-
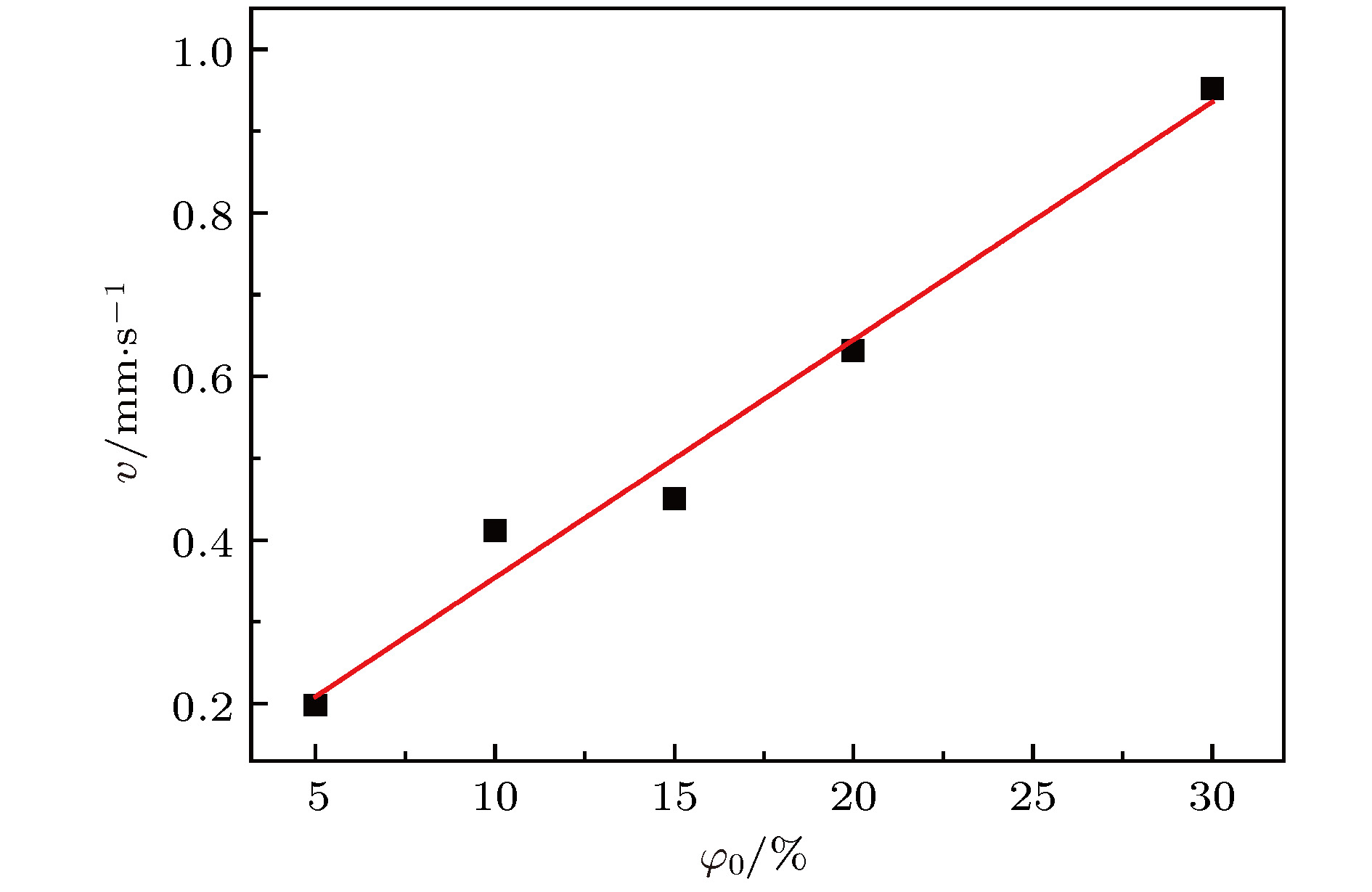
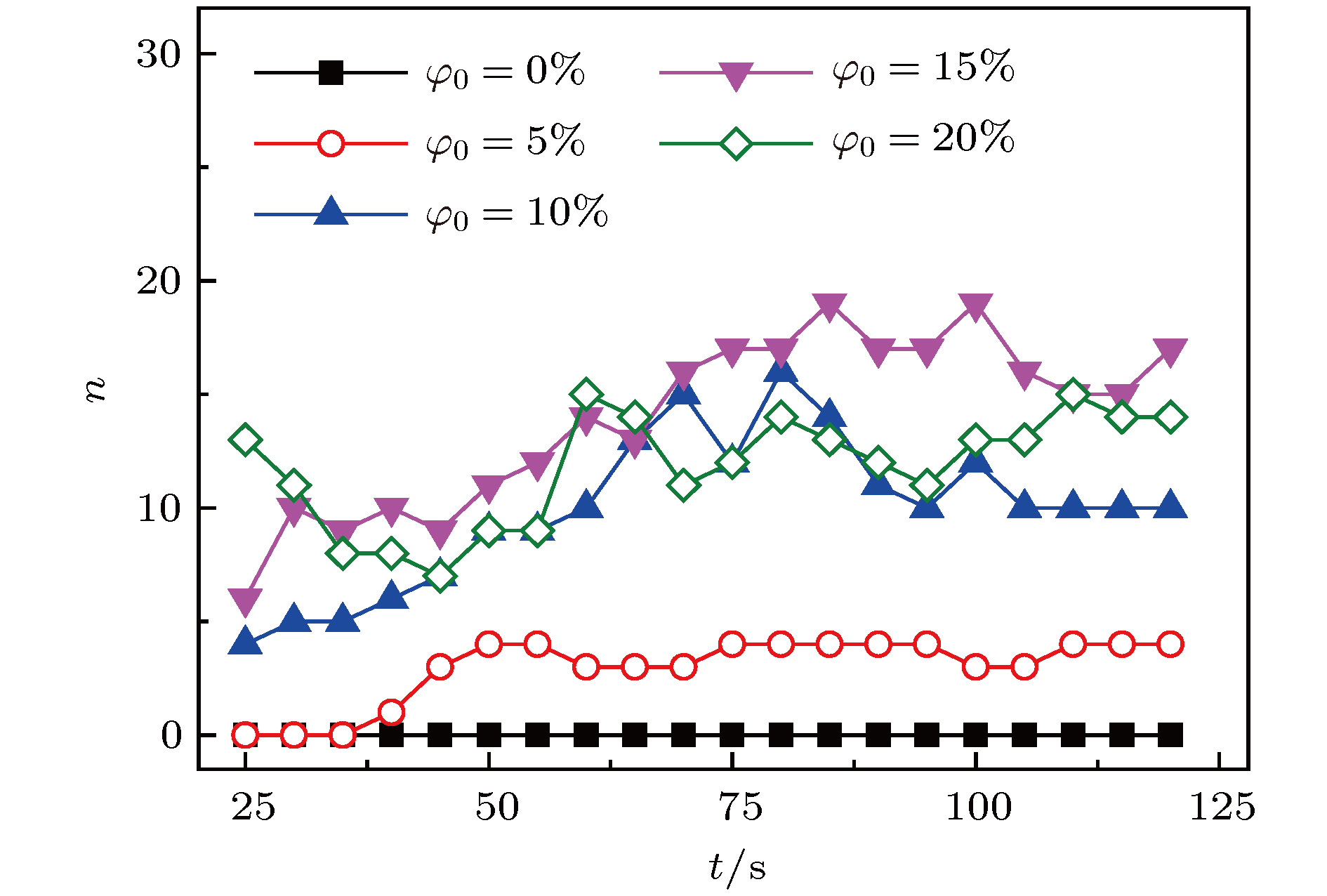
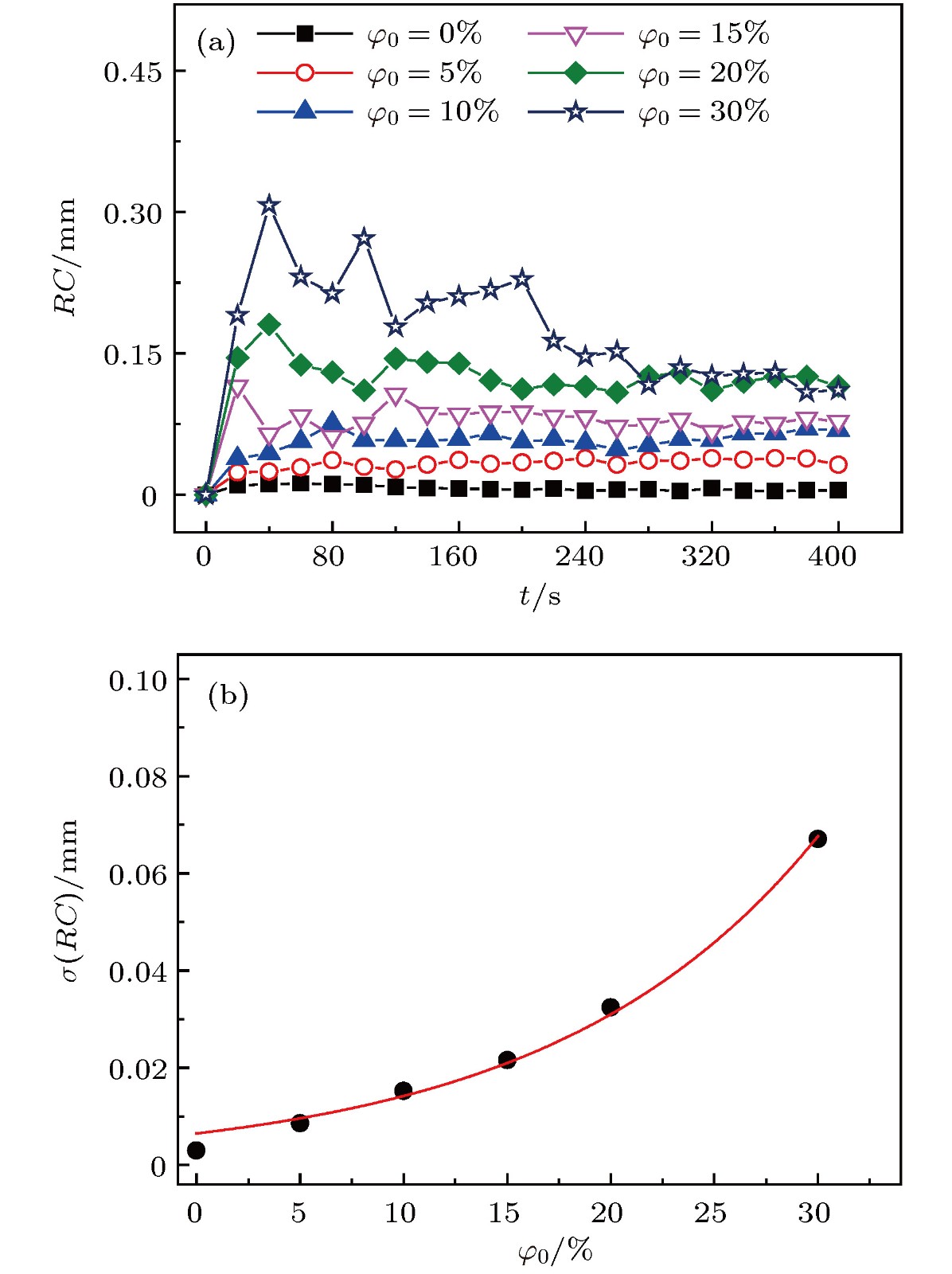
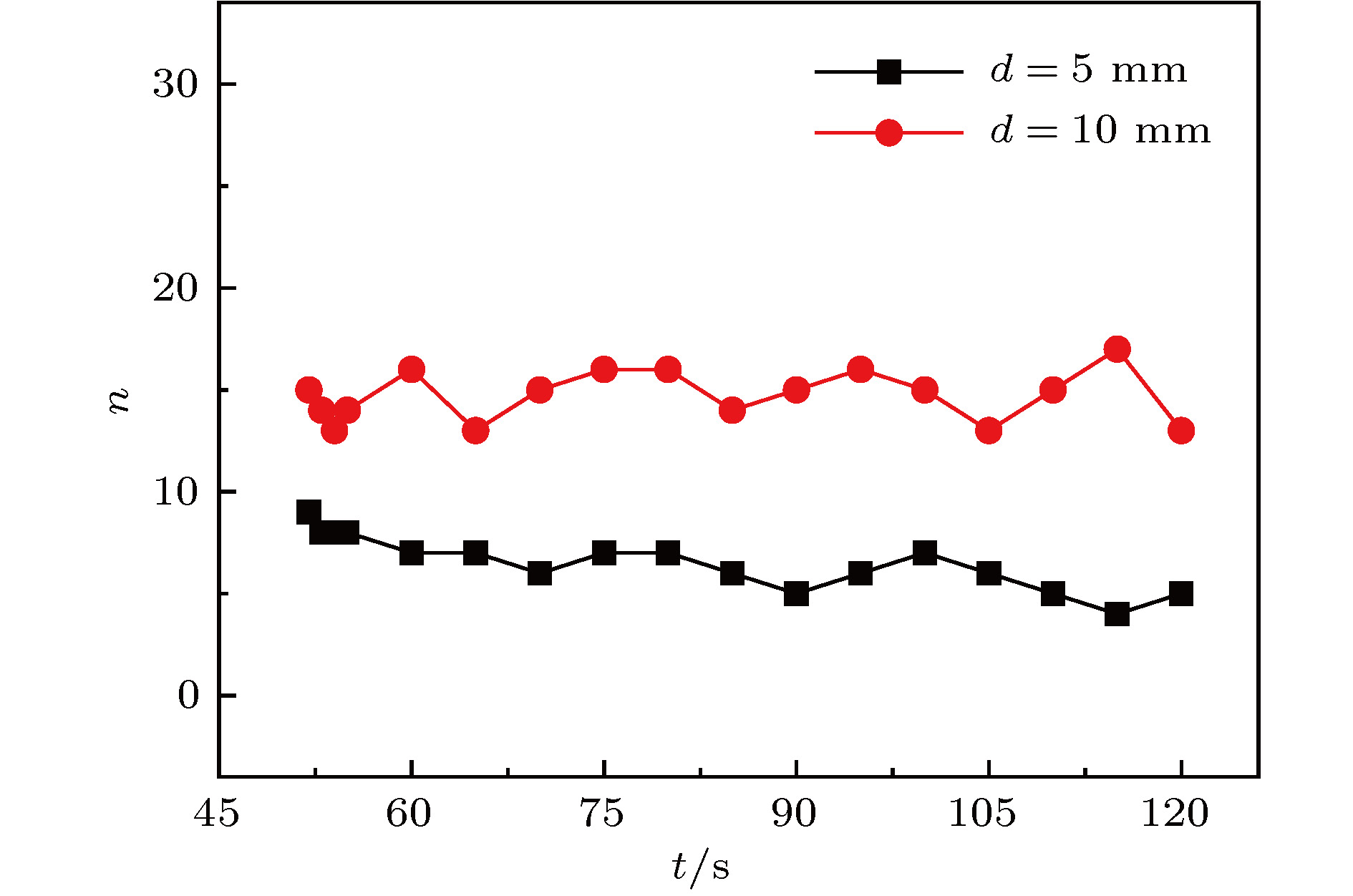
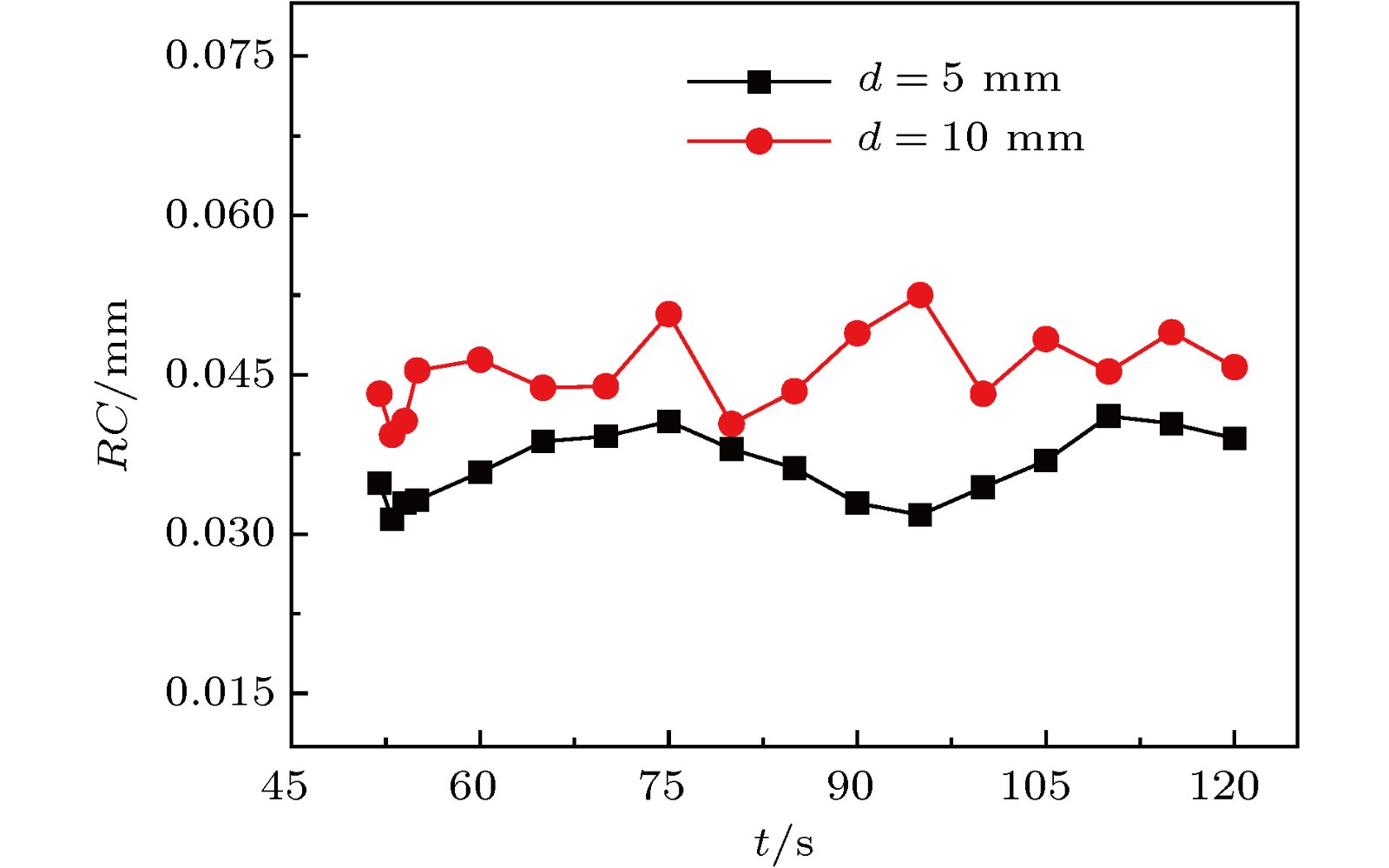
传质引发的Rayleigh-Bénard-Marangoni对流(RBM对流)对化工传递过程有着显著影响. 但是, 已有的相关研究多集中于气-液体系, 并且有限的针对液-液体系的相关研究尚缺乏对RBM对流演化及其引发的界面扰动行为的深入分析. 因此, 本文基于阴影法设计搭建了竖直狭缝内液-液两相液层间传质过程的RBM对流特性可视化实验平台, 并实验观测了水-甲苯-丙酮三元体系中丙酮组分扩散传质时出现的RBM对流结构以及其向下层水相主体的发展演变过程, 探讨了水相丙酮初始浓度、甲苯相丙酮初始浓度以及甲苯层厚度对RBM对流特性和液-液界面形貌的影响. 研究表明: 在Rayleigh-Taylor不稳定性作用下, 水相上层密度(重力)分层“界面”下凸沉降形成波浪形丘状“界面”, 并随着“界面”处密度与压力失调的加剧而演变成羽状流; 因羽流区“界面”不同浓度梯度引起的传质特性差异, 羽状流又可以演变成弱羽状流和强羽状流两种形态; 当丙酮浓度梯度增大到一定程度后, 近界面处短时间内产生大量RBM对流结构, 且结构间相互影响增强而聚并成对流团, 并随着传质过程的进行, 逐渐演变成独立的强羽状流; RBM对流强度与上下液层丙酮浓度梯度大小呈正相关关系, 且液-液界面粗糙度及其非稳态波动随着丙酮浓度梯度的增加而增大.
-
关键词:
- 液-液传质 /
- 阴影法 /
- Rayleigh-Bénard-Marangoni对流结构 /
- 界面形貌
Rayleigh-Bénard-Marangoni convection (RBM convection) induced by the mass transfer has a great influence on the performance of real chemical engineering process. However, the researches of RBM convection characteristics during mass transfer across the interface in liquid-liquid system and their influence on the interface morphology are still limited. In this research, a visualization experiment via the amplified shadowgraph method is conducted to investigate the mass transfer in water-toluene-acetone system in a vertical slit. The convective structure of RBM and its evolution are visually observed. The effects of the initial acetone concentration of aqueous phase and toluene phase, and the thickness of toluene layer on the RBM characteristics and the morphology of the liquid-liquid interface are investigated. The experimental results show that these structures are induced by the interface tension difference along the interface and the vertical density difference caused by non-uniform mass transfer at the interface. As a result of the mass transfer at the interface, the density stratification occurs at the top of the aqueous phase, where the light liquid layer supports heavy one. In addition, non-uniform mass transfer produces perturbation at the top of the aqueous phase, which induces the Rayleigh-Taylor instability at the " interface” between the heavy and light liquid layer. Consequently, a wave-shaped-mound " interface” in the upper aqueous phase is formed as the heavy liquid comes down into the light one, and it can be further evolved into a plume flow with the enhancement of the imbalance between density and pressure at the " interface”. Due to the difference in mass transfer characteristic caused by different concentration gradients in the plume " interface”, the plumes can also evolve into weak plumes and strong plumes. Under the large acetone concentration gradient, a number of RBM convective structures are generated near the interface in a short time and the convective cloud is formed due to the dramatic interaction and coalescence between these structures. With the weakening of mass transfer, the convective cloud disappears and the strong plume is gradually formed. In addition, the strength of RBM convection is demonstrated to be positively correlated with the acetone concentration gradient across the aqueous solution- toluene interface. In addition, the roughness of the interface and its unsteady fluctuation grow up with the increase of acetone concentration gradient across the interface.-
Keywords:
- liquid-liquid mass transfer /
- shadowgraph method /
- Rayleigh-Bénard-Marangoni convective structure /
- interface morphology
[1] Yao F, Chen Y P, Peterson G P 2013 Int. J. Heat Mass Transfer 64 418
 Google Scholar
Google Scholar
[2] Liu X D, Chen Y P, Shi M H 2013 Int. J. Therm. Sci. 65 224
 Google Scholar
Google Scholar
[3] Chen Y P, Cheng P 2005 Int. J. Heat Mass Transfer 32 931
 Google Scholar
Google Scholar
[4] Bodenschatz E, Pesch W, Ahlers G 2000 Annu. Rev. Fluid Mech. 32 709
 Google Scholar
Google Scholar
[5] 王飞, 彭岚, 张全壮, 刘佳 2015 64 140202
 Google Scholar
Google Scholar
Wang F, Peng L, Zhang Q Z, Liu J 2015 Acta Phys. Sin. 64 140202
 Google Scholar
Google Scholar
[6] 翟薇, 王楠, 魏炳波 2007 56 2353
 Google Scholar
Google Scholar
Zhai W, Wang N, Wei B B 2007 Acta Phys. Sin. 56 2353
 Google Scholar
Google Scholar
[7] Schwabe D 1999 Adv. Space Res. 24 1347
 Google Scholar
Google Scholar
[8] Touazi O, Chénier E, Doumenc F, Guerrier B 2010 Int. J. Heat Mass Transfer 53 656
 Google Scholar
Google Scholar
[9] Chen J, Yang C, Mao Z S 2015 Eur. Phys. J. Spec. Top. 224 389
 Google Scholar
Google Scholar
[10] Bo Z, Mao S, Han Z J, Cen K F, Chen J H, Kostya O 2015 Chem. Soc. Rev. 44 2018
[11] 张婷, 施保昌, 柴振华 2015 64 254701
Zhang T, Shi B C, Chai Z H 2015 Acta Phys. Sin. 64 254701
[12] 郑连存, 盛晓艳, 张欣欣 2006 55 5298
 Google Scholar
Google Scholar
Zheng L C, Sheng X Y, Zhang X X 2006 Acta Phys. Sin. 55 5298
 Google Scholar
Google Scholar
[13] Kline J L, Hager J D 2016 Matter Radiat. Extremes 2 16
[14] Dong S X, Han W, Liu M F, Zhang Z W, Li B, Ge L Q 2016 Colloids Surf. A 509 32
 Google Scholar
Google Scholar
[15] Bai L, Zhao S F, Fu Y H, Cheng Y 2016 Biochem. Eng. J. 298 281
[16] Sun Z F 2012 Chem. Eng. Sci. 68 579
 Google Scholar
Google Scholar
[17] Liu C, Zeng A, Yuan X, Yu G 2008 Chem. Eng. Res. Des. 86 201
 Google Scholar
Google Scholar
[18] Alvarez-Herrera C, Moreno-Hernández D, Barrientos-García B, Guerrero-Viramontes J A 2005 Opt. Laser Technol. 41 233
[19] Piekarska W, Kubiak M 2013 Appl. Math. Modell. 37 2051
 Google Scholar
Google Scholar
[20] Szymczyk J A 1991 Can. J. Chem. Eng. 69 1233
[21] Okhotsimskii A, Hozawa M 1998 Chem. Eng. Sci. 53 2547
 Google Scholar
Google Scholar
[22] 王勇, 张泽廷 2002 北京化工大学学报 29 11
 Google Scholar
Google Scholar
Wang Y, Zhang Z T 2002 J. Beijing Univ. Chem. Technol. 29 11
 Google Scholar
Google Scholar
[23] Sun Z F, Yu K T, S Y W, Miao Y Z 2002 Ind. Eng. Chem. Res. 41 1905
 Google Scholar
Google Scholar
[24] 沙勇, 李樟云, 林芬芬, 吐芬, 肖宗源, 叶李艺 2010 化工学报 61 844
Sha Y, Li Z Y, Lin F F, Tu F, Xiao Z Y, Ye L Y 2010 J. Chem. Ind. Eng. 61 844
[25] Orell A, Westwater J W 1961 AlChE J. 8 350
[26] Zhang S H, Wang Z M, Su Y F 1990 Chem. Eng. Res. Des. 68 84
[27] Guzun-Stoica A, Kurzeluk M, Floarea O 2000 Chem. Eng. Sci. 55 3813
 Google Scholar
Google Scholar
[28] Kostarev K G, Shmyrov A V, Zuev A L, Viviani A 2011 Exp. Fluids 51 457
 Google Scholar
Google Scholar
[29] Chen Y, Cheng P 2005 Int. Commun. Heat Mass Transfer 32 175
 Google Scholar
Google Scholar
[30] Agble D, Mendes-Tatsis M A 2000 Int. J. Heat Mass Transfer 43 1025
 Google Scholar
Google Scholar
[31] Shi Y, Kerstin E 2007 Chin. J. Chem. Eng. 15 748
 Google Scholar
Google Scholar
[32] Chen Y P, Liu X D, Shi M H 2013 Appl. Phys. Lett. 102 051609
 Google Scholar
Google Scholar
[33] Chen Y P, Liu X D, Zhao Y J 2015 Appl. Phys. Lett. 106 141601
 Google Scholar
Google Scholar
[34] Chen Y P, Wu L Y, Zhang L 2015 Int. J. Heat Mass Transfer 82 42
 Google Scholar
Google Scholar
[35] Sharp D H 1984 Physica D 12 3
 Google Scholar
Google Scholar
[36] Roberts M S, Jacobs J W 2015 J. Fluid Mech. 787 50
[37] 胡楠, 张会书, 傅强, 李陆星, 袁希钢 2016 化工学报 68 584
Hu L, Zhang H S, Fu Q, Li L X, Yuan X G 2016 J. Chem. Ind. Eng. 68 584
[38] Puthenveettil B A, Arakeri J H 2005 J. Fluid Mech. 542 217
 Google Scholar
Google Scholar
[39] Yang C, Tartaglino U, Persson B N 2006 J. Phys. Rev. Lett. 97 11
-
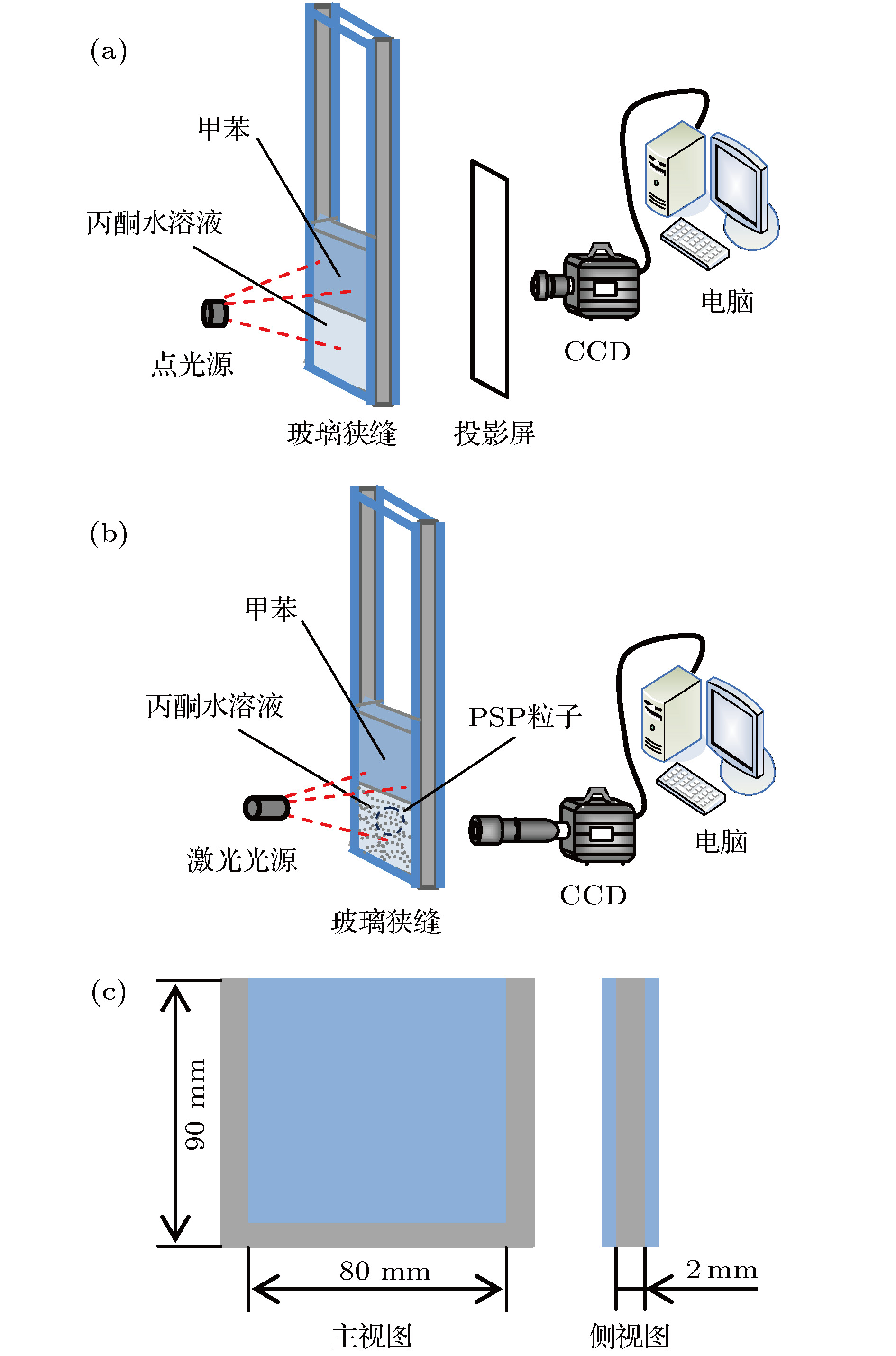
图 1 RBM对流的实验系统图 (a) 阴影法实验系统图; (b) 示踪粒子法实验系统图; (c) 玻璃狭缝尺寸图
Fig. 1. Schematic diagram of the experimental system for RBM convection: (a) Schematic diagram of the experimental system based on shadowgraph method; (b) schematic diagram of the experimental system based on particle tracer method; (c) size of the glass slit.
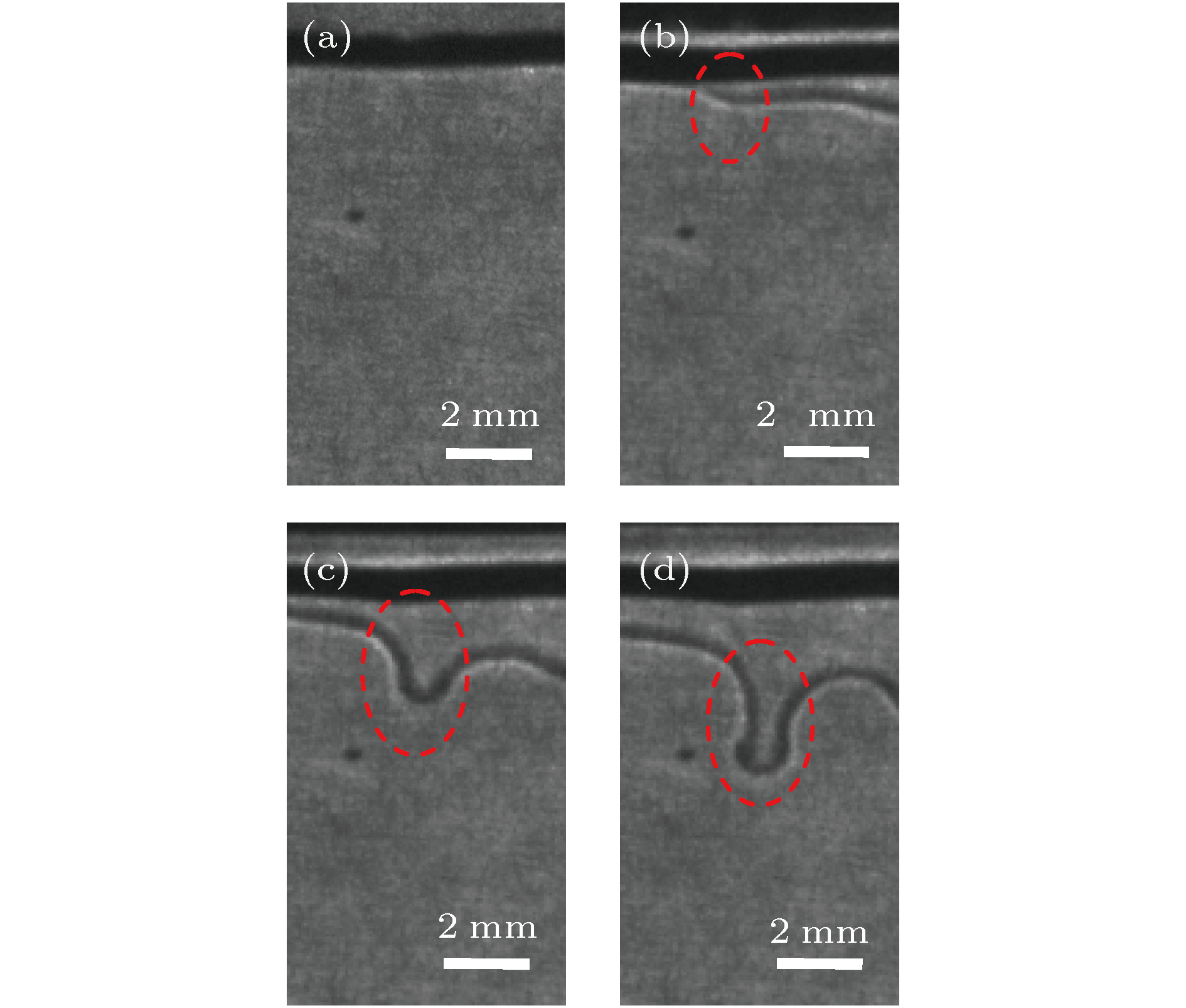
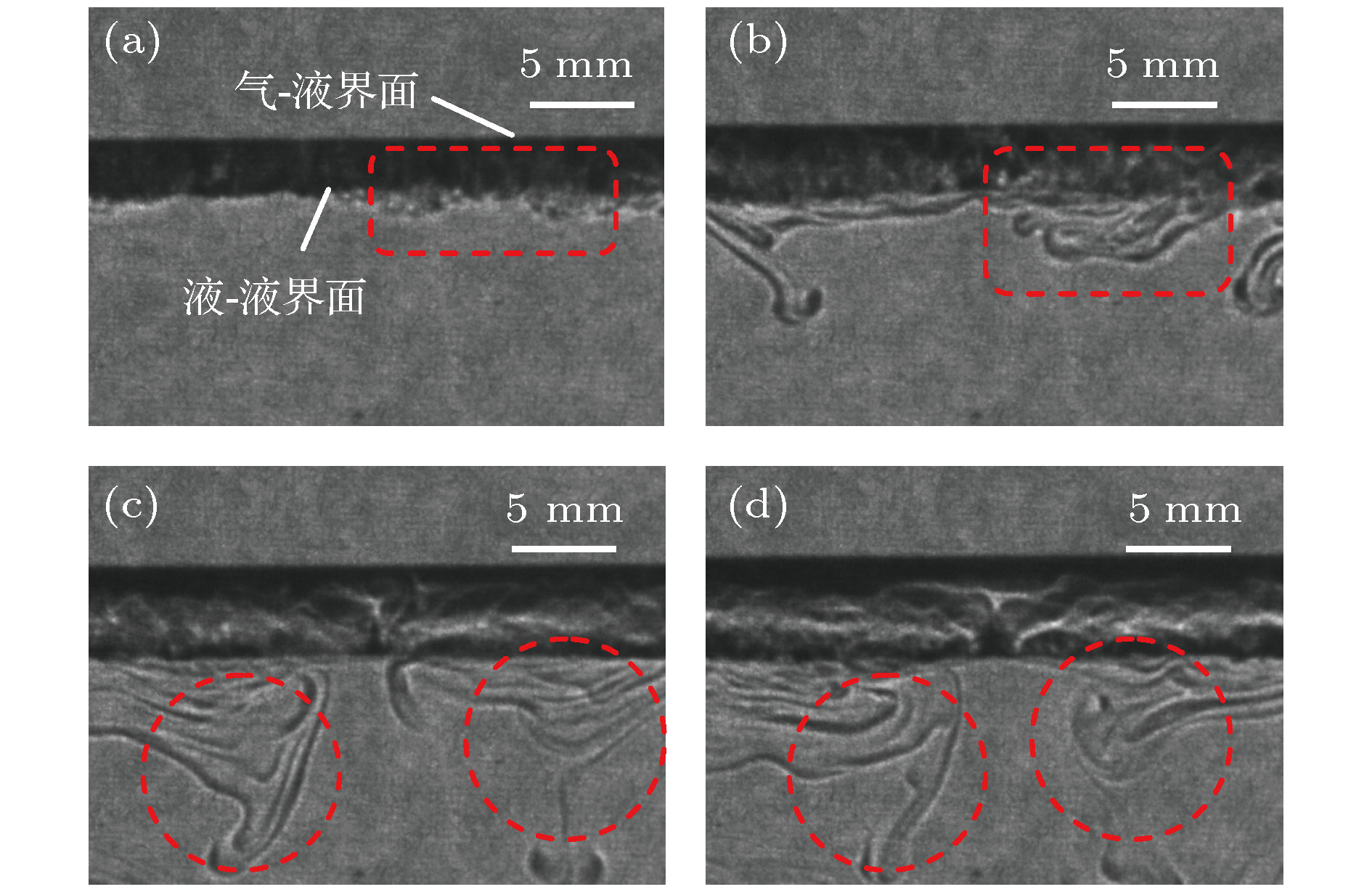
图 6 丘状“界面”的形成过程(水相丙酮初始体积浓度
${\varphi _0} = 5\% $ , 甲苯相丙酮初始体积浓度${\varphi _1} = 0\% $ ) (a) t = 13 s; (b) t = 30 s; (c) t = 36 s; (d) t = 42 sFig. 6. The forming process of the mound “interface” (the initial volume concentration of acetone in aqueous phase
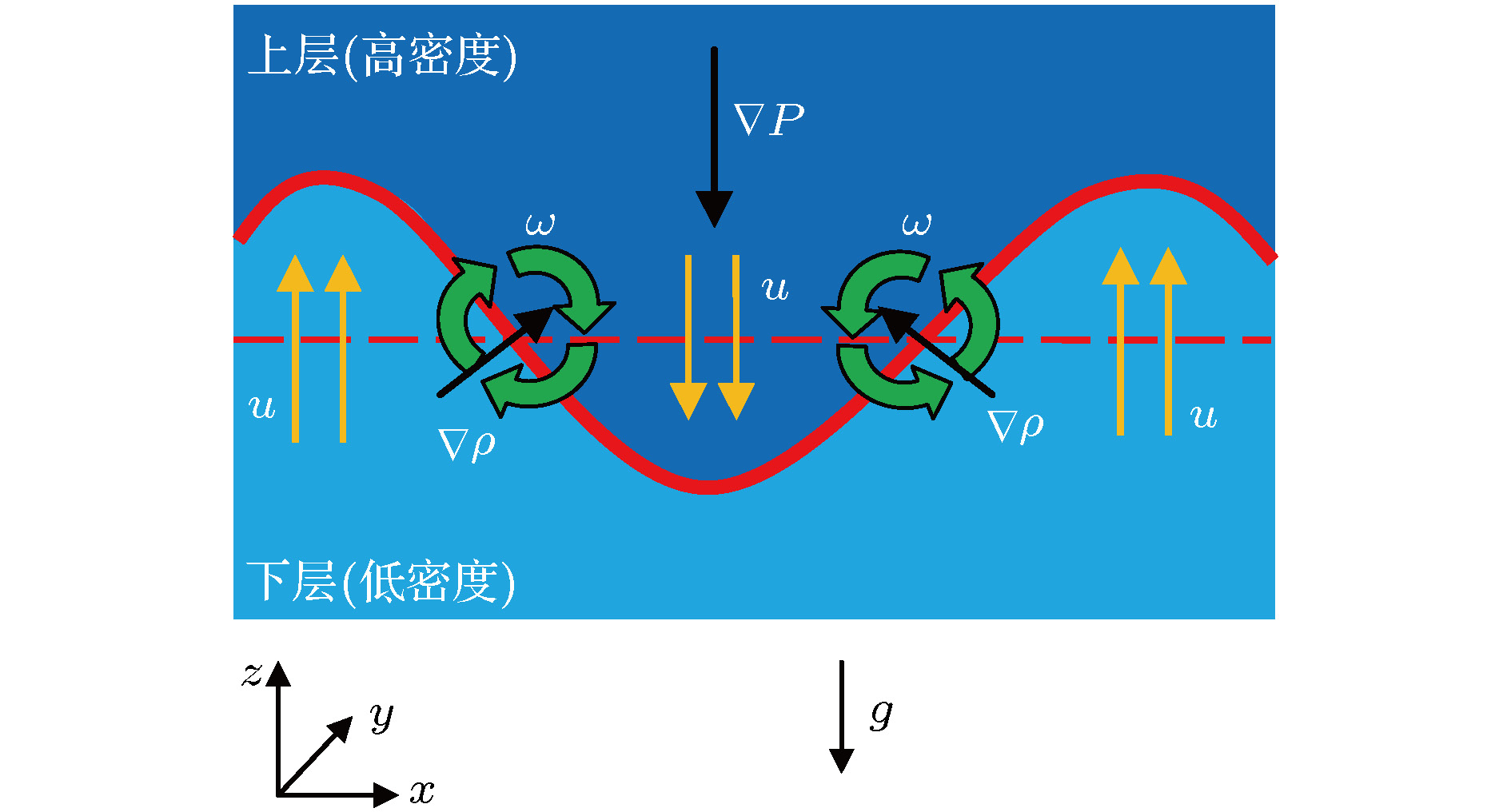
${\varphi _0} = 5\% $ , the initial volume concentration of acetone in the toluene phase${\varphi _1} = 0\% $ ): (a) t = 13 s; (b) t = 30 s; (c) t = 36 s; (d) t = 42 s.图 5 密度分层引起的Rayleigh-Taylor不稳定性示意图
$\omega $ 是涡流, P是压力,$\rho $ 是密度, u是速度, g是重力加速度; 粗的环形箭头表示涡旋产生的速度场Fig. 5. Schematic diagram of Rayleigh-Taylor instability caused by density stratification.
$\omega $ is vorticity, P is pressure,$\rho $ is density, u is velocity and g is acceleration of gravity; the thick circular arrows represent the velocity field created by the vortex.图 11 强羽状流向弱羽状流的演变过程(
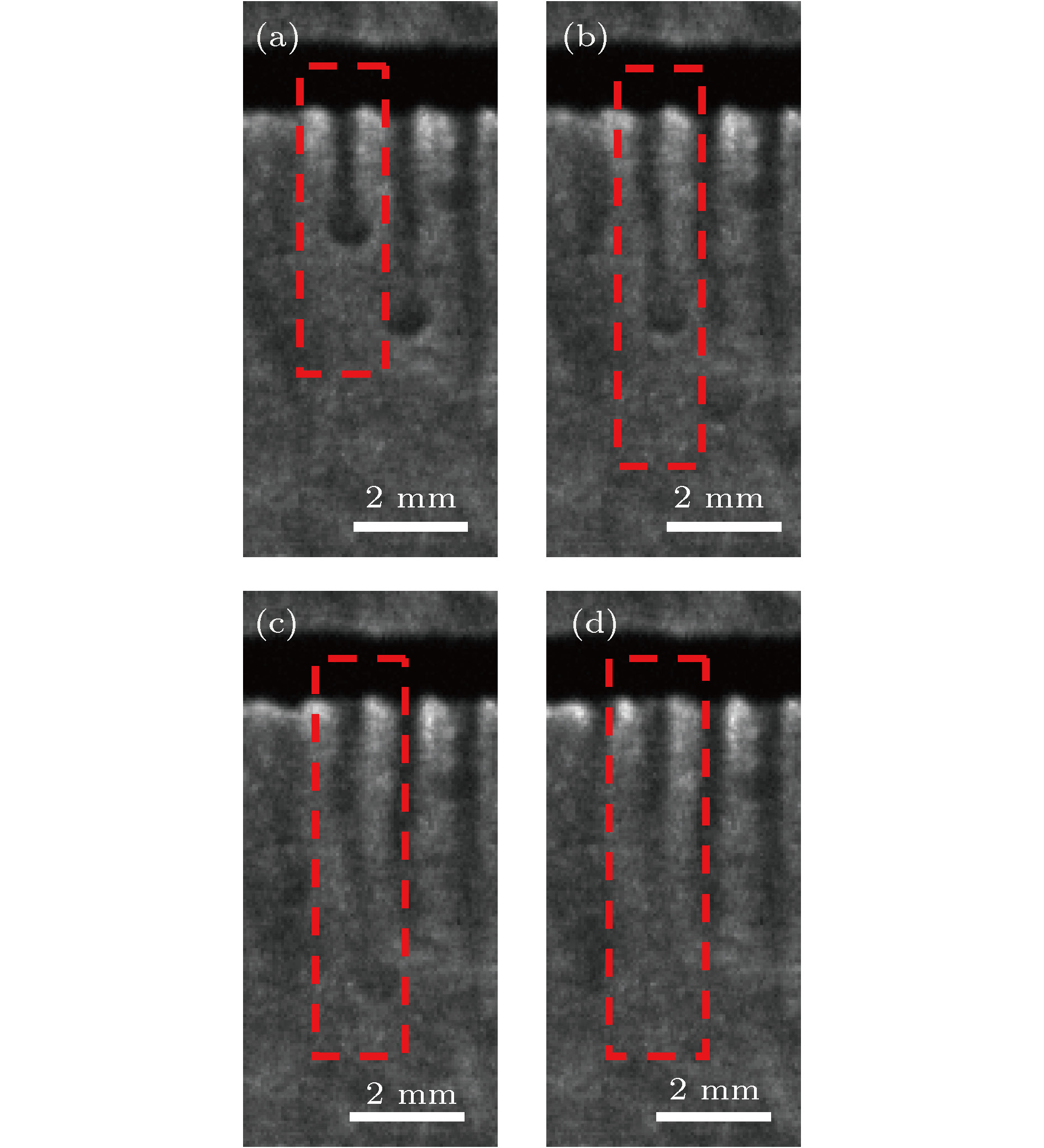
${\varphi _0} = 15\% $ ,${\varphi _1} = 0\% $ ) (a) t = 228 s; (b) t = 234 s; (c) t = 238 s; (d) t = 245 sFig. 11. The evolution of the strong plume flow to the weak plume flow (
${\varphi _0} = 15\% $ ,${\varphi _1} = 0\% $ ): (a) t = 228 s; (b) t = 234 s; (c) t = 238 s; (d) t = 245 s.图 12 传质初期对流结构的聚并过程(
${\varphi _0} \!=\! 30\% $ ,${\varphi _1} \!=\! 0\% $ ) (a) t = 15 s; (b) t = 20 s; (c) t = 24 s; (d) t = 30 sFig. 12. Convergence process of convective structure at the beginning of the mass transfer (
${\varphi _0} = 30\% $ ,${\varphi _1} = 0\% $ ): (a) t = 15 s; (b) t = 20 s; (c) t = 24 s; (d) t = 30 s.图 13 对流团的消失以及强羽状流的出现(
${\varphi _0} = 30\% $ ,${\varphi _1} = 0\% $ ) (a) t = 101 s; (b) t = 196 s; (c) t = 259 s; (d) t = 350 sFig. 13. The disappearance of convective cloud and the appearance of the strong plume (
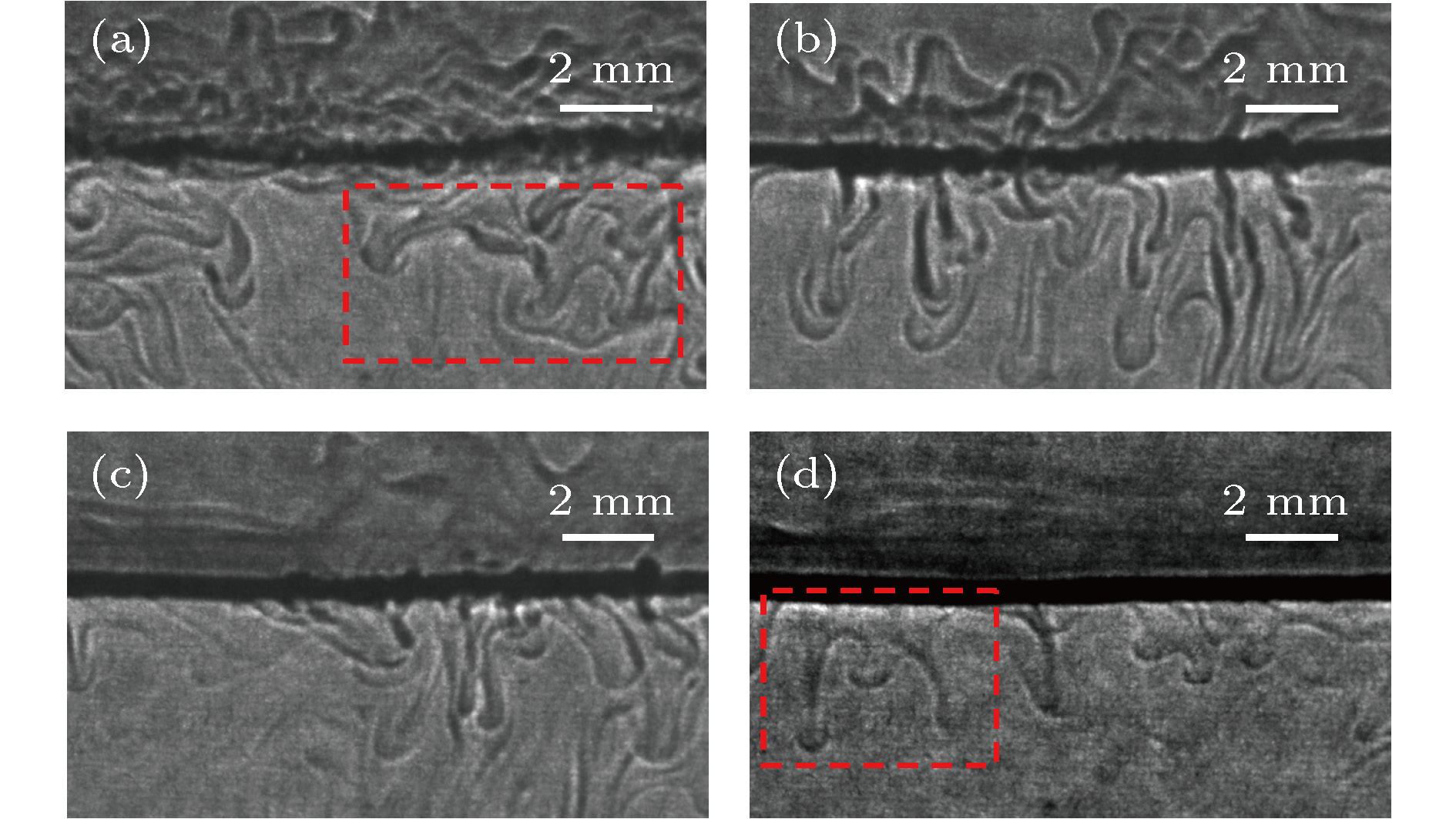
${\varphi _0} = 30\% $ ,${\varphi _1} = 0\% $ ): (a) t = 101 s; (b) t = 196 s; (c) t = 259 s; (d) t = 350 s.图 17 不同甲苯相丙酮初始浓度下的投影图像(t = 35 s,
${\varphi _0} = 20\% $ ) (a)${\varphi _1} = 5\% $ ; (b)${\varphi _1} = 7.5\% $ ; (c)${\varphi _1} = 10\% $ ; (d)${\varphi _1} = 15\% $ Fig. 17. Schlieren images under different initial concentrations of acetone in the toluene phase (t = 35 s,
${\varphi _0} = 20\% $ ): (a)${\varphi _1} = 5\% $ (b)${\varphi _1} = 7.5\% $ ; (c)${\varphi _1} = 10\% $ ; (d)${\varphi _1} = 15\% $ .表 1 实验试剂的物性参数(T = 20 ℃, P = 0.1 MPa)
Table 1. The physical parameters of experimental reagents (T = 20 ℃, P = 0.1 MPa).
实验试剂 ρ/kg·m–3 µ/10–4Pa·s 水 998 10.04 甲苯 867 5.86 丙酮 790 3.26 -
[1] Yao F, Chen Y P, Peterson G P 2013 Int. J. Heat Mass Transfer 64 418
 Google Scholar
Google Scholar
[2] Liu X D, Chen Y P, Shi M H 2013 Int. J. Therm. Sci. 65 224
 Google Scholar
Google Scholar
[3] Chen Y P, Cheng P 2005 Int. J. Heat Mass Transfer 32 931
 Google Scholar
Google Scholar
[4] Bodenschatz E, Pesch W, Ahlers G 2000 Annu. Rev. Fluid Mech. 32 709
 Google Scholar
Google Scholar
[5] 王飞, 彭岚, 张全壮, 刘佳 2015 64 140202
 Google Scholar
Google Scholar
Wang F, Peng L, Zhang Q Z, Liu J 2015 Acta Phys. Sin. 64 140202
 Google Scholar
Google Scholar
[6] 翟薇, 王楠, 魏炳波 2007 56 2353
 Google Scholar
Google Scholar
Zhai W, Wang N, Wei B B 2007 Acta Phys. Sin. 56 2353
 Google Scholar
Google Scholar
[7] Schwabe D 1999 Adv. Space Res. 24 1347
 Google Scholar
Google Scholar
[8] Touazi O, Chénier E, Doumenc F, Guerrier B 2010 Int. J. Heat Mass Transfer 53 656
 Google Scholar
Google Scholar
[9] Chen J, Yang C, Mao Z S 2015 Eur. Phys. J. Spec. Top. 224 389
 Google Scholar
Google Scholar
[10] Bo Z, Mao S, Han Z J, Cen K F, Chen J H, Kostya O 2015 Chem. Soc. Rev. 44 2018
[11] 张婷, 施保昌, 柴振华 2015 64 254701
Zhang T, Shi B C, Chai Z H 2015 Acta Phys. Sin. 64 254701
[12] 郑连存, 盛晓艳, 张欣欣 2006 55 5298
 Google Scholar
Google Scholar
Zheng L C, Sheng X Y, Zhang X X 2006 Acta Phys. Sin. 55 5298
 Google Scholar
Google Scholar
[13] Kline J L, Hager J D 2016 Matter Radiat. Extremes 2 16
[14] Dong S X, Han W, Liu M F, Zhang Z W, Li B, Ge L Q 2016 Colloids Surf. A 509 32
 Google Scholar
Google Scholar
[15] Bai L, Zhao S F, Fu Y H, Cheng Y 2016 Biochem. Eng. J. 298 281
[16] Sun Z F 2012 Chem. Eng. Sci. 68 579
 Google Scholar
Google Scholar
[17] Liu C, Zeng A, Yuan X, Yu G 2008 Chem. Eng. Res. Des. 86 201
 Google Scholar
Google Scholar
[18] Alvarez-Herrera C, Moreno-Hernández D, Barrientos-García B, Guerrero-Viramontes J A 2005 Opt. Laser Technol. 41 233
[19] Piekarska W, Kubiak M 2013 Appl. Math. Modell. 37 2051
 Google Scholar
Google Scholar
[20] Szymczyk J A 1991 Can. J. Chem. Eng. 69 1233
[21] Okhotsimskii A, Hozawa M 1998 Chem. Eng. Sci. 53 2547
 Google Scholar
Google Scholar
[22] 王勇, 张泽廷 2002 北京化工大学学报 29 11
 Google Scholar
Google Scholar
Wang Y, Zhang Z T 2002 J. Beijing Univ. Chem. Technol. 29 11
 Google Scholar
Google Scholar
[23] Sun Z F, Yu K T, S Y W, Miao Y Z 2002 Ind. Eng. Chem. Res. 41 1905
 Google Scholar
Google Scholar
[24] 沙勇, 李樟云, 林芬芬, 吐芬, 肖宗源, 叶李艺 2010 化工学报 61 844
Sha Y, Li Z Y, Lin F F, Tu F, Xiao Z Y, Ye L Y 2010 J. Chem. Ind. Eng. 61 844
[25] Orell A, Westwater J W 1961 AlChE J. 8 350
[26] Zhang S H, Wang Z M, Su Y F 1990 Chem. Eng. Res. Des. 68 84
[27] Guzun-Stoica A, Kurzeluk M, Floarea O 2000 Chem. Eng. Sci. 55 3813
 Google Scholar
Google Scholar
[28] Kostarev K G, Shmyrov A V, Zuev A L, Viviani A 2011 Exp. Fluids 51 457
 Google Scholar
Google Scholar
[29] Chen Y, Cheng P 2005 Int. Commun. Heat Mass Transfer 32 175
 Google Scholar
Google Scholar
[30] Agble D, Mendes-Tatsis M A 2000 Int. J. Heat Mass Transfer 43 1025
 Google Scholar
Google Scholar
[31] Shi Y, Kerstin E 2007 Chin. J. Chem. Eng. 15 748
 Google Scholar
Google Scholar
[32] Chen Y P, Liu X D, Shi M H 2013 Appl. Phys. Lett. 102 051609
 Google Scholar
Google Scholar
[33] Chen Y P, Liu X D, Zhao Y J 2015 Appl. Phys. Lett. 106 141601
 Google Scholar
Google Scholar
[34] Chen Y P, Wu L Y, Zhang L 2015 Int. J. Heat Mass Transfer 82 42
 Google Scholar
Google Scholar
[35] Sharp D H 1984 Physica D 12 3
 Google Scholar
Google Scholar
[36] Roberts M S, Jacobs J W 2015 J. Fluid Mech. 787 50
[37] 胡楠, 张会书, 傅强, 李陆星, 袁希钢 2016 化工学报 68 584
Hu L, Zhang H S, Fu Q, Li L X, Yuan X G 2016 J. Chem. Ind. Eng. 68 584
[38] Puthenveettil B A, Arakeri J H 2005 J. Fluid Mech. 542 217
 Google Scholar
Google Scholar
[39] Yang C, Tartaglino U, Persson B N 2006 J. Phys. Rev. Lett. 97 11
计量
- 文章访问数: 12095
- PDF下载量: 202
- 被引次数: 0













 下载:
下载: