-
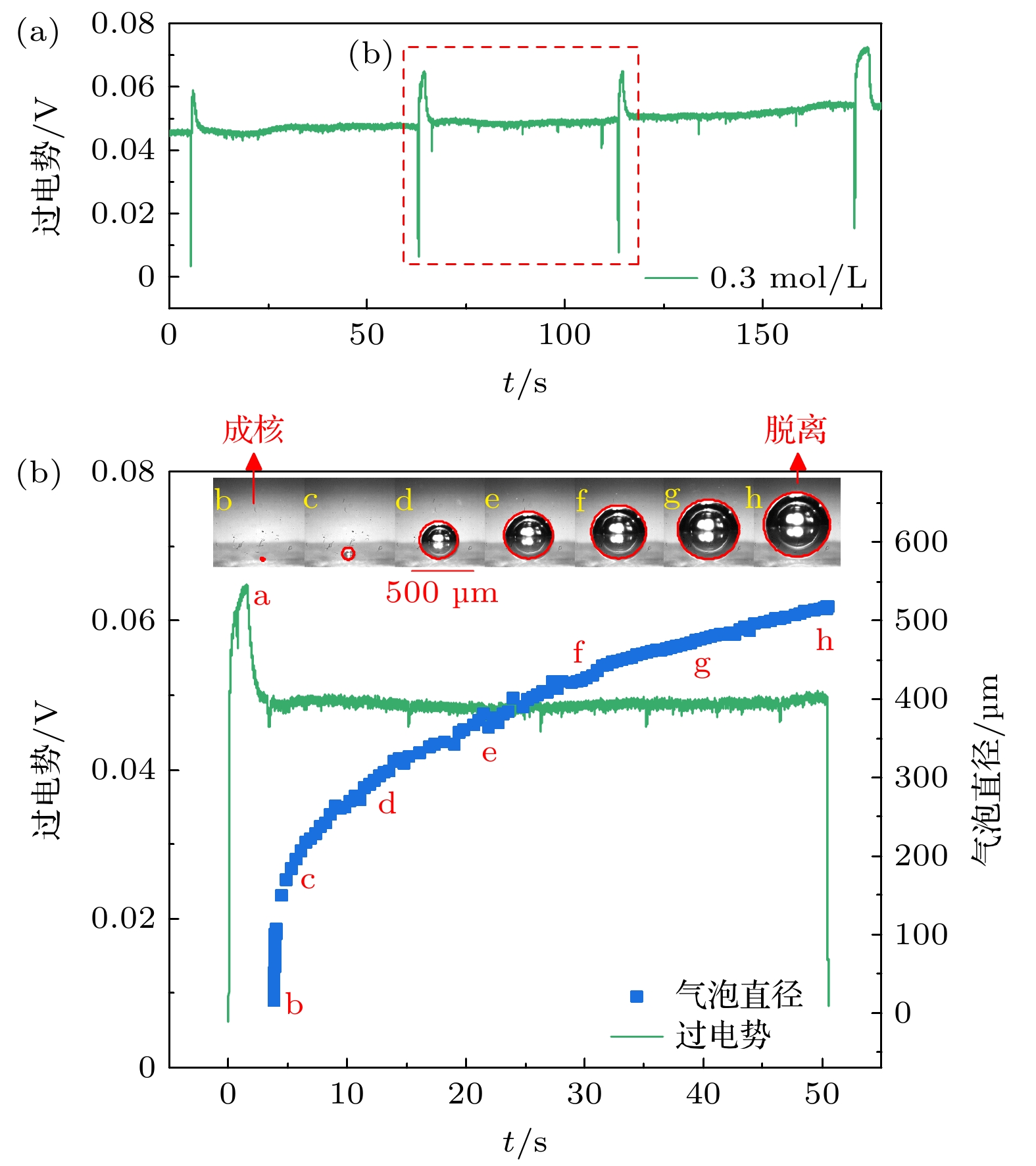
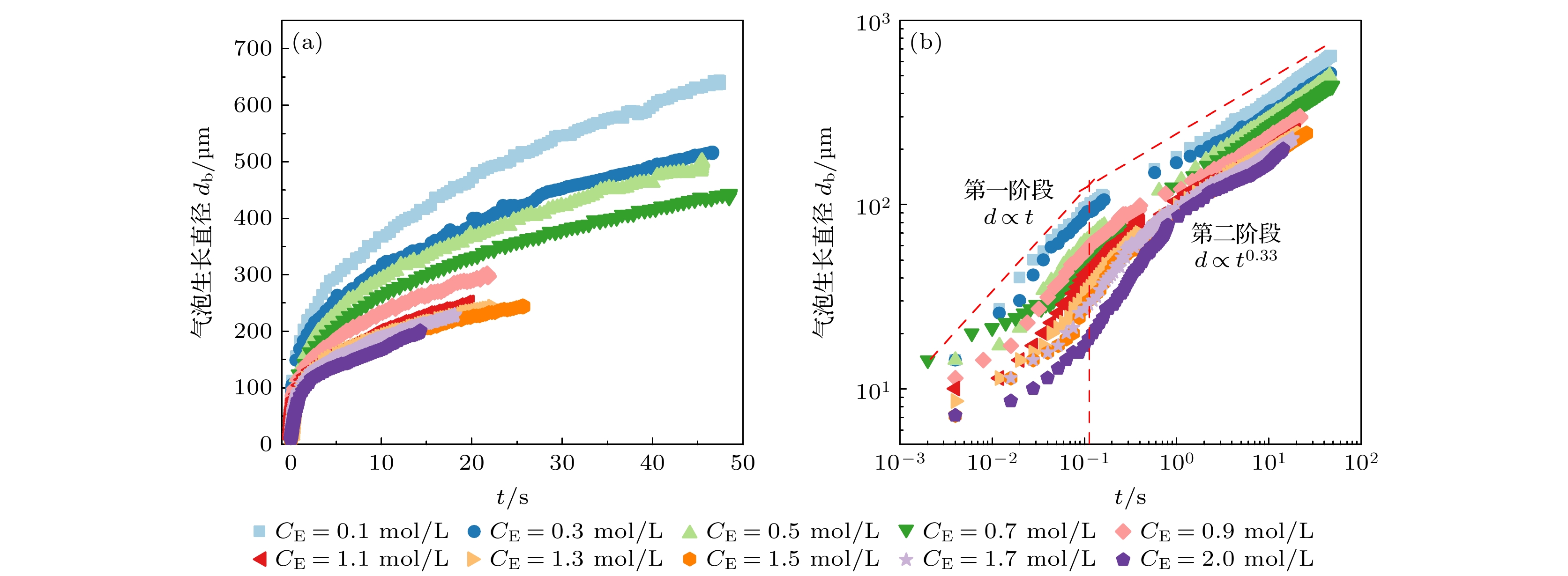
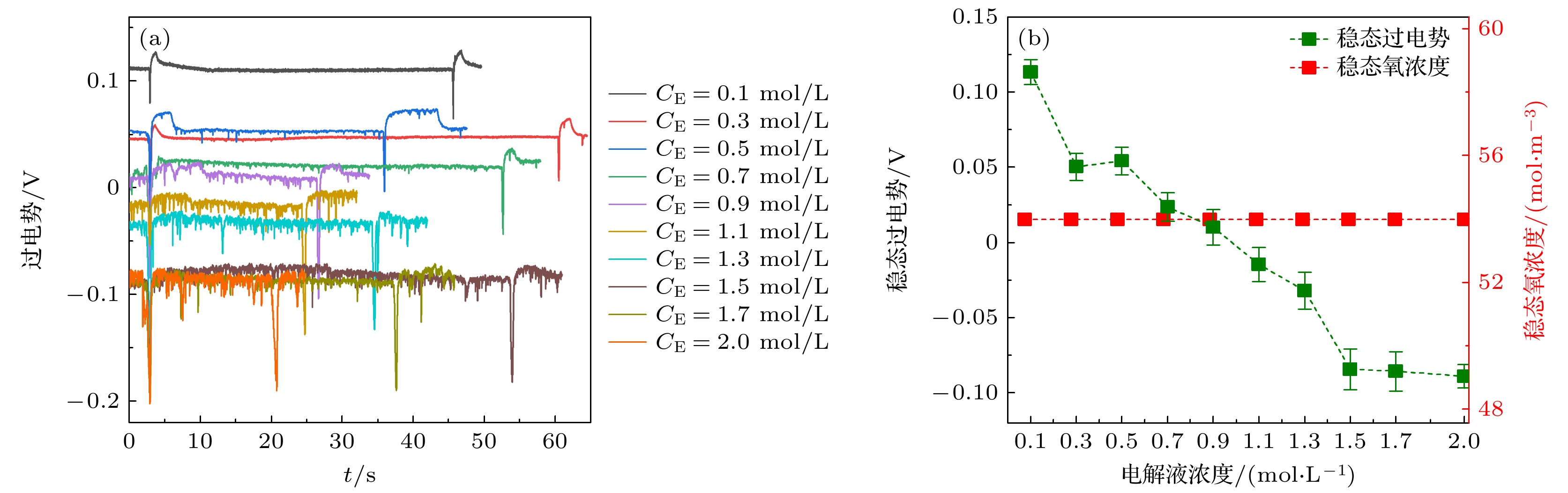
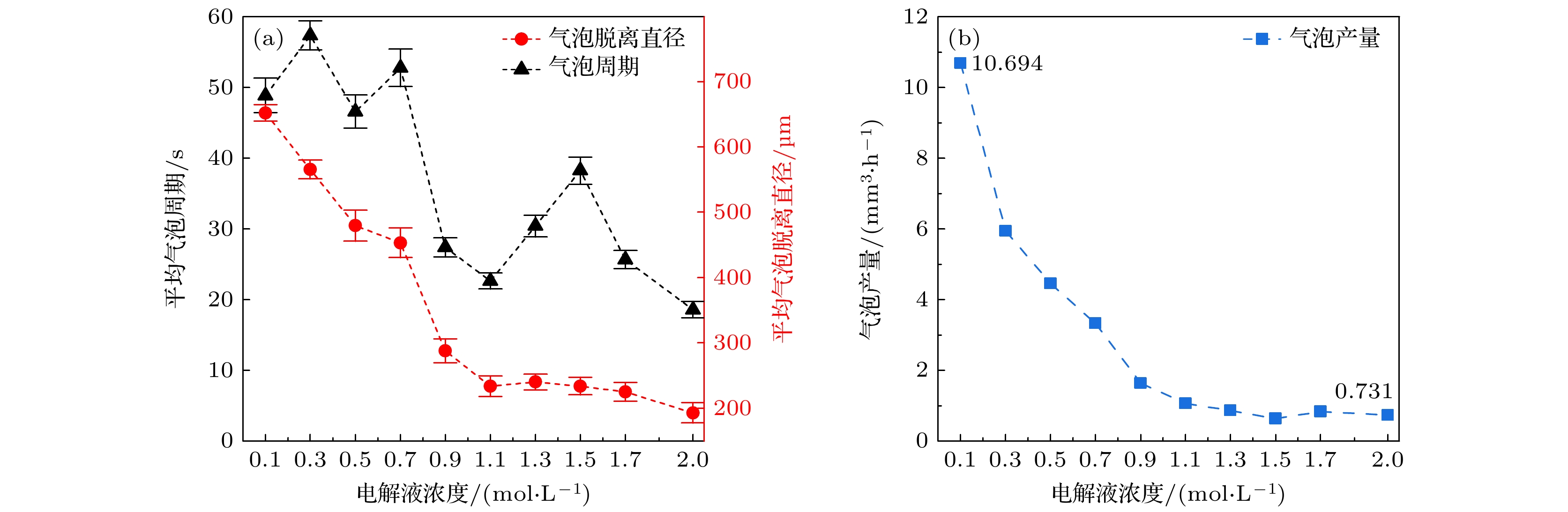
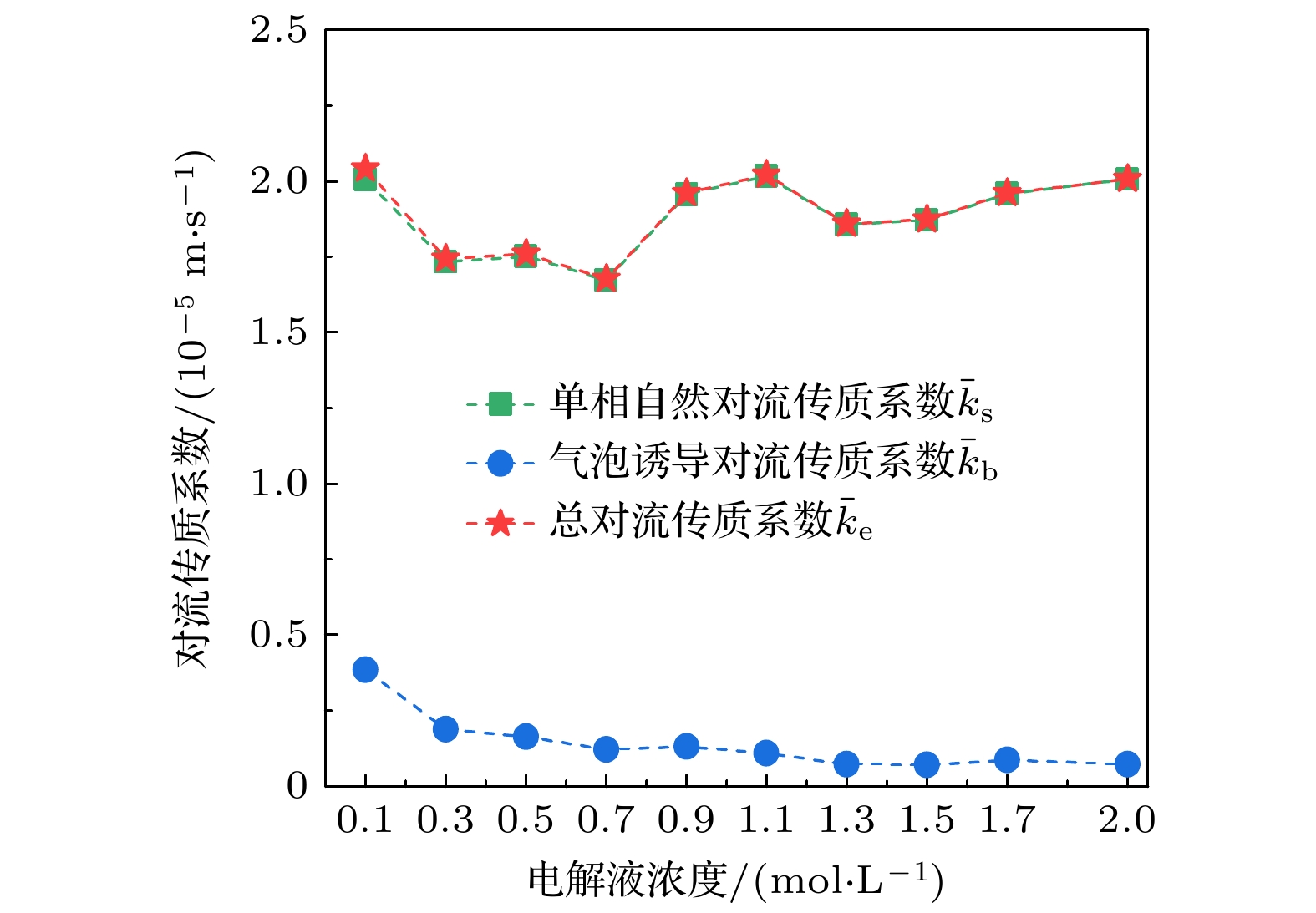
在光电化学分解水反应中, 气泡会覆盖光电极表面的反应区域, 从而影响反应阻抗和气液传质. 本文搭建了激光照射系统, 并将其与电化学工作站、高速显微成像系统耦合. 在不同电解液浓度下(Na2SO4, 0.1—2.0 mol/L), 研究了TiO2析气光电极表面单个氧气泡的演化行为及其传质特性. 随着电解液浓度从0.1 mol/L升高到2.0 mol/L, 溶液电阻与气泡附加电阻降低, 气泡稳定生长阶段的过电势从0.113 V下降到–0.089 V. 气泡在成核、生长和脱离阶段会引起过电势的波动, 这与液相中溶解气浓度的变化引起的阻抗变化相吻合. 通过分析析气效率与气泡覆盖率的关联, 发现电解液浓度提高会导致气泡覆盖率和析气效率同步下降. 通过计算舍伍德无量纲数, 发现总对流传质系数随着电解液浓度的增大而增大; 单相自然对流在气体产物传递过程中占据主导地位, 其传质系数比气泡诱导对流传质系数大一个数量级.In the photoelectrochemical water splitting reaction system, bubbles will cover the reaction area on the photoelectrode surface, affecting the reaction impedance and gas-liquid mass transfer. A laser irradiation system is built and it is coupled with an electrochemical workstation and high-speed microscopic imaging system. The evolution behavior and mass transfer characteristics of single O2 bubble on the TiO2 photoelectrode are studied at different electrolyte concentrations (Na2SO4, 0.1–2.0 mol/L). With the increase of electrolyte concentration from 0.1 mol/L to 2.0 mol/L, the solution resistance and bubble additional resistance decrease, and the overpotential in the stable growth stage of bubble decreases from 0.113 V to –0.089 V. The bubble will cause the fluctuation of overpotential in the nucleation, growth and detachment stages, which is consistent with the impedance change caused by the change of dissolved oxygen concentration in the liquid phase. By analyzing the correlation between gas evolution efficiency and bubble coverage, it is found that the increase of electrolyte concentration will lead the bubble coverage and gas evolution efficiency to decrease simultaneously. By calculating the Sherwood dimensionless number, the results show that the total convective mass transfer coefficient increases with the electrolyte concentration increasing. Single-phase natural convection plays a dominant role in the process of gas product transfer, and its mass transfer coefficient is one order of magnitude larger than that of bubble-induced convection. In summary, by adjusting the electrolyte concentration, the bubble on the gas evolution photoelectrode surface can be effectively removed and the mass transfer of the system can be optimized, which is of great significance in improving the efficiency of photoelectrochemical water splitting.
[1] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[2] Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T 1997 Nature 388 431
 Google Scholar
Google Scholar
[3] 黄柏标, 王朋, 张晓阳, 秦晓燕, 戴瑛 2009 科学通报 54 847
 Google Scholar
Google Scholar
Huang B B, Wang P, Zhang X Y, Qin X Y, Dai Y 2009 Sci. Bull. 54 847
 Google Scholar
Google Scholar
[4] 陈娟雯, 郭烈锦, 胡晓玮, 曹振山 2018 工程热 39 550
Chen J W, Guo L J, Hu X W, Cao Z S 2018 J. Eng. Thermophys. 39 550
[5] 郭烈锦, 曹振山, 王晔春, 张博, 冯雨杨, 徐强 2023 西安交通大学学报 57 1
 Google Scholar
Google Scholar
Guo L J, Cao Z S, Wang Y C, Zhang B, Feng Y Y, Xu Q 2023 J. Xi'an Jiaotong Univ. 57 1
 Google Scholar
Google Scholar
[6] Tawfik M E, Diez F J 2014 Electrochim. Acta 146 792
 Google Scholar
Google Scholar
[7] Matsushima H, Iida T, Fukunaka Y 2012 J. Solid State Electrochem. 16 617
 Google Scholar
Google Scholar
[8] Li Y J, Zhang H C, Xu T H, Lu Z Y, Wu X C, Wan P B, Sun X M, Jiang L 2015 Adv. Funct. Mater. 25 1737
 Google Scholar
Google Scholar
[9] Chen J W, Guo L J 2019 Appl. Phys. Lett. 115 101602
 Google Scholar
Google Scholar
[10] Lu X, Lalwani S, Yuan L, Abdelsalam M A, AlMarzooqi F, Zhang T 2022 Int. J. Hydrogen Energ. 47 36504
 Google Scholar
Google Scholar
[11] Ansari S A, Khan M M, Ansari M O, Cho M H 2016 New J. Chem. 40 3000
 Google Scholar
Google Scholar
[12] Piontek S, Andronescu C, Zaichenko A, Konkena B, Puring K J, Marler B, Antoni H, Sinev I, Muhler M, Mollenhauer D, Roldan Cuenya B, Schuhmann W, Apfel U P 2018 ACS Catal. 8 987
 Google Scholar
Google Scholar
[13] Ye X, Xu Q, Nie T F, Luo X Y, Jiang S, Guo L J 2023 J. Phys. Chem. C 127 22085
 Google Scholar
Google Scholar
[14] Bashkatov A, Yang X, Mutschke G, Fritzsche B, Hossain S S, Eckert K 2021 Phys. Chem. Chem. Phys. 23 11818
 Google Scholar
Google Scholar
[15] Matsushima H, Fukunaka Y, Kuribayashi K 2006 Electrochim. Acta 51 4190
 Google Scholar
Google Scholar
[16] Lubetkin S D, Akhtar M 1996 J. Colloid Interf. Sci. 180 43
 Google Scholar
Google Scholar
[17] Baczyzmalski D, Karnbach F, Yang X, Mutschke G, Uhlemann M, Eckert K, Cierpka C 2016 J. Electrochem. Soc. 163 E248
 Google Scholar
Google Scholar
[18] Cao Z S, Zhang B, Feng Y Y, Xu Q, Wang Y C, Guo L J 2022 Electrochim. Acta 434 141293
 Google Scholar
Google Scholar
[19] Wang H P, Xu Z X, Lin W, Yang X, Gu X R, Zhu W, Zhuang Z B 2023 Nano Res. 16 420
 Google Scholar
Google Scholar
[20] Obata K, Stegenburga L, Takanabe K 2019 J. Phys. Chem. C 123 21554
 Google Scholar
Google Scholar
[21] Liu Y, Jiang J G, Xu Q, Li M T, Guo L J 2013 Mater. Res. Bull. 48 4548
 Google Scholar
Google Scholar
[22] Hu X W, Cao Z S, Wang Y C, Shen S, Guo L J, Chen J W 2016 Electrochim. Acta 202 175
 Google Scholar
Google Scholar
[23] Thoroddsen S T, Etoh T G, Takehara K 2008 Annu. Rev. Fluid Mech. 40 257
 Google Scholar
Google Scholar
[24] Rox H, Bashkatov A, Yang X, Loos S, Mutschke G, Gerbeth G, Eckert K 2022 Int. J. Hydrog. Energ. 48 2892
 Google Scholar
Google Scholar
[25] Vogt H 1993 Electrochim. Acta 38 1421
 Google Scholar
Google Scholar
[26] Lubetkin S, Blackwell M 1988 J. Colloid Interf. Sci. 126 610
 Google Scholar
Google Scholar
[27] Scriven L E 1959 Chem. Eng. Sci. 10 1
 Google Scholar
Google Scholar
[28] Chandran P, Bakshi S, Chatterjee D 2015 Chem. Eng. Sci. 138 99
 Google Scholar
Google Scholar
[29] Vogt H, Balzer R J 2005 Electrochim. Acta 50 2073
 Google Scholar
Google Scholar
[30] Wei J J, Xue Y F, Zhao J F, Li J 2011 Chin. Phys. Lett. 28 016401
 Google Scholar
Google Scholar
[31] Chen J W, Guo L J, Hu X W, Cao Z S, Wang Y C 2018 Electrochim. Acta 274 57
 Google Scholar
Google Scholar
[32] Wang M S, Nie T F, She Y, Tao L, Luo X Y, Xu Q, Guo L J 2023 Int. J. Hydrog. Energ. 48 23387
 Google Scholar
Google Scholar
[33] Bashkatov A, Hossain S S, Yang X, Mutschke G, Eckert K 2019 Phys. Rev. Lett. 123 214503
 Google Scholar
Google Scholar
[34] Vogt H 1993 Electrochim. Acta 38 1427
 Google Scholar
Google Scholar
[35] Vogt H 2017 Electrochim. Acta 235 495
 Google Scholar
Google Scholar
[36] Vogt H 2013 Electrochim. Acta 87 611
 Google Scholar
Google Scholar
[37] Shah A, Jorne J 1989 J. Electrochem. Soc. 136 144
 Google Scholar
Google Scholar
[38] Lochiel A C, Calderbank P H 1964 Chem. Eng. Sci. 19 471
 Google Scholar
Google Scholar
-
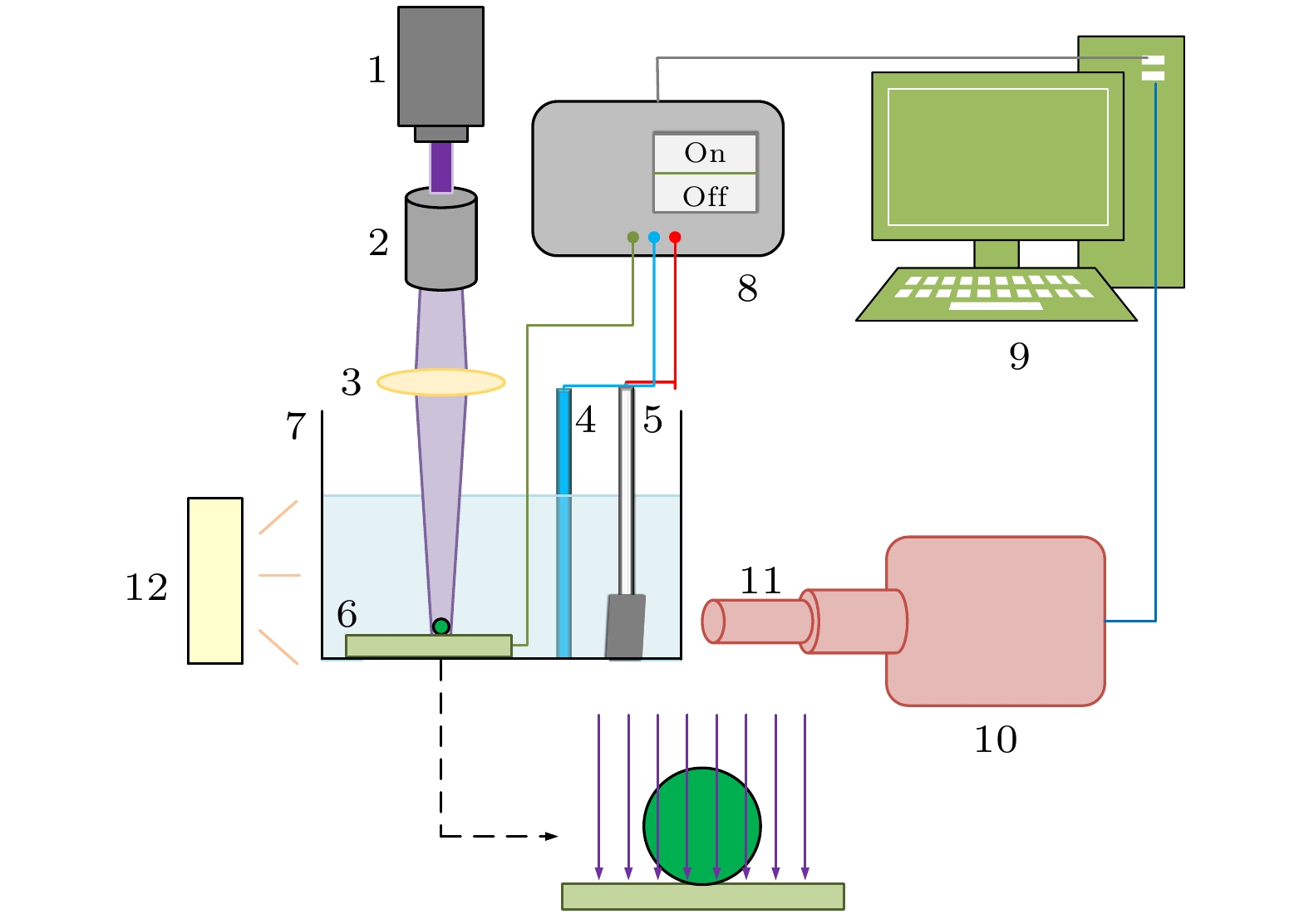
图 1 光电化学分解水实验系统图(1-紫外激光发生器; 2-光束扩展器; 3-透镜; 4-Ag/AgCl参比电极; 5-铂片对电极; 6-纳米棒状TiO2薄膜工作电极; 7-石英反应池; 8-电化学工作站; 9-计算机; 10-高速摄像机; 11-显微镜头; 12-LED照明光源)
Fig. 1. Experimental measurement system diagram (1-UV laser generator; 2-beam expander; 3-lens; 4-Ag/AgCl reference electrode; 5-platinum counter electrode; 6-nanorod TiO2 film working electrode; 7-quartz reaction cell; 8-electrochemical workstation; 9-computer; 10-high speed camera; 11-micro-lens; 12-LED lighting source).
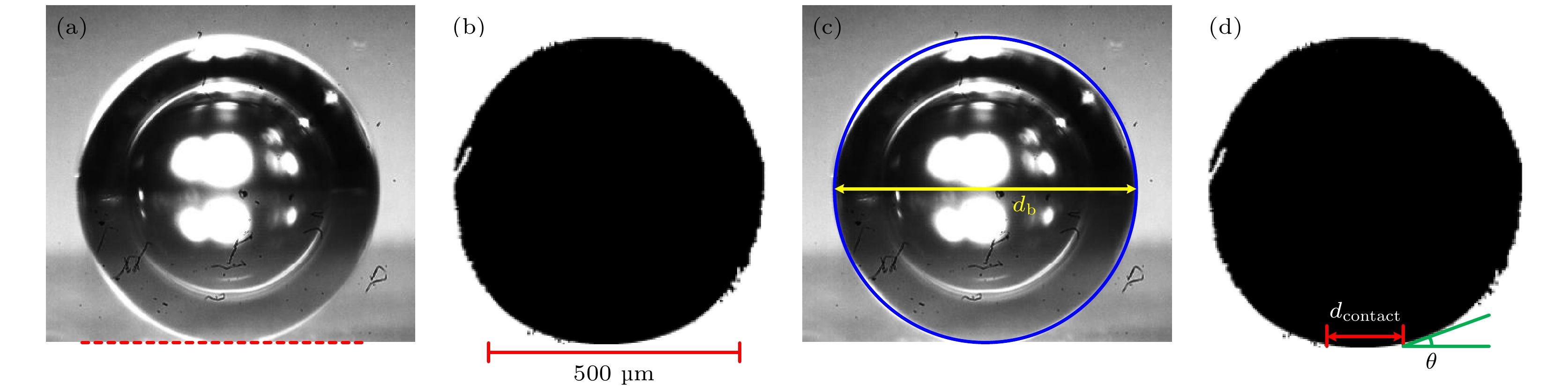
图 2 MATLAB程序处理气泡图像 (a)裁剪高速相机拍摄的气泡图像; (b)将图像转换为黑白二值图; (c)在黑白二值图上识别气泡直径的像素点; (d)在黑白二值图上识别气泡的接触直径和接触角
Fig. 2. Bubble images processed by Matlab program: (a) Cutting bubble image; (b) converting image into a black and white binary image; (c) identifying pixels of bubble diameter on the black and white binary image; (d) identifying contact diameter and contact angle of bubble.
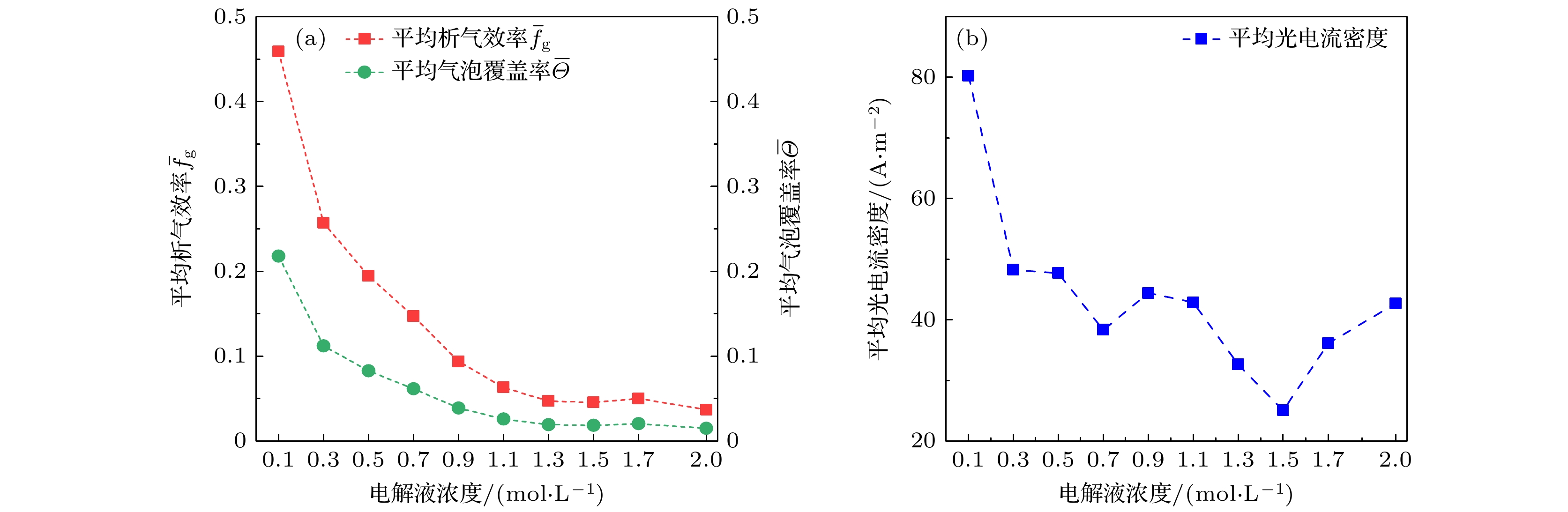
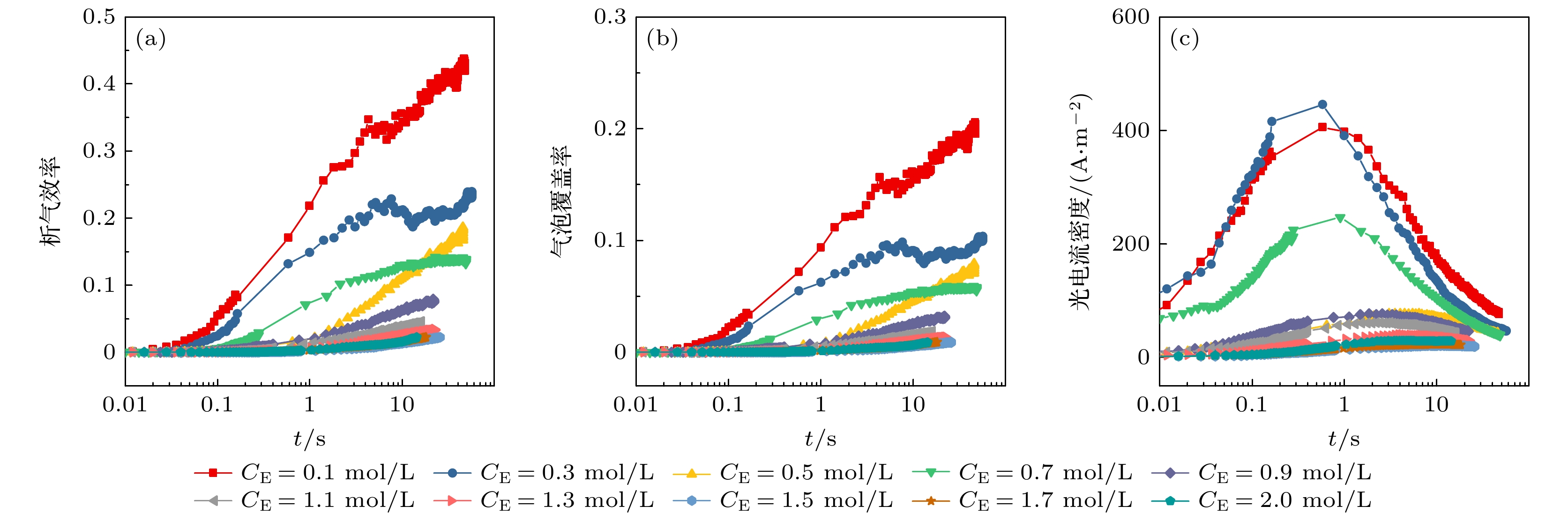
图 7 不同电解液浓度下的析气效率和气泡覆盖率 (a)电解液浓度为0.5 mol/L时析气效率和气泡覆盖率随气泡生长时间的变化; (b)平均析气效率和气泡覆盖率随电解液浓度的变化
Fig. 7. Gas evolution efficiency fg and bubble coverage Θ at different electrolyte concentration: (a) The variation curves of fg and Θ with bubble growth time when the electrolyte concentration is 0.5 mol/L; (b) the variation curves of $ {{\bar f}_{{\mathrm{g}}}} $ and $ \overline{\varTheta } $ with electrolyte concentration.
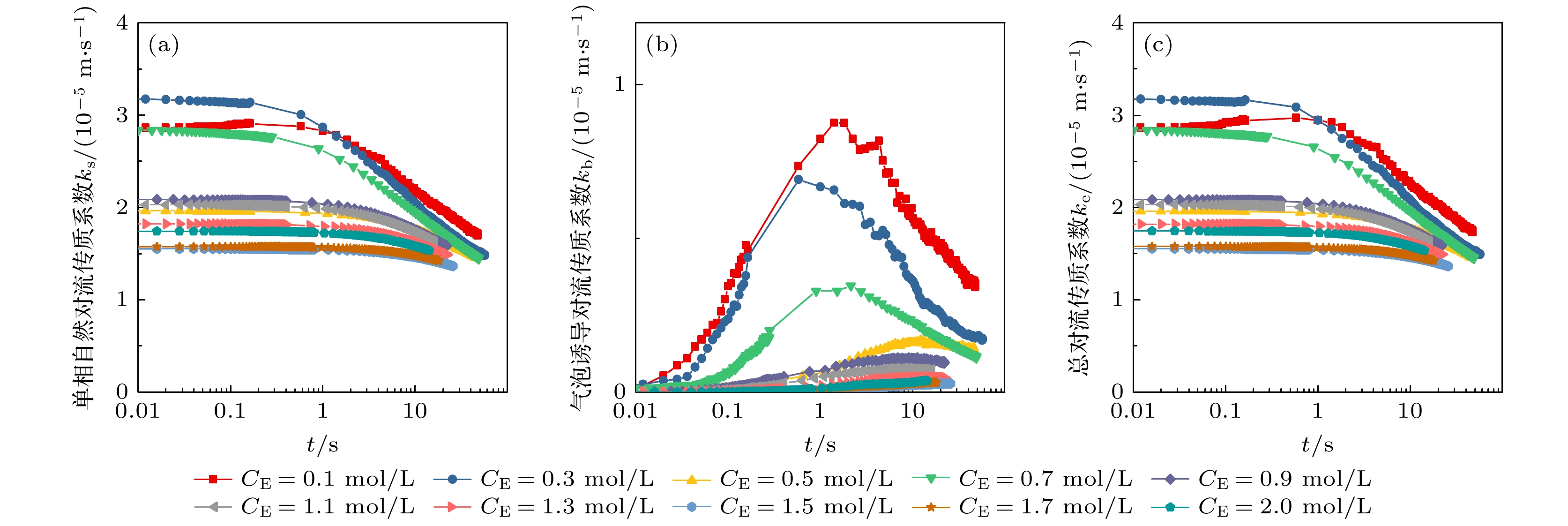
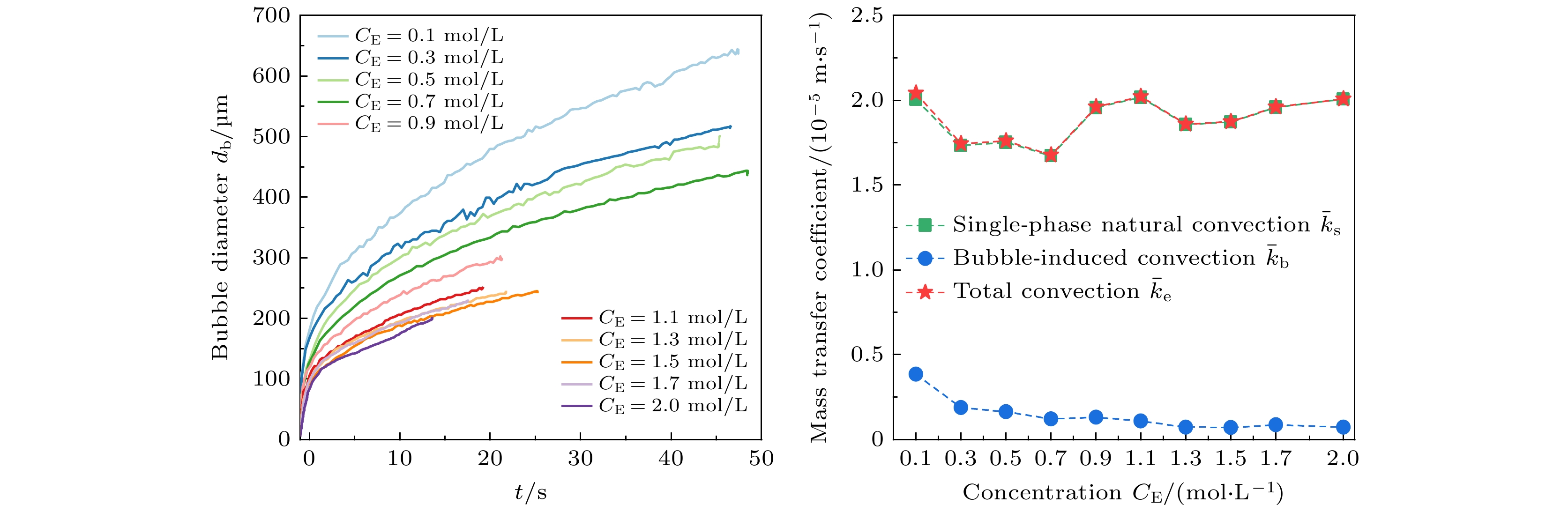
图 9 平均单相自然对流传质系数$ {{\bar k}_{{\mathrm{s}}}} $、气泡诱导对流传质系数$ {{\bar k}_{{\mathrm{b}}}} $和总对流传质系数$ {{\bar k}_{{\mathrm{e}}}} $随电解液浓度的变化
Fig. 9. Variation curves of average mass transfer coefficients of single-phase natural convection $ {{\bar k}_{{\mathrm{s}}}} $, bubble-induced convection $ {{\bar k}_{{\mathrm{b}}}} $ and total convection ${{\bar k}_{{\mathrm{e}}}} $ with electrolyte concentration.
-
[1] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[2] Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T 1997 Nature 388 431
 Google Scholar
Google Scholar
[3] 黄柏标, 王朋, 张晓阳, 秦晓燕, 戴瑛 2009 科学通报 54 847
 Google Scholar
Google Scholar
Huang B B, Wang P, Zhang X Y, Qin X Y, Dai Y 2009 Sci. Bull. 54 847
 Google Scholar
Google Scholar
[4] 陈娟雯, 郭烈锦, 胡晓玮, 曹振山 2018 工程热 39 550
Chen J W, Guo L J, Hu X W, Cao Z S 2018 J. Eng. Thermophys. 39 550
[5] 郭烈锦, 曹振山, 王晔春, 张博, 冯雨杨, 徐强 2023 西安交通大学学报 57 1
 Google Scholar
Google Scholar
Guo L J, Cao Z S, Wang Y C, Zhang B, Feng Y Y, Xu Q 2023 J. Xi'an Jiaotong Univ. 57 1
 Google Scholar
Google Scholar
[6] Tawfik M E, Diez F J 2014 Electrochim. Acta 146 792
 Google Scholar
Google Scholar
[7] Matsushima H, Iida T, Fukunaka Y 2012 J. Solid State Electrochem. 16 617
 Google Scholar
Google Scholar
[8] Li Y J, Zhang H C, Xu T H, Lu Z Y, Wu X C, Wan P B, Sun X M, Jiang L 2015 Adv. Funct. Mater. 25 1737
 Google Scholar
Google Scholar
[9] Chen J W, Guo L J 2019 Appl. Phys. Lett. 115 101602
 Google Scholar
Google Scholar
[10] Lu X, Lalwani S, Yuan L, Abdelsalam M A, AlMarzooqi F, Zhang T 2022 Int. J. Hydrogen Energ. 47 36504
 Google Scholar
Google Scholar
[11] Ansari S A, Khan M M, Ansari M O, Cho M H 2016 New J. Chem. 40 3000
 Google Scholar
Google Scholar
[12] Piontek S, Andronescu C, Zaichenko A, Konkena B, Puring K J, Marler B, Antoni H, Sinev I, Muhler M, Mollenhauer D, Roldan Cuenya B, Schuhmann W, Apfel U P 2018 ACS Catal. 8 987
 Google Scholar
Google Scholar
[13] Ye X, Xu Q, Nie T F, Luo X Y, Jiang S, Guo L J 2023 J. Phys. Chem. C 127 22085
 Google Scholar
Google Scholar
[14] Bashkatov A, Yang X, Mutschke G, Fritzsche B, Hossain S S, Eckert K 2021 Phys. Chem. Chem. Phys. 23 11818
 Google Scholar
Google Scholar
[15] Matsushima H, Fukunaka Y, Kuribayashi K 2006 Electrochim. Acta 51 4190
 Google Scholar
Google Scholar
[16] Lubetkin S D, Akhtar M 1996 J. Colloid Interf. Sci. 180 43
 Google Scholar
Google Scholar
[17] Baczyzmalski D, Karnbach F, Yang X, Mutschke G, Uhlemann M, Eckert K, Cierpka C 2016 J. Electrochem. Soc. 163 E248
 Google Scholar
Google Scholar
[18] Cao Z S, Zhang B, Feng Y Y, Xu Q, Wang Y C, Guo L J 2022 Electrochim. Acta 434 141293
 Google Scholar
Google Scholar
[19] Wang H P, Xu Z X, Lin W, Yang X, Gu X R, Zhu W, Zhuang Z B 2023 Nano Res. 16 420
 Google Scholar
Google Scholar
[20] Obata K, Stegenburga L, Takanabe K 2019 J. Phys. Chem. C 123 21554
 Google Scholar
Google Scholar
[21] Liu Y, Jiang J G, Xu Q, Li M T, Guo L J 2013 Mater. Res. Bull. 48 4548
 Google Scholar
Google Scholar
[22] Hu X W, Cao Z S, Wang Y C, Shen S, Guo L J, Chen J W 2016 Electrochim. Acta 202 175
 Google Scholar
Google Scholar
[23] Thoroddsen S T, Etoh T G, Takehara K 2008 Annu. Rev. Fluid Mech. 40 257
 Google Scholar
Google Scholar
[24] Rox H, Bashkatov A, Yang X, Loos S, Mutschke G, Gerbeth G, Eckert K 2022 Int. J. Hydrog. Energ. 48 2892
 Google Scholar
Google Scholar
[25] Vogt H 1993 Electrochim. Acta 38 1421
 Google Scholar
Google Scholar
[26] Lubetkin S, Blackwell M 1988 J. Colloid Interf. Sci. 126 610
 Google Scholar
Google Scholar
[27] Scriven L E 1959 Chem. Eng. Sci. 10 1
 Google Scholar
Google Scholar
[28] Chandran P, Bakshi S, Chatterjee D 2015 Chem. Eng. Sci. 138 99
 Google Scholar
Google Scholar
[29] Vogt H, Balzer R J 2005 Electrochim. Acta 50 2073
 Google Scholar
Google Scholar
[30] Wei J J, Xue Y F, Zhao J F, Li J 2011 Chin. Phys. Lett. 28 016401
 Google Scholar
Google Scholar
[31] Chen J W, Guo L J, Hu X W, Cao Z S, Wang Y C 2018 Electrochim. Acta 274 57
 Google Scholar
Google Scholar
[32] Wang M S, Nie T F, She Y, Tao L, Luo X Y, Xu Q, Guo L J 2023 Int. J. Hydrog. Energ. 48 23387
 Google Scholar
Google Scholar
[33] Bashkatov A, Hossain S S, Yang X, Mutschke G, Eckert K 2019 Phys. Rev. Lett. 123 214503
 Google Scholar
Google Scholar
[34] Vogt H 1993 Electrochim. Acta 38 1427
 Google Scholar
Google Scholar
[35] Vogt H 2017 Electrochim. Acta 235 495
 Google Scholar
Google Scholar
[36] Vogt H 2013 Electrochim. Acta 87 611
 Google Scholar
Google Scholar
[37] Shah A, Jorne J 1989 J. Electrochem. Soc. 136 144
 Google Scholar
Google Scholar
[38] Lochiel A C, Calderbank P H 1964 Chem. Eng. Sci. 19 471
 Google Scholar
Google Scholar
计量
- 文章访问数: 3448
- PDF下载量: 69
- 被引次数: 0













 下载:
下载: