-
超冷长程Rydberg-基态分子由一个Rydberg原子与一个或多个基态原子通过Rydberg电子与基态原子的低能散射形成. 这类分子具有尺寸大和永久电偶极矩大等优良特性, 是近年来人们研究的热点课题. 本文综述了超冷Rydberg-基态分子的最新理论和实验进展, 包括Rydberg电子与基态原子低能散射的理论模型和势能曲线, 光缔合Rydberg分子的实验制备和光谱特性, Rydberg-基态分子永久电偶极矩的测量等. 最新的研究发现Cs nDJ -型Rydberg-基态分子的永久电偶极矩为负值, 这与以往的此类分子(电偶极矩为正)不同. 负电偶极矩反映了Rydberg电子的概率密度在基态(微扰)原子附近是减少的, 由微扰原子附近Rydberg电子波函数的相消干涉产生.
-
关键词:
- 超冷长程Rydberg-基态分子 /
- Fermi赝势 /
- 绝热势能曲线 /
- 永久电偶极矩
Ultra-cold long-range Rydberg-ground molecule consisting of a Rydberg atom and one or more ground-state atoms is formed by low-energy scattering between the Rydberg electron and ground-state atoms located inside the Rydberg electron’s wave function. The low-energy scattering interaction, initially investigated by Fermi and Omont, has been predicted to lead to molecular binding in a novel type of Rydberg molecules, including the trilobite and butterfly molecules. Their unconventional binding mechanism, which is unlike covalent, or ionic, or van der Waals bonds, results in loosely bound molecules with bond lengths on the order of thousands of Bohr radius. This kind of molecule with large size and huge permanent electric dipole moment is a good candidate for realizing the certain strongly correlated many-body gases and for quantum information processing, as well as for dipolar quantum gases and spin systems with long-range interactions. Consequently, these molecules have received considerable attention in recent years. In this paper, we review the recent theoretical and experimental investigations of ultra-cold long-range Rydberg-ground molecules, including the scattering interaction between the Rydberg electron and ground-state atom and the resulting adiabatic potential curves, experimental observations of photo-associated Rydberg-ground molecules spectra, as well as the measurements of permanent electric dipole moment. Ultra-cold long-range Rydberg-ground molecules are prepared by photoassociation in a high-density cold atom sample. Therefore, the Rydberg electron can bind several ground-state atoms to form a polyatomic Rydberg-ground molecule. The permanent molecular electric-dipole moments are revealed by spectral line broadening in the electric fields. The latest research pointed out that the permanent electric dipole moments of the Cs nDJ -type Rydberg-ground molecules are negative, which is different from the previous reports (the electric dipole moments are positive). The negative sign reflects a deficiency of Rydberg-electron density near the ground-state perturber, which is caused by electronic configuration mixing. -
Keywords:
- ultra-cold long-range Rydberg-ground molecules /
- Fermi pseudopotential /
- adiabatic potential curves /
- permanent electric dipole moment
[1] Amaldi E, Segré E 1934 Il Nuovo Cimento. 11 145
[2] Fermi E 1934 Il Nuovo Cimento. 11 157
 Google Scholar
Google Scholar
[3] Omont A 1977 J. Phys. 38 1343
 Google Scholar
Google Scholar
[4] Greene C H, Dickinson A S, Sadeghpour H R 2000 Phys. Rev. Lett. 85 2458
 Google Scholar
Google Scholar
[5] Chibisov M I, Khuskivadze A A, Fabrikant I I 2002 J. Phys. B: At. Mol. Opt. Phys. 35 L193
 Google Scholar
Google Scholar
[6] Hamilton E L, Greene C H, Sadeghpour H R 2002 J. Phys. B: At. Mol. Opt. Phys. 35 L199
 Google Scholar
Google Scholar
[7] Bendkowsky V, Butscher B, Nipper J, Shaffer J P, Löw R, Pfau T 2009 Nature 458 1005
 Google Scholar
Google Scholar
[8] Bellos M A, Carollo R, Banerjee J, Eyler E E, Gould P L, Stwalley W C 2013 Phys. Rev. Lett. 111 053001
 Google Scholar
Google Scholar
[9] Anderson D A, Miller S A, Raithel G 2014 Phys. Rev. Lett. 112 163201
 Google Scholar
Google Scholar
[10] Krupp A T, Gaj A, Balewski J B, Ilzhöfer P, Hofferberth S, Löw R, Pfau T, Kurz M, Schmelcher P 2014 Phys. Rev. Lett. 112 143008
 Google Scholar
Google Scholar
[11] Maclennan J L, Chen Y J, Raithel G 2019 Phys. Rev. A 99 033407
 Google Scholar
Google Scholar
[12] Booth D, Rittenhouse S T, Yang J, Sadeghpour H R, Shaffer J P 2015 Science 348 99
 Google Scholar
Google Scholar
[13] Tallant J, Rittenhouse S T, Booth D, Sadeghpour H R, Shaffer J P 2012 Phys. Rev. Lett. 109 173202
 Google Scholar
Google Scholar
[14] Saßmannshausen H, Merkt F, Deiglmayr J 2015 Phys. Rev. Lett. 114 133201
 Google Scholar
Google Scholar
[15] Fey C, Yang J, Rittenhouse S T, Munkes F, Baluktsian M, Schmelcher P, Sadeghpour H R, Shaffer J P 2019 Phys. Rev. Lett. 122 103001
 Google Scholar
Google Scholar
[16] Bai S, Han X, Bai J, Jiao Y, Zhao J, Jia S, Raithel G 2020 Phys. Rev. Res. 2 033525
 Google Scholar
Google Scholar
[17] Bai S, Han X, Bai J, Jiao Y, Wang H, Zhao J, Jia S 2020 J. Chem. Phys. 152 084302
 Google Scholar
Google Scholar
[18] Engel F, Dieterle T, Hummel F, Fey C, Schmelcher P, Löw R, Pfau T, Meinert F 2019 Phys. Rev. Lett. 123 073003
 Google Scholar
Google Scholar
[19] Deiß M, Haze S, Wolf J, Wang L, Meinert F, Fey C, Hummel F, Schmelcher P, Denschlag J H 2020 Phys. Rev. Res. 2 013047
 Google Scholar
Google Scholar
[20] Peper M, Deiglmayr J 2020 J. Phys. B: At. Mol. Opt. Phys. 53 064001
 Google Scholar
Google Scholar
[21] Reinhold E, Ubachs W 2005 Mol. Phys. 103 1329
 Google Scholar
Google Scholar
[22] Weimer H, Müller M, Lesanovsky I, Zoller P, Büchler H P 2010 Nat. Phys. 6 382
 Google Scholar
Google Scholar
[23] Lukin M D, Fleischhauer M, Cote R, Duan L M, Jaksch D, Cirac J I, Zoller P 2001 Phys. Rev. Lett. 87 037901
 Google Scholar
Google Scholar
[24] Demille D 2002 Phys. Rev. Lett. 88 067901
 Google Scholar
Google Scholar
[25] Rabl P, Demille D, Doyle J M, Lukin M D, Schoelkopf R J, Zoller P 2006 Phys. Rev. Lett. 97 033003
 Google Scholar
Google Scholar
[26] Anderson D A, Miller S A, Raithel G 2014 Phys. Rev. A 90 062518
 Google Scholar
Google Scholar
[27] Yang Y, Kühn O 2008 Mol. Phys. 106 2445
 Google Scholar
Google Scholar
[28] Yang Y, Meuwly M 2010 J. Chem. Phys. 133 064503
 Google Scholar
Google Scholar
[29] Yang Y, Liu X, Meuwly M, Xiao L, Jia S 2012 J. Phys. Chem. A 116 11134
 Google Scholar
Google Scholar
[30] Yang Y, Kühn O 2011 Chem. Phys. Lett. 505 1
 Google Scholar
Google Scholar
[31] Liu C, Manz J, Yang Y 2016 Phys. Chem. Chem. Phys. 18 5048
 Google Scholar
Google Scholar
[32] Yang Y, Jia D, Wang Y J, Zhai H J, Man Y, Li S D 2017 Nanoscale 9 1443
 Google Scholar
Google Scholar
[33] Jia D, Manz J, Yang Y 2019 J. Phys. Chem. Lett. 10 4273
 Google Scholar
Google Scholar
[34] Johnston A R, Burrow P D 1982 J. Phys. B: At. Mol. Opt. Phys. 15 L745
 Google Scholar
Google Scholar
[35] Bendkowsky V, Butscher B, Nipper J, Balewski J B, Shaffer J P, Löw R, Pfau T, Li W, Stanojevic J, Pohl T, Rost J M 2010 Phys. Rev. Lett. 105 163201
 Google Scholar
Google Scholar
[36] Böttcher F, Gaj A, Westphal K M, Schlagmüller M, Kleinbach K S, Löw R, Liebisch T C, Pfau T, Hofferberth S 2016 Phys. Rev. A 93 032512
 Google Scholar
Google Scholar
[37] Schlagmüller M, Liebisch T C, Nguyen H, Lochead G, Engel F, Böttcher F, Westphal K M, Kleinbach K S, Löw R, Hofferberth S, Pfau T, PérezRíos J, Greene C H 2016 Phys. Rev. Lett. 116 053001
 Google Scholar
Google Scholar
[38] Manthey T, Niederprüm T, Thomas O, Ott H 2015 New J. Phys. 17 103024
 Google Scholar
Google Scholar
[39] Whalen J D, Ding R, Kanungo S K, Killian T C, Yoshida S, Burgdörfer J, Dunning F B 2019 Mol. Phys. 117 3088
 Google Scholar
Google Scholar
[40] Whalen J D, Kanungo S K, Ding R, Wagner M, Schmidt R, Sadeghpour H R, Yoshida S, Burgdörfer J, Dunning F B, Killian T C 2019 Phys. Rev. A 100 011402(R
 Google Scholar
Google Scholar
[41] DeSalvo B J, Aman J A, Dunning F B, Killian T C, Sadeghpour H R, Yoshida S, Burgdörfer J 2015 Phys. Rev. A 92 031403(R
 Google Scholar
Google Scholar
[42] Camargo F, Whalen J D, Ding R, Sadeghpour H R, Yoshida S, Burgdörfer J, Dunning F B, Killian T C 2016 Phys. Rev. A 93 022702
 Google Scholar
Google Scholar
[43] Liu I C, Rost J M 2006 Eur. Phys. J. D 40 65
 Google Scholar
Google Scholar
[44] Liu I C, Stanojevic J, Rost J M 2009 Phys. Rev. Lett. 102 173001
 Google Scholar
Google Scholar
[45] Gaj A, Krupp A T, Balewski J B, Löw R, Hofferberth S, Pfau T 2014 Nat. Commun. 5 4546
 Google Scholar
Google Scholar
[46] Schmidt R, Sadeghpour H R, Demler E 2016 Phys. Rev. Lett. 116 105302
 Google Scholar
Google Scholar
[47] Amaldi E, Segré E 1934 Nature 133 141
[48] Füchtbauer C, Schulz P, Brandt A F 1934 Ztschrift Für Physik. 90 403
[49] Eiles M T, Pérez-Ríos J, Robicheaux F, Greene C H 2016 J. Phys. B: At. Mol. Opt. Phys. 49 114005
 Google Scholar
Google Scholar
[50] Fey C, Kurz M, Schmelcher P 2016 Phys. Rev. A 94 012516
 Google Scholar
Google Scholar
[51] Fey C, Hummel F, Schmelcher P 2019 Phys. Rev. A 99 022506
 Google Scholar
Google Scholar
[52] Ospelkaus S, Ni K-K, Wang D, de Miranda M H G, Neyenhuis B, Quéméner G, Julienne P S, Bohn J L, Jin D S, Ye J 2010 Science 327 853
 Google Scholar
Google Scholar
[53] Carr L D, DeMille D, Krems R V, Ye J 2009 New J. Phys. 11 055049
 Google Scholar
Google Scholar
[54] Li W, Pohl T, Rost J M, Rittenhouse S T, Sadeghpour H R, Nipper J, Butscher B, Balewski J B, Bendkowsky V, Löw R, Pfau T 2011 Science 334 1110
 Google Scholar
Google Scholar
[55] Niederprüm T, Thomas O, Eichert T, Lippe C, Pérez-Ríos J, Greene C H, Ott H 2016 Nat. Commun. 7 12820
 Google Scholar
Google Scholar
[56] Markson S, Rittenhouse S T, Schmidt R, Shaffer J P, Sadeghpour H R 2016 Chem. Phys. Chem. 17 3683
 Google Scholar
Google Scholar
-
图 1 Rydberg-基态分子示意图. 自旋为
$ { \widehat{{S}}}_{1} $ 的Rydberg电子和基态原子分别位于以Cs+离子为参考系的位置r和$R\hat{{z}}$ 处.$ {\widehat{{S}}}_{2} $ 和$ {\widehat{{I}}}_{2} $ 分别是基态原子的电子自旋和核自旋Fig. 1. Schematic of the molecular system. The Rydberg electron with spin
$ {\widehat{{S}}}_{1} $ sits at position r relative to the ionic core. A cesium ground state atom is located at position$R\hat{{z}}$ relative to the ionic core of the Rydberg atom.$ {\widehat{{S}}}_{2} $ and$\hat{ I}_2$ are electronic and nuclear spin of the ground state atom, respectively.图 2 理论计算的n =19附近的Cs分子势能曲线, 其中插图是橙色原点标记I (II)处势阱对应的“三叶虫型”(“蝴蝶型”)分子的电子概率密度
Fig. 2. Calculations of potential energy curves of the Cs Rydberg-ground molecule near n = 19. Inset I (II) displays the electronic wave function densities of “Trilobite” (“Butterfly”) molecules at the orange points marked I (II) in the figure.
图 3 数值计算的37D5/2 + 6S1/2 (F = 4) Rydberg-基态分子的绝热势能曲线. 深势阱(灰色线)主要由自旋三重态T形成, 浅势阱(蓝色线)主要由自旋单态S和自旋三重态T超精细结构混合而形成. 实线和虚线分别是考虑和不考虑p-波散射相互作用时的分子绝热势能曲线. 彩色填充线分别是最外层深势阱TΣ (v = 0)和TΣ (v = 1)的振动波函数, 黑色填充线是最外层浅势阱STΣ (v = 0)的振动波函数[17]
Fig. 3. Calculations of potential energy curves of the Rydberg-ground molecule that is asymptotically related to the 37D5/2 Rydberg atomic line with (solid lines) and without (dashed lines) taking the p-wave scattering interaction into account. The deep potential (gray) for the triplet state and shallow wells (blue) for hyperfine-mixed singlet-triplet potential state. The vibrational wave functions in the outmost wells are indicated in filled curves for TΣ (v = 0) and TΣ (v = 1) of deep potential and STΣ (v = 0) of shallow potential[17].
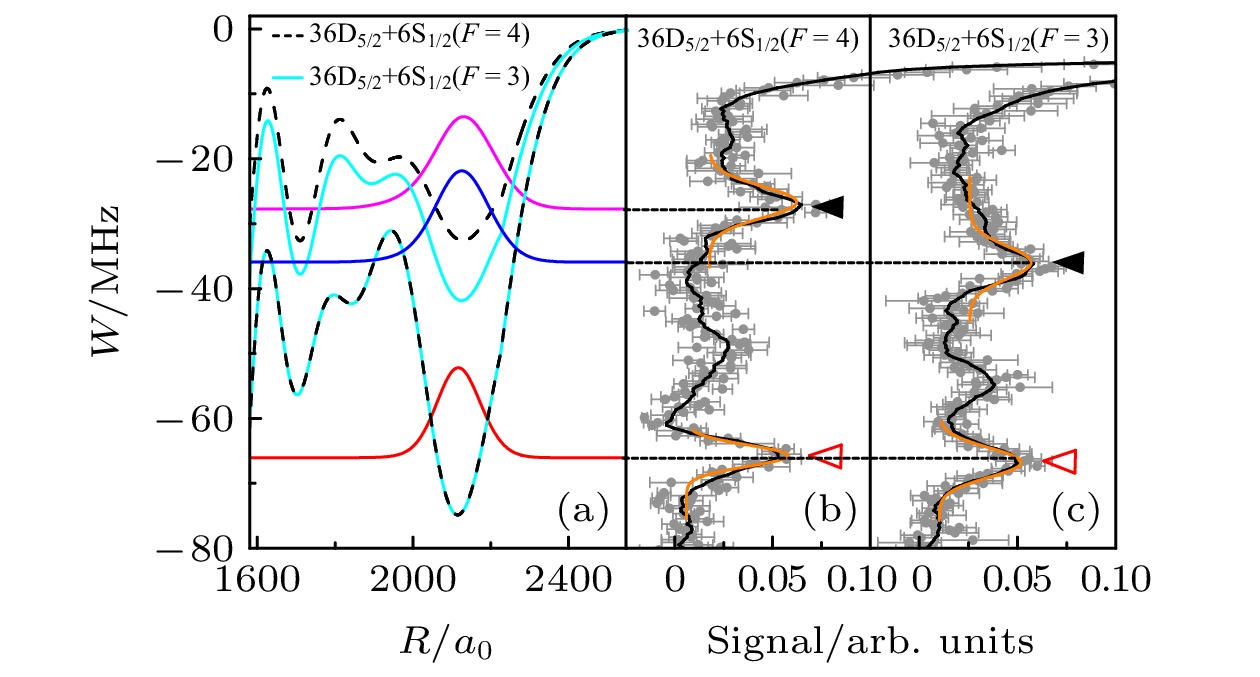
图 4 (a) 数值计算的Cs原子36D5/2 + 6S1/2 (F = 4)(黑色虚线)和36D5/2 + 6S1/2 (F = 3)(青色实线) Rydberg-基态分子的绝热势能曲线. 深势阱(TΣ)主要由自旋三重态T的s-波散射形成, 势阱深度不随基态原子的超精细能级F的变化而变化. 浅势阱(STΣ)主要由自旋单态S和自旋三重态T超精细结构混合而形成, 其势阱深度与基态原子所处的超精细能级F有关, 基态原子处于超精细能级F = 3的势阱深度大于F = 4的势阱深度. 彩色的实线表示v = 0的振动波函数. (b), (c)为实验测得的36D5/2 + 6S1/2 (F = 4)和36D5/2 + 6S1/2 (F = 3)的光缔合光谱. 实心(空心)三角表示由浅势阱(深势阱)形成的分子信号. 黄色线是高斯拟合曲线[16]
Fig. 4. (a) Calculations of potential energy curves (PECs) for 36D5/2 + 6S1/2 (F = 4) (black dashed lines) and PECs for 36D5/2 + 6S1/2 (F = 3) (cyan solid lines) molecules, respectively. The deep potentials (TΣ) mostly arise from triplet s-wave scattering and do not depend on F. The shallow potentials (STΣ) mostly arise from mixed singlet-triplet s-wave scattering and depend on F; The potential energy curves for F = 3 is deeper than that for F = 4. The colored lines show the v = 0 vibrational wave functions of the potential energy curves. (b), (c) Experimental photo-association spectra for 36D5/2 + 6S1/2 (F = 4) and 36D5/2 + 6S1/2 (F = 3) molecules. Filled (open) triangles mark the molecular signals formed by mixed (triplet) potentials. The yellow lines display Gaussian fittings[16].
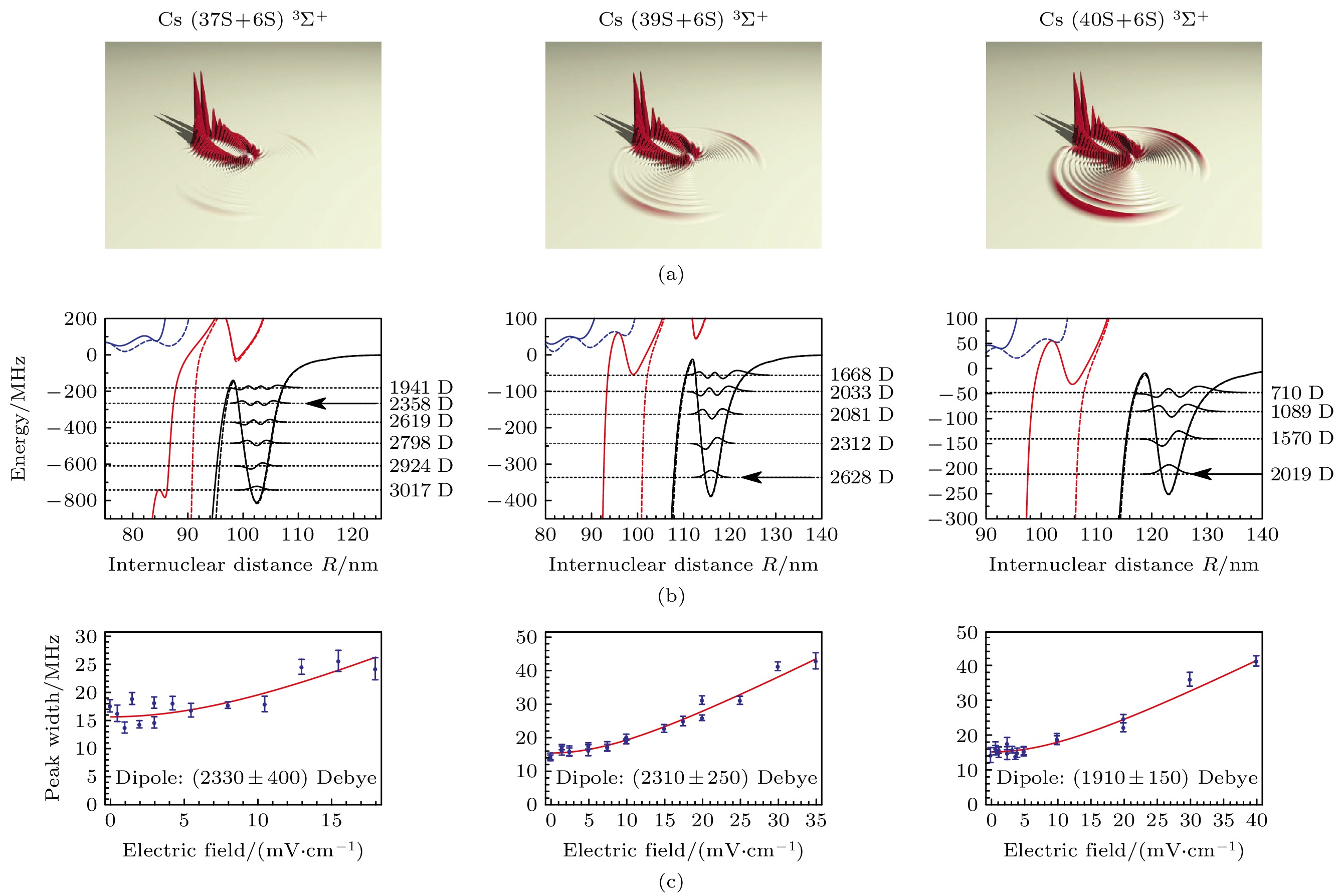
图 7 (a) 理论计算的Cs nS + 6S (n = 37, 39, 40) TΣ+态电子波函数密度[12]; (b) 相应于(a)图的势能曲线和振动能级[12]; (c)为(b)图中箭头标注的振动能级在外电场中分子谱线的展宽[12]
Fig. 7. (a) Calculated densities of electron wave functions for the TΣ+ states of Cs nS + 6S (n = 37, 39, 40)[12]; (b) the corresponding potential energy curves for the states shown in panel (a) with calculated vibrational levels[12]; (c) the broadening of the vibrational levels indicated by an arrow in panel (b) as a function of applied electric field[12].
图 8 不同电场E下的37D5/2 + 6S1/2 (F = 4) Rydberg-基态分子的光缔合光谱. 分子信号在外电场的作用下向蓝失谐处平移, 且在电场E ≥ 0.27 V/cm时开始展宽. 右图是放大的E = 0.37 V/cm下的分子信号, 红色实线是理论拟合线[16]
Fig. 8. Spectra of 36D5/2 + 6S1/2 (F = 4) Rydberg-ground molecules with indicated electric fields E. The molecular peaks are blue shifted by E and substantially broadened in fields E ≥ 0.27 V/cm. The right panel shows zoom-ins of molecules peaks with E = 0.37 V/cm. The red solid lines show model spectra[16].
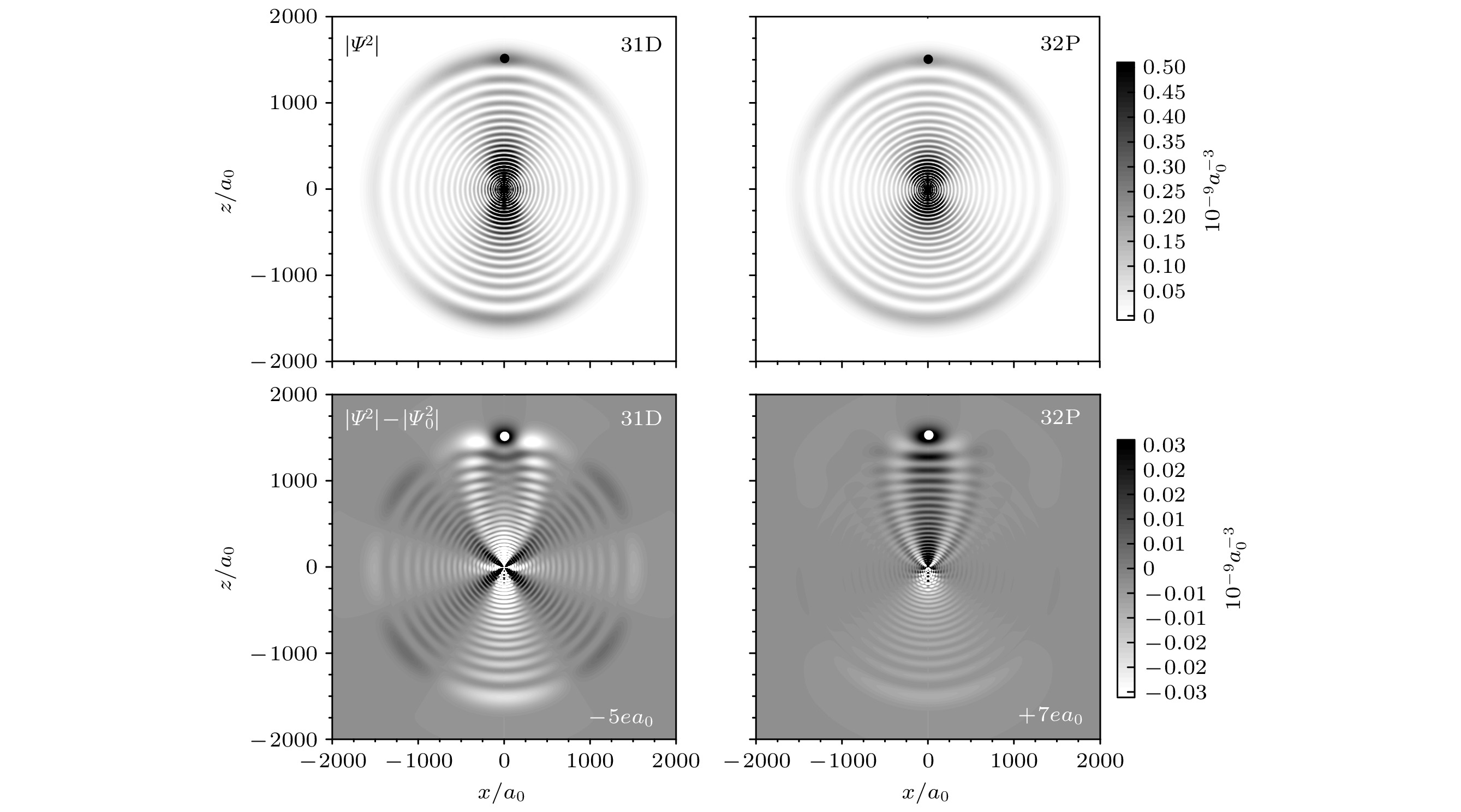
图 9 Cs 31D5/2 + 6S1/2 (左) 和32P3/2 + 6S1/2 (右) Rydberg分子的绝热电子波函数密度, 对应的基态原子位于1500a0附近(图中点的位置). 上图为波函数密度, 下图为分子减去原子的电子波函数密度, 白色和黑色表示密度的减少和增加[16]
Fig. 9. Densities of adiabatic wave functions for Cs 31D5/2 + 6S1/2 (F = 4) triplet (left) and 32P5/2 + 6S1/2 (F = 4) triplet (right), with the perturber located at ≈1500
$ {a}_{0} $ (dot). Top panel shows the wave function densities. Bottom panels shows the difference between electronic wave function densities of molecules and atoms on a linear grayscale, with white and black indicating reductions and increases by amounts shown on the gray-scale bar[16]. -
[1] Amaldi E, Segré E 1934 Il Nuovo Cimento. 11 145
[2] Fermi E 1934 Il Nuovo Cimento. 11 157
 Google Scholar
Google Scholar
[3] Omont A 1977 J. Phys. 38 1343
 Google Scholar
Google Scholar
[4] Greene C H, Dickinson A S, Sadeghpour H R 2000 Phys. Rev. Lett. 85 2458
 Google Scholar
Google Scholar
[5] Chibisov M I, Khuskivadze A A, Fabrikant I I 2002 J. Phys. B: At. Mol. Opt. Phys. 35 L193
 Google Scholar
Google Scholar
[6] Hamilton E L, Greene C H, Sadeghpour H R 2002 J. Phys. B: At. Mol. Opt. Phys. 35 L199
 Google Scholar
Google Scholar
[7] Bendkowsky V, Butscher B, Nipper J, Shaffer J P, Löw R, Pfau T 2009 Nature 458 1005
 Google Scholar
Google Scholar
[8] Bellos M A, Carollo R, Banerjee J, Eyler E E, Gould P L, Stwalley W C 2013 Phys. Rev. Lett. 111 053001
 Google Scholar
Google Scholar
[9] Anderson D A, Miller S A, Raithel G 2014 Phys. Rev. Lett. 112 163201
 Google Scholar
Google Scholar
[10] Krupp A T, Gaj A, Balewski J B, Ilzhöfer P, Hofferberth S, Löw R, Pfau T, Kurz M, Schmelcher P 2014 Phys. Rev. Lett. 112 143008
 Google Scholar
Google Scholar
[11] Maclennan J L, Chen Y J, Raithel G 2019 Phys. Rev. A 99 033407
 Google Scholar
Google Scholar
[12] Booth D, Rittenhouse S T, Yang J, Sadeghpour H R, Shaffer J P 2015 Science 348 99
 Google Scholar
Google Scholar
[13] Tallant J, Rittenhouse S T, Booth D, Sadeghpour H R, Shaffer J P 2012 Phys. Rev. Lett. 109 173202
 Google Scholar
Google Scholar
[14] Saßmannshausen H, Merkt F, Deiglmayr J 2015 Phys. Rev. Lett. 114 133201
 Google Scholar
Google Scholar
[15] Fey C, Yang J, Rittenhouse S T, Munkes F, Baluktsian M, Schmelcher P, Sadeghpour H R, Shaffer J P 2019 Phys. Rev. Lett. 122 103001
 Google Scholar
Google Scholar
[16] Bai S, Han X, Bai J, Jiao Y, Zhao J, Jia S, Raithel G 2020 Phys. Rev. Res. 2 033525
 Google Scholar
Google Scholar
[17] Bai S, Han X, Bai J, Jiao Y, Wang H, Zhao J, Jia S 2020 J. Chem. Phys. 152 084302
 Google Scholar
Google Scholar
[18] Engel F, Dieterle T, Hummel F, Fey C, Schmelcher P, Löw R, Pfau T, Meinert F 2019 Phys. Rev. Lett. 123 073003
 Google Scholar
Google Scholar
[19] Deiß M, Haze S, Wolf J, Wang L, Meinert F, Fey C, Hummel F, Schmelcher P, Denschlag J H 2020 Phys. Rev. Res. 2 013047
 Google Scholar
Google Scholar
[20] Peper M, Deiglmayr J 2020 J. Phys. B: At. Mol. Opt. Phys. 53 064001
 Google Scholar
Google Scholar
[21] Reinhold E, Ubachs W 2005 Mol. Phys. 103 1329
 Google Scholar
Google Scholar
[22] Weimer H, Müller M, Lesanovsky I, Zoller P, Büchler H P 2010 Nat. Phys. 6 382
 Google Scholar
Google Scholar
[23] Lukin M D, Fleischhauer M, Cote R, Duan L M, Jaksch D, Cirac J I, Zoller P 2001 Phys. Rev. Lett. 87 037901
 Google Scholar
Google Scholar
[24] Demille D 2002 Phys. Rev. Lett. 88 067901
 Google Scholar
Google Scholar
[25] Rabl P, Demille D, Doyle J M, Lukin M D, Schoelkopf R J, Zoller P 2006 Phys. Rev. Lett. 97 033003
 Google Scholar
Google Scholar
[26] Anderson D A, Miller S A, Raithel G 2014 Phys. Rev. A 90 062518
 Google Scholar
Google Scholar
[27] Yang Y, Kühn O 2008 Mol. Phys. 106 2445
 Google Scholar
Google Scholar
[28] Yang Y, Meuwly M 2010 J. Chem. Phys. 133 064503
 Google Scholar
Google Scholar
[29] Yang Y, Liu X, Meuwly M, Xiao L, Jia S 2012 J. Phys. Chem. A 116 11134
 Google Scholar
Google Scholar
[30] Yang Y, Kühn O 2011 Chem. Phys. Lett. 505 1
 Google Scholar
Google Scholar
[31] Liu C, Manz J, Yang Y 2016 Phys. Chem. Chem. Phys. 18 5048
 Google Scholar
Google Scholar
[32] Yang Y, Jia D, Wang Y J, Zhai H J, Man Y, Li S D 2017 Nanoscale 9 1443
 Google Scholar
Google Scholar
[33] Jia D, Manz J, Yang Y 2019 J. Phys. Chem. Lett. 10 4273
 Google Scholar
Google Scholar
[34] Johnston A R, Burrow P D 1982 J. Phys. B: At. Mol. Opt. Phys. 15 L745
 Google Scholar
Google Scholar
[35] Bendkowsky V, Butscher B, Nipper J, Balewski J B, Shaffer J P, Löw R, Pfau T, Li W, Stanojevic J, Pohl T, Rost J M 2010 Phys. Rev. Lett. 105 163201
 Google Scholar
Google Scholar
[36] Böttcher F, Gaj A, Westphal K M, Schlagmüller M, Kleinbach K S, Löw R, Liebisch T C, Pfau T, Hofferberth S 2016 Phys. Rev. A 93 032512
 Google Scholar
Google Scholar
[37] Schlagmüller M, Liebisch T C, Nguyen H, Lochead G, Engel F, Böttcher F, Westphal K M, Kleinbach K S, Löw R, Hofferberth S, Pfau T, PérezRíos J, Greene C H 2016 Phys. Rev. Lett. 116 053001
 Google Scholar
Google Scholar
[38] Manthey T, Niederprüm T, Thomas O, Ott H 2015 New J. Phys. 17 103024
 Google Scholar
Google Scholar
[39] Whalen J D, Ding R, Kanungo S K, Killian T C, Yoshida S, Burgdörfer J, Dunning F B 2019 Mol. Phys. 117 3088
 Google Scholar
Google Scholar
[40] Whalen J D, Kanungo S K, Ding R, Wagner M, Schmidt R, Sadeghpour H R, Yoshida S, Burgdörfer J, Dunning F B, Killian T C 2019 Phys. Rev. A 100 011402(R
 Google Scholar
Google Scholar
[41] DeSalvo B J, Aman J A, Dunning F B, Killian T C, Sadeghpour H R, Yoshida S, Burgdörfer J 2015 Phys. Rev. A 92 031403(R
 Google Scholar
Google Scholar
[42] Camargo F, Whalen J D, Ding R, Sadeghpour H R, Yoshida S, Burgdörfer J, Dunning F B, Killian T C 2016 Phys. Rev. A 93 022702
 Google Scholar
Google Scholar
[43] Liu I C, Rost J M 2006 Eur. Phys. J. D 40 65
 Google Scholar
Google Scholar
[44] Liu I C, Stanojevic J, Rost J M 2009 Phys. Rev. Lett. 102 173001
 Google Scholar
Google Scholar
[45] Gaj A, Krupp A T, Balewski J B, Löw R, Hofferberth S, Pfau T 2014 Nat. Commun. 5 4546
 Google Scholar
Google Scholar
[46] Schmidt R, Sadeghpour H R, Demler E 2016 Phys. Rev. Lett. 116 105302
 Google Scholar
Google Scholar
[47] Amaldi E, Segré E 1934 Nature 133 141
[48] Füchtbauer C, Schulz P, Brandt A F 1934 Ztschrift Für Physik. 90 403
[49] Eiles M T, Pérez-Ríos J, Robicheaux F, Greene C H 2016 J. Phys. B: At. Mol. Opt. Phys. 49 114005
 Google Scholar
Google Scholar
[50] Fey C, Kurz M, Schmelcher P 2016 Phys. Rev. A 94 012516
 Google Scholar
Google Scholar
[51] Fey C, Hummel F, Schmelcher P 2019 Phys. Rev. A 99 022506
 Google Scholar
Google Scholar
[52] Ospelkaus S, Ni K-K, Wang D, de Miranda M H G, Neyenhuis B, Quéméner G, Julienne P S, Bohn J L, Jin D S, Ye J 2010 Science 327 853
 Google Scholar
Google Scholar
[53] Carr L D, DeMille D, Krems R V, Ye J 2009 New J. Phys. 11 055049
 Google Scholar
Google Scholar
[54] Li W, Pohl T, Rost J M, Rittenhouse S T, Sadeghpour H R, Nipper J, Butscher B, Balewski J B, Bendkowsky V, Löw R, Pfau T 2011 Science 334 1110
 Google Scholar
Google Scholar
[55] Niederprüm T, Thomas O, Eichert T, Lippe C, Pérez-Ríos J, Greene C H, Ott H 2016 Nat. Commun. 7 12820
 Google Scholar
Google Scholar
[56] Markson S, Rittenhouse S T, Schmidt R, Shaffer J P, Sadeghpour H R 2016 Chem. Phys. Chem. 17 3683
 Google Scholar
Google Scholar
计量
- 文章访问数: 6683
- PDF下载量: 95
- 被引次数: 0






















 下载:
下载: