between the CH4 adsorption selectivity coefficient of the equimolar CH4/H2 mixed component and the single-component CH4 adsorption capacity. By using the grand canonical Monte Carlo simulation method, physical quantities such as adsorption isotherms and isosteric heats of adsorption for CH4 and H2 of three channel-shaped zeolites are obtained. The result shows that the pore structure (surface area and pore volume) of the channel-shaped zeolite has a greater influence on the CH4 adsorption capacity than the energy effect (heat of adsorption), under the same external environment. Combining with the industrial background of steam methane reforming hydrogen production, the separation and selectivity performance of the CH4/H2 mixed system under different components are further studied. The result reveals that there is no correlation between adsorption selectivity of ultra-microporous zeolite material for CH4 and bulk pressure or feed ratio. According to the centroid distribution density of gas molecules, it is found that CH4 preferentially occupies the space of smaller pore windows in the channel-shaped zeolite, while the distribution range of H2 is larger but there is no unambiguous preferential adsorption site.
-
Keywords:
- zeolite molecular sieve /
- high-throughput computing /
- grand canonical Monte Carlo simulation /
- adsorption separation
[1] Nijem N, Veyan J F, Kong L Z, Li K, Pramanik S, Zhao Y, Li J, Langreth D, Chabal Y J 2010 J. Am. Chem. Soc. 132 1654
 Google Scholar
Google Scholar
[2] Liu X B, Li W X, Zhao X D, Fan L Z 2019 Adv. Funct. Mater. 29 1901510
 Google Scholar
Google Scholar
[3] 陈玉红, 刘婷婷, 张梅玲, 元丽华, 张材荣 2017 化学学报 75 708
 Google Scholar
Google Scholar
Chen Y H, Liu T T, Zhang M L, Yuan L H, Zhang C R 2017 Acta Chim. Sin. 75 708
 Google Scholar
Google Scholar
[4] 刘秀英, 王朝阳, 唐永建, 孙卫国, 吴卫东, 张厚琼, 刘淼, 袁磊, 徐嘉靖 2009 58 1126
 Google Scholar
Google Scholar
Liu X Y, Wang C Y, Tang Y J, Sun W G, Wu W D, Zhang H Q, Liu M, Yuan L, Xu J J 2009 Acta Phys. Sin. 58 1126
 Google Scholar
Google Scholar
[5] Chen J J, Gao X H, Yan L F, Xu D G 2018 Int. J. Hydrogen Energy 43 12948
 Google Scholar
Google Scholar
[6] LeValley T L, Richard A R, Fan M H 2014 Int. J. Hydrogen Energy 39 16983
 Google Scholar
Google Scholar
[7] Yang Q Y, Wiersum A D, Jobic H, Guillerm V, Serre C, Llewellyn P L, Maurin G 2011 J. Phys. Chem. C 115 13768
 Google Scholar
Google Scholar
[8] Bastos-Neto M, Patzschke C, Lange M, Guillerm V, Serre C, Llewellyn P L, Maurin G 2012 Energy Environ. Sci. 5 8294
 Google Scholar
Google Scholar
[9] Zhou D, Cheng Q Y, Cui Y, Wang T, Li X X, Han B H 2014 Carbon 66 592
 Google Scholar
Google Scholar
[10] 吴选军, 郑佶, 李江, 蔡卫权 2013 物理化学学报 29 2207
 Google Scholar
Google Scholar
Wu X J, Zheng J, Li J, Cai W Q 2013 Acta Phys.-Chim. Sin. 29 2207
 Google Scholar
Google Scholar
[11] Chue K T, Kim J N, Yoo Y J, Cho S H, Yang R T 1995 Ind. Eng. Chem. Res. 34 591
 Google Scholar
Google Scholar
[12] Ho M T, Allinson G W, Wiley D E 2008 Ind. Eng. Chem. Res. 47 1562
 Google Scholar
Google Scholar
[13] Li Y, Yu J H 2014 Chem. Rev. 114 7268
 Google Scholar
Google Scholar
[14] Li J Y, Corma A, Yu J H 2015 Chem. Soc. Rev. 44 7112
 Google Scholar
Google Scholar
[15] Weckhuysen B M, Yu J H 2015 Chem. Soc. Rev. 44 7022
 Google Scholar
Google Scholar
[16] Ferri P, Li C G, Paris C, Vidal-Moya A, Moliner M, Boronat M, Corma A 2019 ACS Catal. 9 11542
 Google Scholar
Google Scholar
[17] Margarit V J, Osman M, Al-Khattaf S, Martínez C, Boronat M, Corma A 2019 ACS Catal. 9 5935
 Google Scholar
Google Scholar
[18] McCusker L B, Liebau F, Engelhardt G 2001 Pure Appl. Chem. 73 381
 Google Scholar
Google Scholar
[19] Li Y, Cao H, Yu J H 2018 ACS Nano 12 4096
 Google Scholar
Google Scholar
[20] Stückenschneider K, Merz J, Schembecker G 2013 J. Mol. Model. 19 5611
 Google Scholar
Google Scholar
[21] Fu H, Wang Y J, Zhang T H, Yang C H, Shan H H 2017 J. Phys. Chem. C 121 25818
 Google Scholar
Google Scholar
[22] Perez-Carbajo J, Gómez-Álvarez P, Bueno-Perez R, Merkling P J, Calero S 2014 Phys. Chem. Chem. Phys. 16 5678
 Google Scholar
Google Scholar
[23] 刘秀英, 李晓凤, 张丽英, 樊志琴, 马兴科 2012 61 146802
 Google Scholar
Google Scholar
Liu X Y, Li X F, Zhang L Y, Fan Z Q, Ma X K 2012 Acta Phys. Sin. 61 146802
 Google Scholar
Google Scholar
[24] Curtarolo S, Hart G L W, Nardelli M B, Mingo N, Sanvito S, Levy O 2013 Nat. Mater. 12 191
 Google Scholar
Google Scholar
[25] 刘治鲁, 李炜, 刘昊, 庄旭东, 李松 2019 化学学报 77 323
 Google Scholar
Google Scholar
Liu Z L, Li W, Liu H, Zhuang X D, Li S 2019 Acta Chim. Sin. 77 323
 Google Scholar
Google Scholar
[26] Wollmann P, Leistner M, Stoeck U, Grünker R, Gedrich K, Klein N, Throl O, Grählert W, Senkovska I, Dreisbach F, Kaskel S 2011 Chem. Commun. 47 5151
 Google Scholar
Google Scholar
[27] Database of Zeolite Structures http://www.iza-structure.org/databases/
[28] Martin R L, Smit B, Haranczyk M 2012 J. Chem. Inf. Model. 52 308
 Google Scholar
Google Scholar
[29] Pinheiro M, Martin R L, Rycroft C H, Jones A, Iglesia E, Haranczyk M 2013 J. Mol. Graphics Modell. 44 208
 Google Scholar
Google Scholar
[30] Bai P, Tsapatsis M, Siepmann J I 2013 J. Phys. Chem. C 117 24375
 Google Scholar
Google Scholar
[31] Goodbody S J, Watanabe K, MacGowan D, Walton J P R B, Quirke N 1991 Faraday Trans. 87 1951
 Google Scholar
Google Scholar
[32] Levesque D, Gicquel A, Darkrim F L, Kayiran S B 2002 J. Phys. Condens. Matter 14 9285
 Google Scholar
Google Scholar
[33] Peng X, Cheng X, Cao D P 2011 J. Mater. Chem. 21 11259
 Google Scholar
Google Scholar
[34] Dubbeldam D, Torres-Knoop A, Walton K S 2013 Mol. Simulat. 39 1253
 Google Scholar
Google Scholar
[35] Dubbeldam D, Snurr R Q 2007 Mol. Simul. 33 305
 Google Scholar
Google Scholar
[36] Bao Z B, Alnemrat S, Yu L, Vasiliev I, Ren Q L, Lu X Y, Deng S G 2011 Langmuir 27 13554
 Google Scholar
Google Scholar
-
表 1 沸石的结构参数
Table 1. Structural parameters of zeolite.
Zeolite Unit cell/Å Cell angle/(°) PLD/Å ASA/(m2·g–1) POAV/(cm3·g–1) ASV a = b = 8.674, c = 13.919 α = β = γ = 90 4.0279 1323.9 0.1923 BOF a = 7.503, b = 12.773, c = 13.706 α = β = γ = 90 4.1832 1408.0 0.2207 FER a = 19.018, b = 14.303, c = 7.541 α = β = γ = 90 4.2893 1545.5 0.2375 表 2 吸附质分子和沸石的力场参数
Table 2. Force field parameters of adsorbate molecules and zeolites.
原子 (ε/kB)/K σ/Å q/e CH4 148.0 3.73 0 H2 H 36.7 2.958 0.468 com 0 0 –0.936 Zeolite Si 22 2.3 1.5 O 53 3.3 –0.75 -
[1] Nijem N, Veyan J F, Kong L Z, Li K, Pramanik S, Zhao Y, Li J, Langreth D, Chabal Y J 2010 J. Am. Chem. Soc. 132 1654
 Google Scholar
Google Scholar
[2] Liu X B, Li W X, Zhao X D, Fan L Z 2019 Adv. Funct. Mater. 29 1901510
 Google Scholar
Google Scholar
[3] 陈玉红, 刘婷婷, 张梅玲, 元丽华, 张材荣 2017 化学学报 75 708
 Google Scholar
Google Scholar
Chen Y H, Liu T T, Zhang M L, Yuan L H, Zhang C R 2017 Acta Chim. Sin. 75 708
 Google Scholar
Google Scholar
[4] 刘秀英, 王朝阳, 唐永建, 孙卫国, 吴卫东, 张厚琼, 刘淼, 袁磊, 徐嘉靖 2009 58 1126
 Google Scholar
Google Scholar
Liu X Y, Wang C Y, Tang Y J, Sun W G, Wu W D, Zhang H Q, Liu M, Yuan L, Xu J J 2009 Acta Phys. Sin. 58 1126
 Google Scholar
Google Scholar
[5] Chen J J, Gao X H, Yan L F, Xu D G 2018 Int. J. Hydrogen Energy 43 12948
 Google Scholar
Google Scholar
[6] LeValley T L, Richard A R, Fan M H 2014 Int. J. Hydrogen Energy 39 16983
 Google Scholar
Google Scholar
[7] Yang Q Y, Wiersum A D, Jobic H, Guillerm V, Serre C, Llewellyn P L, Maurin G 2011 J. Phys. Chem. C 115 13768
 Google Scholar
Google Scholar
[8] Bastos-Neto M, Patzschke C, Lange M, Guillerm V, Serre C, Llewellyn P L, Maurin G 2012 Energy Environ. Sci. 5 8294
 Google Scholar
Google Scholar
[9] Zhou D, Cheng Q Y, Cui Y, Wang T, Li X X, Han B H 2014 Carbon 66 592
 Google Scholar
Google Scholar
[10] 吴选军, 郑佶, 李江, 蔡卫权 2013 物理化学学报 29 2207
 Google Scholar
Google Scholar
Wu X J, Zheng J, Li J, Cai W Q 2013 Acta Phys.-Chim. Sin. 29 2207
 Google Scholar
Google Scholar
[11] Chue K T, Kim J N, Yoo Y J, Cho S H, Yang R T 1995 Ind. Eng. Chem. Res. 34 591
 Google Scholar
Google Scholar
[12] Ho M T, Allinson G W, Wiley D E 2008 Ind. Eng. Chem. Res. 47 1562
 Google Scholar
Google Scholar
[13] Li Y, Yu J H 2014 Chem. Rev. 114 7268
 Google Scholar
Google Scholar
[14] Li J Y, Corma A, Yu J H 2015 Chem. Soc. Rev. 44 7112
 Google Scholar
Google Scholar
[15] Weckhuysen B M, Yu J H 2015 Chem. Soc. Rev. 44 7022
 Google Scholar
Google Scholar
[16] Ferri P, Li C G, Paris C, Vidal-Moya A, Moliner M, Boronat M, Corma A 2019 ACS Catal. 9 11542
 Google Scholar
Google Scholar
[17] Margarit V J, Osman M, Al-Khattaf S, Martínez C, Boronat M, Corma A 2019 ACS Catal. 9 5935
 Google Scholar
Google Scholar
[18] McCusker L B, Liebau F, Engelhardt G 2001 Pure Appl. Chem. 73 381
 Google Scholar
Google Scholar
[19] Li Y, Cao H, Yu J H 2018 ACS Nano 12 4096
 Google Scholar
Google Scholar
[20] Stückenschneider K, Merz J, Schembecker G 2013 J. Mol. Model. 19 5611
 Google Scholar
Google Scholar
[21] Fu H, Wang Y J, Zhang T H, Yang C H, Shan H H 2017 J. Phys. Chem. C 121 25818
 Google Scholar
Google Scholar
[22] Perez-Carbajo J, Gómez-Álvarez P, Bueno-Perez R, Merkling P J, Calero S 2014 Phys. Chem. Chem. Phys. 16 5678
 Google Scholar
Google Scholar
[23] 刘秀英, 李晓凤, 张丽英, 樊志琴, 马兴科 2012 61 146802
 Google Scholar
Google Scholar
Liu X Y, Li X F, Zhang L Y, Fan Z Q, Ma X K 2012 Acta Phys. Sin. 61 146802
 Google Scholar
Google Scholar
[24] Curtarolo S, Hart G L W, Nardelli M B, Mingo N, Sanvito S, Levy O 2013 Nat. Mater. 12 191
 Google Scholar
Google Scholar
[25] 刘治鲁, 李炜, 刘昊, 庄旭东, 李松 2019 化学学报 77 323
 Google Scholar
Google Scholar
Liu Z L, Li W, Liu H, Zhuang X D, Li S 2019 Acta Chim. Sin. 77 323
 Google Scholar
Google Scholar
[26] Wollmann P, Leistner M, Stoeck U, Grünker R, Gedrich K, Klein N, Throl O, Grählert W, Senkovska I, Dreisbach F, Kaskel S 2011 Chem. Commun. 47 5151
 Google Scholar
Google Scholar
[27] Database of Zeolite Structures http://www.iza-structure.org/databases/
[28] Martin R L, Smit B, Haranczyk M 2012 J. Chem. Inf. Model. 52 308
 Google Scholar
Google Scholar
[29] Pinheiro M, Martin R L, Rycroft C H, Jones A, Iglesia E, Haranczyk M 2013 J. Mol. Graphics Modell. 44 208
 Google Scholar
Google Scholar
[30] Bai P, Tsapatsis M, Siepmann J I 2013 J. Phys. Chem. C 117 24375
 Google Scholar
Google Scholar
[31] Goodbody S J, Watanabe K, MacGowan D, Walton J P R B, Quirke N 1991 Faraday Trans. 87 1951
 Google Scholar
Google Scholar
[32] Levesque D, Gicquel A, Darkrim F L, Kayiran S B 2002 J. Phys. Condens. Matter 14 9285
 Google Scholar
Google Scholar
[33] Peng X, Cheng X, Cao D P 2011 J. Mater. Chem. 21 11259
 Google Scholar
Google Scholar
[34] Dubbeldam D, Torres-Knoop A, Walton K S 2013 Mol. Simulat. 39 1253
 Google Scholar
Google Scholar
[35] Dubbeldam D, Snurr R Q 2007 Mol. Simul. 33 305
 Google Scholar
Google Scholar
[36] Bao Z B, Alnemrat S, Yu L, Vasiliev I, Ren Q L, Lu X Y, Deng S G 2011 Langmuir 27 13554
 Google Scholar
Google Scholar
计量
- 文章访问数: 9426
- PDF下载量: 150
- 被引次数: 0














 下载:
下载: