-
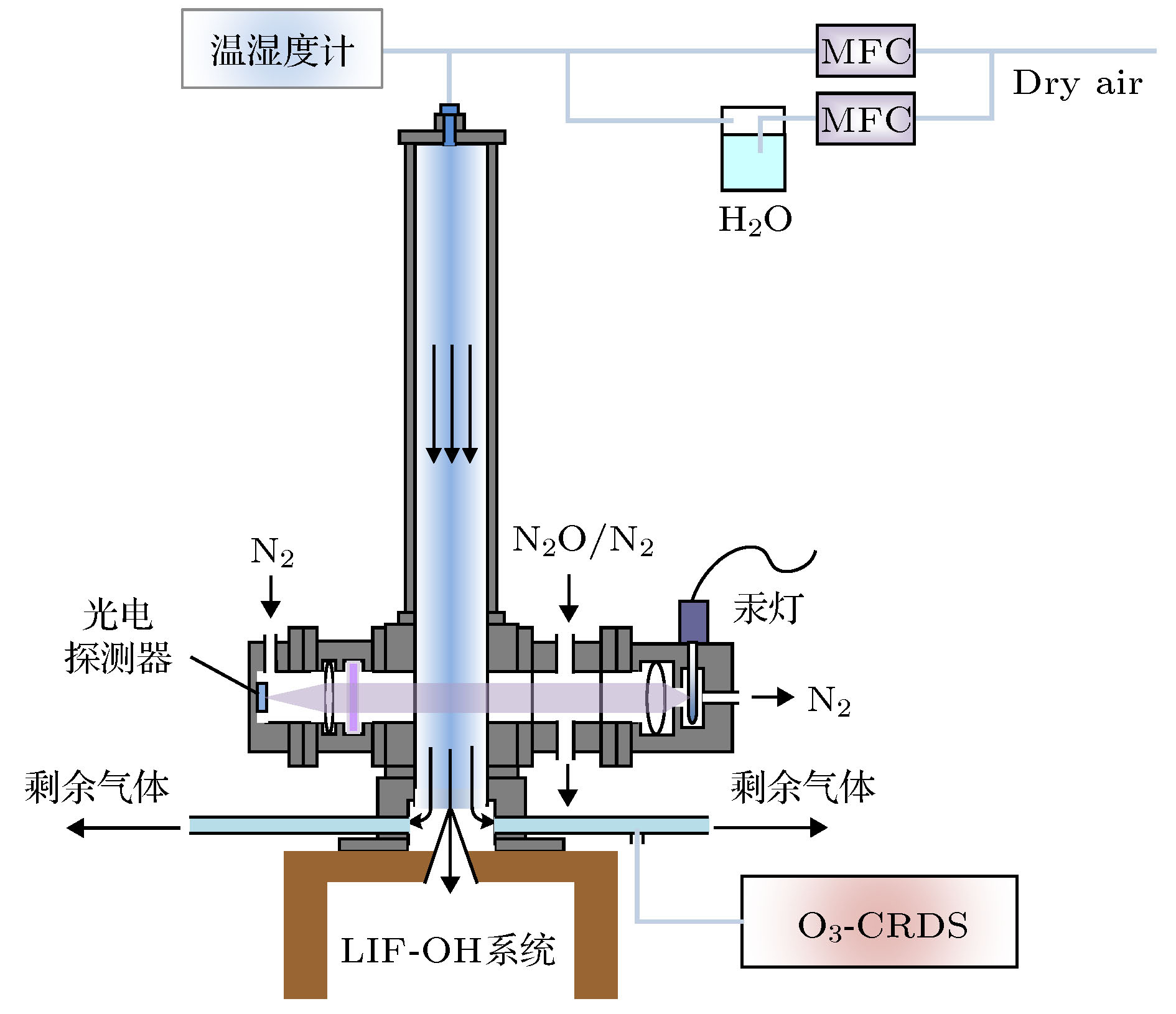
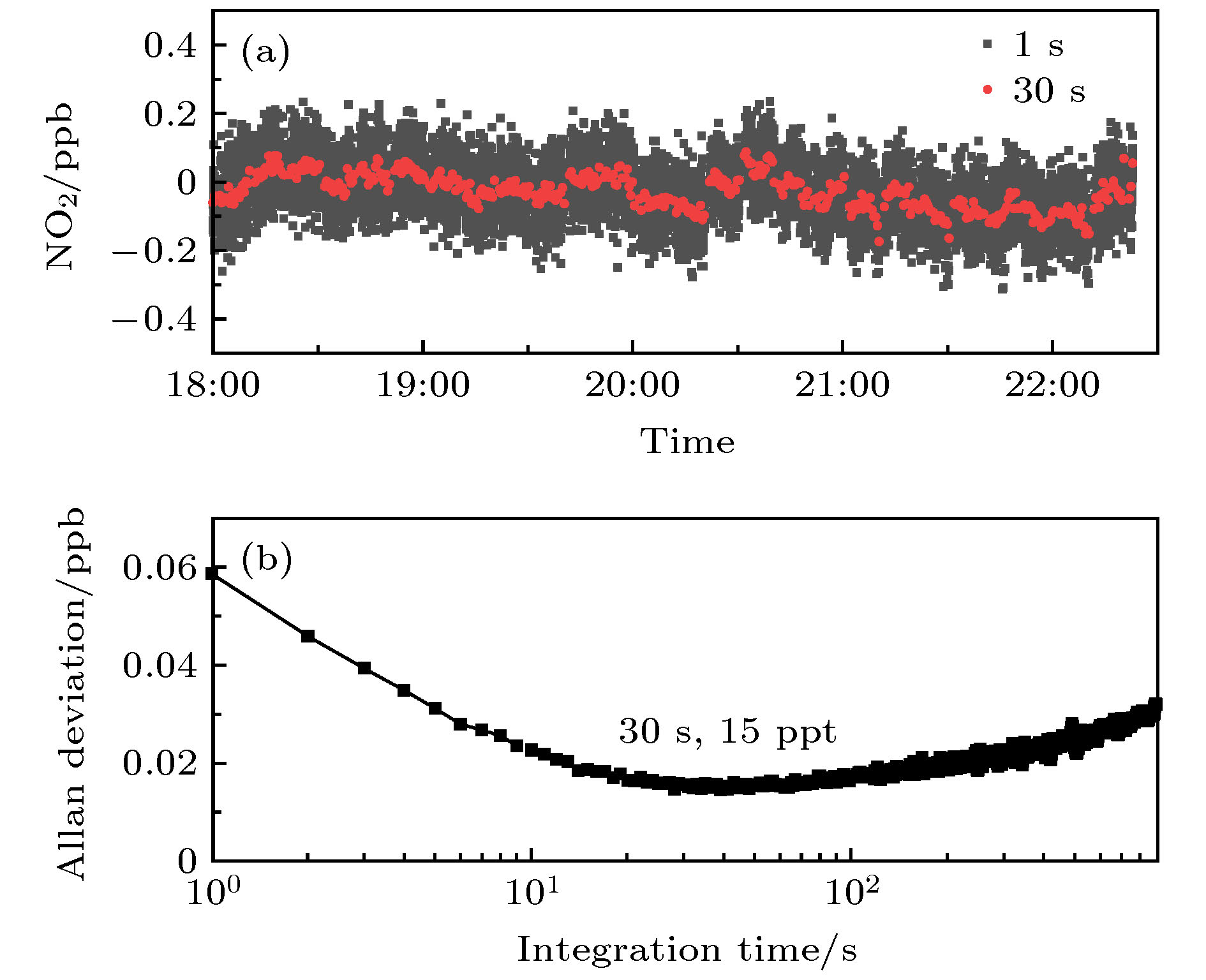
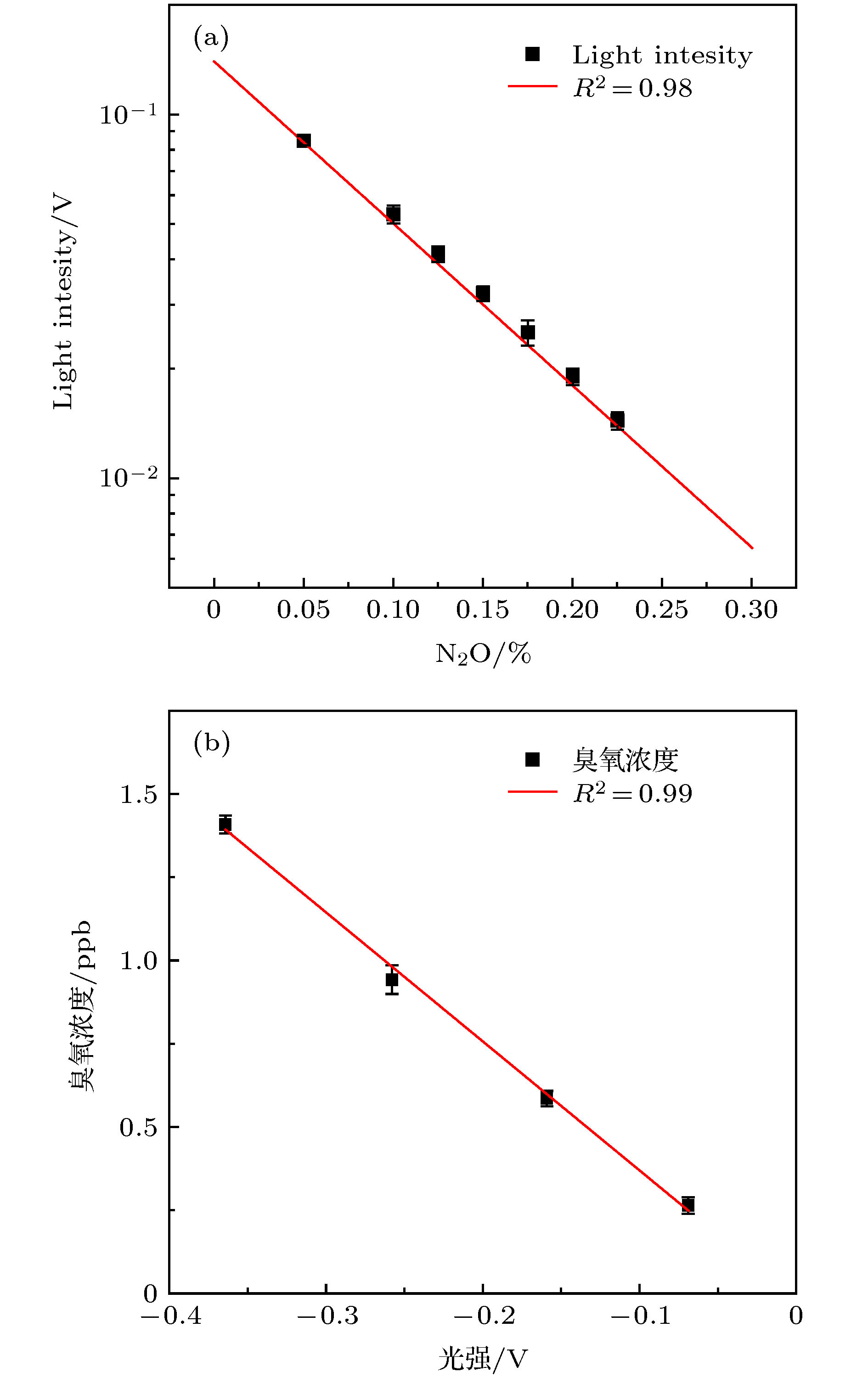
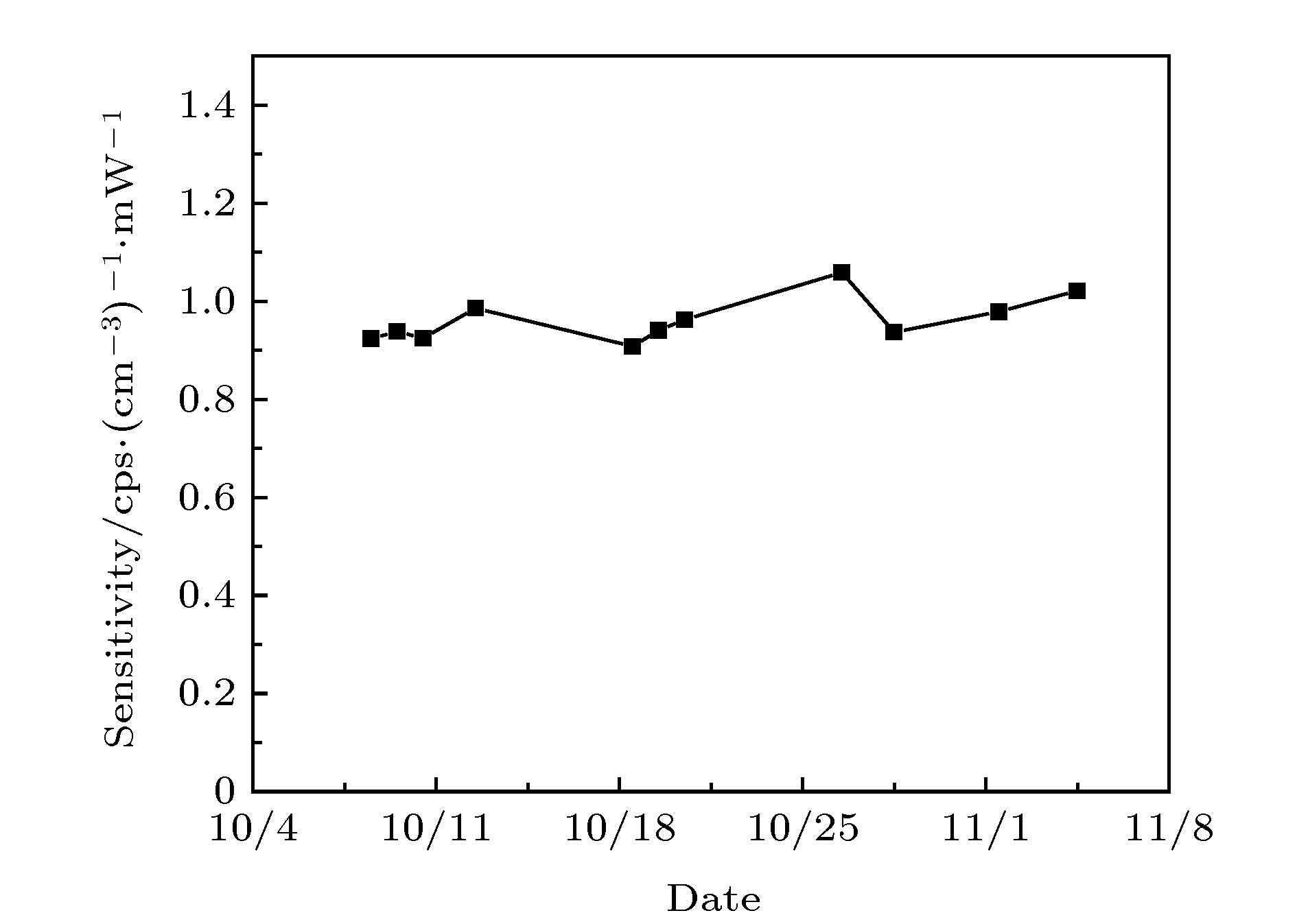
OH radical is the most important oxidant in the atmosphere, and controls the tropospheric concentration of tropospheric trace gases such as CO, SO2, NO2, CH4 and other volatile organic compounds. Accurate measurement of the concentration of OH radical in troposphere is the key to clarifying the formation mechanism of secondary pollution in China. The laser-induced fluorescence (LIF) technique is widely used in tropospheric OH radical field observation due to its high sensitivity, high selectivity, and small interference. However, the LIF technique is not an absolute measurement technology. In recent years, OH radical measurements and simulations in many field observations show that the improvement of accuracy of calibration is a way to reduce the differences. Currently, the common calibration methods are ozone-alkene method and water photolysis method. Further improving the accuracy of calibration is a key factor to ensure the accurate measurement of OH radicals. In this paper, a portable calibration method of OH radicals based on simultaneous photolysis is introduced. The synthetic air with a certain water vapor concentration is irradiated in laminar flow by 185 nm light of mercury lamp, and the photolysis of water vapor and O2 produce OH, HO2 radicals and O3. The concentration of OH radicals is calculated by oxygen concentration, water vapor concentration, ozone concentration, oxygen absorption cross section and water vapor absorption cross section. The water vapor is measured by a high-precision temperature and humidity probe, and the systematic error of the probe is corrected by 911-0016 ammonia (NH3, H2O) analyzer. As the ozone concentration is only 0.5-1 ppb in the calibration, the commercial ozone analyzer cannot meet the requirement for the measurement. A high-precision ozone analyzer O3-CRDS based on cavity-ring-down spectrocopy is built to achieve the detection limit of 15 ppt (1σ). Using the O3-CRDS analyzer, the concentration distribution coefficient of ozone in laminar flow along the radial direction of the flow tube (P = 1.9) is measured. Because the absorption cross section of oxygen at 185 nm is seriously affected by oxygen column concentration and the characteristics of mercury lamp, the oxygen absorption cross section is remeasured based on Lambert’s law, which is $ \sigma_{\rm O_2} $ = (1.25 ± 0.08)×10–20 cm2. The portable calibration device is established by establishing the corresponding relationship between ozone concentration and light intensity. By changing the concentration of water vapor in the flow tube, the OH radicals with concentrations in a range of 3×108-2.8×109 cm–3 are produced, which are used to calibrate the atmospheric OH radical measurement instrument based on LIF technique. The fluorescence signal has a good correlation with the concentration of OH. The calibration device of OH radical is used to calibrate the LIF system during “a comprehensive study of the ozone formation mechanism in Shenzhen” (STORM) field observation in Autumn 2018. The calibration results under the field condition show that the calibration uncertainty of the calibration device for LIF instrument is 13.0%, which has good stability and accuracy.-
Keywords:
- OH radical /
- simultaneous photolysis /
- ozone distribution coefficient
[1] Guo S, Hu M, Zamora M L, Peng J F, Shang D J, Zheng J, Du Z F, Wu Z, Shao M, Zeng L M, Molina M J, Zhang R Y 2014 Proc. Natl. Acad. Sci. U.S.A. 111 17373
 Google Scholar
Google Scholar
[2] Huang R J, Zhang Y L, Bozzetti C, Ho K F, Cao J J, Han Y M, Daellenbach K R, Slowik J G, Platt S M, Canonaco F, Zotter P, Wolf R, Pieber S M, Bruns E A, Crippa M, Ciarelli G, Piazzalunga A, Schwikowski M, Abbaszade G, Schnelle-Kreis J, Zimmermann R, An Z S, Szidat S, Baltensperger U, El Haddad I, Prevot A S H 2014 Nature 514 218
 Google Scholar
Google Scholar
[3] Ehhalt D H 1999 Phys. Chem. Chem. Phys. 1 5401
 Google Scholar
Google Scholar
[4] Jaegle L, Jacob D J, Brune W H, Faloona I, Tan D, Heikes B G, Kondo Y, Sachse G W, Anderson B, Gregory G L, Singh H B, Pueschel R, Ferry G, Blake D R, Shetter R E 2000 J. Geophys. Res. Atmos. 105 3877
 Google Scholar
Google Scholar
[5] 陆克定, 张远航 2010 化学进展 22 500
Lu K D, Zhang Y H 2010 Prog. Chem. 22 500
[6] Hofzumahaus A, Rohrer F, Lu K D, Bohn B, Brauers T, Chang C, Fuchs H, Holland F, Kita K, Kondo Y, Li X, Lou S R, Shao M, Zeng L M, Wahner A, Zhang Y H 2009 Science 324 1702
 Google Scholar
Google Scholar
[7] Brauers T, Aschmutat U, Brandenburger U, Dorn H P, Hausmann M, Heßling M, Hofzumahaus A, Holland F, Plass-Dülmer C, Ehhalt D H 1996 Geophys. Res. Lett. 23 2545
 Google Scholar
Google Scholar
[8] Mauldin R L, Cantrell C A, Zondlo M, Kosciuch E, Eisele F L, Chen G, Davis D, Weber R, Crawford J, Blake D, Bandy A, Thornton D 2003 J. Geophys. Res. Atmos. 108 8796
 Google Scholar
Google Scholar
[9] Thomas L A G, Hard M 1995 Atmos. Sci. 52 3354
 Google Scholar
Google Scholar
[10] Stone D, Whalley L K, Heard D E 2012 Chem. Soc. Rev. 41 6348
 Google Scholar
Google Scholar
[11] Novelli A, Hens K, Ernest C T, Kubistin D, Regelin E, Elste T, Plass-Duelmer C, Martinez M, Lelieveld J, Harder H 2014 Atmos. Meas. Tech. 7 3413
 Google Scholar
Google Scholar
[12] Lu K D, Hofzumahaus A, Holland F, Bohn B, Brauers T, Fuchs H, Hu M, Haeseler R, Kita K, Kondo Y, Li X, Lou S R, Oebel A, Shao M, Zeng L M, Wahner A, Zhu T, Zhang Y H, Rohrer F 2013 Atmos. Chem. Phys. 13 1057
 Google Scholar
Google Scholar
[13] Ren X R, Olson J R, Crawford J H, Brune W H, Mao J Q, Long R B, Chen Z, Chen G, Avery M A, Sachse G W, Barrick J D, Diskin G S, Huey L G, Fried A, Cohen R C, Heikes B, Wennberg P O, Singh H B, Blake D R, Shetter R E 2008 J. Geophys. Res. Atmos. 113 D05310
 Google Scholar
Google Scholar
[14] Whalley L K, Edwards P M, Furneaux K L, Goddard A, Ingham T, Evans M J, Stone D, Hopkins J R, Jones C E, Karunaharan A, Lee J D, Lewis A C, Monks P S, Moller S J, Heard D E 2011 Atmos. Chem. Phys. 11 7223
 Google Scholar
Google Scholar
[15] Faloona I C, Tan D, Lesher R L, Hazen N L, Frame C L, Simpas J B, Harder H, Martinez M, Di Carlo P, Ren X R, Brune W H 2004 J. Atmos. Chem. 47 139
 Google Scholar
Google Scholar
[16] [17] Hard T M, George L A, O'Brien R J 2002 Environ. Sci. Technol. 36 1783
 Google Scholar
Google Scholar
[18] Dusanter D V S, Stevens P S 2008 Atmos. Chem. Phys. 8 321
 Google Scholar
Google Scholar
[19] Bloss W J, Lee J D, Bloss C, Heard D E, Pilling M J, Wirtz K, Martin-Reviejo M, Siese M 2004 Atmos. Chem. Phys. 4 571
 Google Scholar
Google Scholar
[20] Schultz M, Heitlinger M, Mihelcic D, Volz-Thomas A 1995 J. Geophys. Res. 100 18811
 Google Scholar
Google Scholar
[21] Kanaya Y, Sadanaga Y, Hirokawa J, Kajii Y, Akimoto H 2001 J. Atmos. Chem. 38 73
 Google Scholar
Google Scholar
[22] Kono M, Lewis B R, Baldwin K G H, Gibson S T 2003 J. Chem. Phys. 118 10924
 Google Scholar
Google Scholar
[23] Lanzendorf E J, Hanisco T F, Donahue N M, Wennberg P O 1997 Geophys. Res. Lett. 24 3037
 Google Scholar
Google Scholar
[24] Creasey D J, Heard D E, Lee J D 2000 Geophys. Res. Lett. 27 1651
 Google Scholar
Google Scholar
[25] Hofzumahaus A, Brauers T, Aschmutat U, Brandenburger U, Dorn H P, Hausmann M, Heßling M, Holland F, Plass-Dülmer C, Sedlacek M, Weber M, Ehhalt D H 1997 Geophys. Res. Lett. 24 3039
 Google Scholar
Google Scholar
[26] Li Z Y, Hu R Z, Xie P H, Chen H, Liu X Y, Liang S X, Wang D, Wang F Y, Wang Y H, Lin C, Liu J G, Liu W Q 2019 Atmos. Meas. Tech. 12 3223
 Google Scholar
Google Scholar
[27] Cantrell C A, Zimmer A, Tyndall G S 1997 Geophys. Res. Lett. 24 2687
 Google Scholar
Google Scholar
-
表 1 OH自由基标定装置不确定度
Table 1. Uncertainty of OH radical calibration source.
误差源 不确定度 来源 臭氧分布系数P 6.0% 测量 臭氧灵敏度Qv 2.9% 测量 PD光强 I' 1.0% 测量 水汽浓度[H2O] 2.0% 测量 氧气吸收截面$ \sigma _{\rm O_2} $ 7.0% 测量 水汽吸收截面$ {\sigma _{{{\rm{H}}_2}{\rm{O}}}} $ 3.0% 引用 标定装置产生OH自由基误差 10.4% 计算 -
[1] Guo S, Hu M, Zamora M L, Peng J F, Shang D J, Zheng J, Du Z F, Wu Z, Shao M, Zeng L M, Molina M J, Zhang R Y 2014 Proc. Natl. Acad. Sci. U.S.A. 111 17373
 Google Scholar
Google Scholar
[2] Huang R J, Zhang Y L, Bozzetti C, Ho K F, Cao J J, Han Y M, Daellenbach K R, Slowik J G, Platt S M, Canonaco F, Zotter P, Wolf R, Pieber S M, Bruns E A, Crippa M, Ciarelli G, Piazzalunga A, Schwikowski M, Abbaszade G, Schnelle-Kreis J, Zimmermann R, An Z S, Szidat S, Baltensperger U, El Haddad I, Prevot A S H 2014 Nature 514 218
 Google Scholar
Google Scholar
[3] Ehhalt D H 1999 Phys. Chem. Chem. Phys. 1 5401
 Google Scholar
Google Scholar
[4] Jaegle L, Jacob D J, Brune W H, Faloona I, Tan D, Heikes B G, Kondo Y, Sachse G W, Anderson B, Gregory G L, Singh H B, Pueschel R, Ferry G, Blake D R, Shetter R E 2000 J. Geophys. Res. Atmos. 105 3877
 Google Scholar
Google Scholar
[5] 陆克定, 张远航 2010 化学进展 22 500
Lu K D, Zhang Y H 2010 Prog. Chem. 22 500
[6] Hofzumahaus A, Rohrer F, Lu K D, Bohn B, Brauers T, Chang C, Fuchs H, Holland F, Kita K, Kondo Y, Li X, Lou S R, Shao M, Zeng L M, Wahner A, Zhang Y H 2009 Science 324 1702
 Google Scholar
Google Scholar
[7] Brauers T, Aschmutat U, Brandenburger U, Dorn H P, Hausmann M, Heßling M, Hofzumahaus A, Holland F, Plass-Dülmer C, Ehhalt D H 1996 Geophys. Res. Lett. 23 2545
 Google Scholar
Google Scholar
[8] Mauldin R L, Cantrell C A, Zondlo M, Kosciuch E, Eisele F L, Chen G, Davis D, Weber R, Crawford J, Blake D, Bandy A, Thornton D 2003 J. Geophys. Res. Atmos. 108 8796
 Google Scholar
Google Scholar
[9] Thomas L A G, Hard M 1995 Atmos. Sci. 52 3354
 Google Scholar
Google Scholar
[10] Stone D, Whalley L K, Heard D E 2012 Chem. Soc. Rev. 41 6348
 Google Scholar
Google Scholar
[11] Novelli A, Hens K, Ernest C T, Kubistin D, Regelin E, Elste T, Plass-Duelmer C, Martinez M, Lelieveld J, Harder H 2014 Atmos. Meas. Tech. 7 3413
 Google Scholar
Google Scholar
[12] Lu K D, Hofzumahaus A, Holland F, Bohn B, Brauers T, Fuchs H, Hu M, Haeseler R, Kita K, Kondo Y, Li X, Lou S R, Oebel A, Shao M, Zeng L M, Wahner A, Zhu T, Zhang Y H, Rohrer F 2013 Atmos. Chem. Phys. 13 1057
 Google Scholar
Google Scholar
[13] Ren X R, Olson J R, Crawford J H, Brune W H, Mao J Q, Long R B, Chen Z, Chen G, Avery M A, Sachse G W, Barrick J D, Diskin G S, Huey L G, Fried A, Cohen R C, Heikes B, Wennberg P O, Singh H B, Blake D R, Shetter R E 2008 J. Geophys. Res. Atmos. 113 D05310
 Google Scholar
Google Scholar
[14] Whalley L K, Edwards P M, Furneaux K L, Goddard A, Ingham T, Evans M J, Stone D, Hopkins J R, Jones C E, Karunaharan A, Lee J D, Lewis A C, Monks P S, Moller S J, Heard D E 2011 Atmos. Chem. Phys. 11 7223
 Google Scholar
Google Scholar
[15] Faloona I C, Tan D, Lesher R L, Hazen N L, Frame C L, Simpas J B, Harder H, Martinez M, Di Carlo P, Ren X R, Brune W H 2004 J. Atmos. Chem. 47 139
 Google Scholar
Google Scholar
[16] [17] Hard T M, George L A, O'Brien R J 2002 Environ. Sci. Technol. 36 1783
 Google Scholar
Google Scholar
[18] Dusanter D V S, Stevens P S 2008 Atmos. Chem. Phys. 8 321
 Google Scholar
Google Scholar
[19] Bloss W J, Lee J D, Bloss C, Heard D E, Pilling M J, Wirtz K, Martin-Reviejo M, Siese M 2004 Atmos. Chem. Phys. 4 571
 Google Scholar
Google Scholar
[20] Schultz M, Heitlinger M, Mihelcic D, Volz-Thomas A 1995 J. Geophys. Res. 100 18811
 Google Scholar
Google Scholar
[21] Kanaya Y, Sadanaga Y, Hirokawa J, Kajii Y, Akimoto H 2001 J. Atmos. Chem. 38 73
 Google Scholar
Google Scholar
[22] Kono M, Lewis B R, Baldwin K G H, Gibson S T 2003 J. Chem. Phys. 118 10924
 Google Scholar
Google Scholar
[23] Lanzendorf E J, Hanisco T F, Donahue N M, Wennberg P O 1997 Geophys. Res. Lett. 24 3037
 Google Scholar
Google Scholar
[24] Creasey D J, Heard D E, Lee J D 2000 Geophys. Res. Lett. 27 1651
 Google Scholar
Google Scholar
[25] Hofzumahaus A, Brauers T, Aschmutat U, Brandenburger U, Dorn H P, Hausmann M, Heßling M, Holland F, Plass-Dülmer C, Sedlacek M, Weber M, Ehhalt D H 1997 Geophys. Res. Lett. 24 3039
 Google Scholar
Google Scholar
[26] Li Z Y, Hu R Z, Xie P H, Chen H, Liu X Y, Liang S X, Wang D, Wang F Y, Wang Y H, Lin C, Liu J G, Liu W Q 2019 Atmos. Meas. Tech. 12 3223
 Google Scholar
Google Scholar
[27] Cantrell C A, Zimmer A, Tyndall G S 1997 Geophys. Res. Lett. 24 2687
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 14261
- PDF Downloads: 131
- Cited By: 0
















 DownLoad:
DownLoad: