-
Hydrogen-induced cracking (HIC) is a key problem restricting the application of ultra-high strength steel. It is necessary to analyze the distribution of diffusible hydrogen to reveal the mechanism of HIC. The site occupation tendency of H in interstitial and vacancy positions are investigated by the ab-initio method, and the stable configuration and steady state energy are obtained. The solution tendency of H atom in interstitial and vacancy positions is analyzed based on the aforementioned results. Specifically, the Mulliken population, density of states, charge density difference are calculated and used to analyze the interaction between α-Fe metal and H atom. The results show that the dissolved H tends to occupy the interstitial sites of the body-centered cubic, the weak hybridization interaction between the interstitial hydrogen and its nearest neighbour Fe atom is contributed by the H 1s orbital and Fe 4s orbital. Vacancies can capture H atoms easily and H atoms tend to occupy the isoelectric surface near the inwall of the vacancies. A vacancy defect can hold up to three H atoms which are difficult to combine with each other to form H2 molecule by covalent bond. H atoms in vacancies and at interstitial positions change the charge distribution of the Fe lattice, which weakens the binding force of the atoms and forms anti-bonding orbital in local area. The proposed thermodynamical model allows the determining of the equilibrium vacancy and the dissolved H concentration for a given temperature and H chemical potential in the reservoir, and the calculated results are in good agreement with the actual results.
-
Keywords:
- density functional theory /
- thermodynamic analysis /
- ferrite /
- diffusible hydrogen
[1] 张文钺 1999 焊接冶金学(基本原理)(北京: 机械工业出版社) 第241—244页
Zhang W Y 2012 Welding Metallurgy (Fundamental Principle) (Beijing: China Machine Press) pp241–244 (in Chinese)
[2] 张敬强 2015 博士学位论文 (哈尔滨: 哈尔滨工业大学)
Zhang J Q 2015 Ph.D Dissertation (Harbin: Harbin Institute of Technology) (in Chinese)
[3] Tateyama Y, Ohno T 2003 ISIJ Internation 43 573
 Google Scholar
Google Scholar
[4] Geng W T, Wan L, Du J P, Ogata S 2017 Scripta Materialia 134 105
 Google Scholar
Google Scholar
[5] MonasterioP R, Lau T T, Yip S, van Vliet K J 2009 Phys. Rev. Lett. 103 085501
 Google Scholar
Google Scholar
[6] Nagumo M, Nakamura M, Taika K 2001 Metall. Mater. Trans. A 32 339
 Google Scholar
Google Scholar
[7] Först C J, Slycke J, van Vliet K J, Yip S 2006 Phys. Rev. Lett. 96 175501
 Google Scholar
Google Scholar
[8] Fukai Y 1983 Jpn. J. Appl. Phys. 22 207
 Google Scholar
Google Scholar
[9] 张凤春, 李春福, 文平, 罗强, 冉曾令 2014 63 227101
 Google Scholar
Google Scholar
ZhangF C, Li C F, Wen P, Luo Q, Ran Z L 2014 Acta Phys. Sin. 63 227101
 Google Scholar
Google Scholar
[10] Du Y A, Ismer L, Rogal J, Hickel T, Neugebauer J, Drautz R 2011 Phys. Rev. B 84 144121
 Google Scholar
Google Scholar
[11] Nazarov R, Hickel T, Neugebauer J 2014 Phys. Rev. B 89 144108
 Google Scholar
Google Scholar
[12] Nazarov R, Hickel T, Neugebauer J 2012 Phys. Rev. B 85 144118
 Google Scholar
Google Scholar
[13] Hickel T, Grabowski B, Körmann F, Neugebauer J 2011 J. Phys. Condens. Mater. 24 053202
 Google Scholar
Google Scholar
[14] Ramasubramaniam A, Itakura M, Carter EA 2009 Phys. Rev. B 79 174101
 Google Scholar
Google Scholar
[15] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[16] Milman V, Winkler B, White JA, Pickard C J, Payne M C, Akhmataskaya E V, Nobes R H 2000 Int. J. Quantum Chem. 77 895
 Google Scholar
Google Scholar
[17] Perdew JP, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[18] Methfessel M, Paxton A T 1989 Phys. Rev. B 40 3616
 Google Scholar
Google Scholar
[19] Acet M, Zähres H, Wassermann E F, Pepperhoff W 1994 Phys. Rev. B 49 6012
 Google Scholar
Google Scholar
[20] Hayward E, Fu C C 2013 Phys. Rev. B 87 174103
 Google Scholar
Google Scholar
[21] Baroni S, de Gironcoli S, Dal Corso A C, Giannozzi P 2001 Rev. Mod. Phys. 73 515
 Google Scholar
Google Scholar
[22] Bozzolo G, Ferrante J 1992 Phys. Rev. B 46 8600
 Google Scholar
Google Scholar
[23] Bhatia M A, Groh S, Solanki K N 2014 J. Appl. Phys. 116 064302
 Google Scholar
Google Scholar
[24] Tateyama Y, Ohno T 2003 Phys. Rev. B 67 174105
 Google Scholar
Google Scholar
[25] Mclellan R B, Harkins C G 1975 Mater. Sci. Eng. 18 5
 Google Scholar
Google Scholar
[26] Lynch SP 2013 Metall. Mater. Trans. A 44 1209
 Google Scholar
Google Scholar
[27] Lu G, Kaxiras E 2005 Phys. Rev. Lett. 94 155501
 Google Scholar
Google Scholar
[28] Ismer L, Hickel T, Neugebauer J 2010 Phys. Rev. B 81 094111
 Google Scholar
Google Scholar
[29] Korzhavyi P A, Abrikosov I A, Johansson B, Ruban A V, Skriver H L 1999 Phys. Rev. B 59 11693
 Google Scholar
Google Scholar
[30] Nazarov R, Hickel T, Neugebauer J 2010 Phys. Rev. B 82 224104
 Google Scholar
Google Scholar
[31] Speight J G 2005 Lange’s Handbook of Chemistry (16th Ed.) (New York: The McGraw-Hill Companies, Inc) pp1299−1309
-
图 2 α-Fe+H (T-site)的分波电子态密度 (a) 固溶前后 H 原子; (b) 固溶前后最近邻Fe原子; (c) 四面体间隙H原子; (d) 间隙H原子最近邻Fe原子
Figure 2. Partial electronic density of state of α-Fe+H (T-site): (a) Free H atom and interstitial H atom; (b) Fe atom in perfect α-Fe crystal and the nearest neighbour of interstitial H atom; (c) H atom in tetrahedral interstice; (d) the nearest neighbour Fe atom of interstitial H atom.
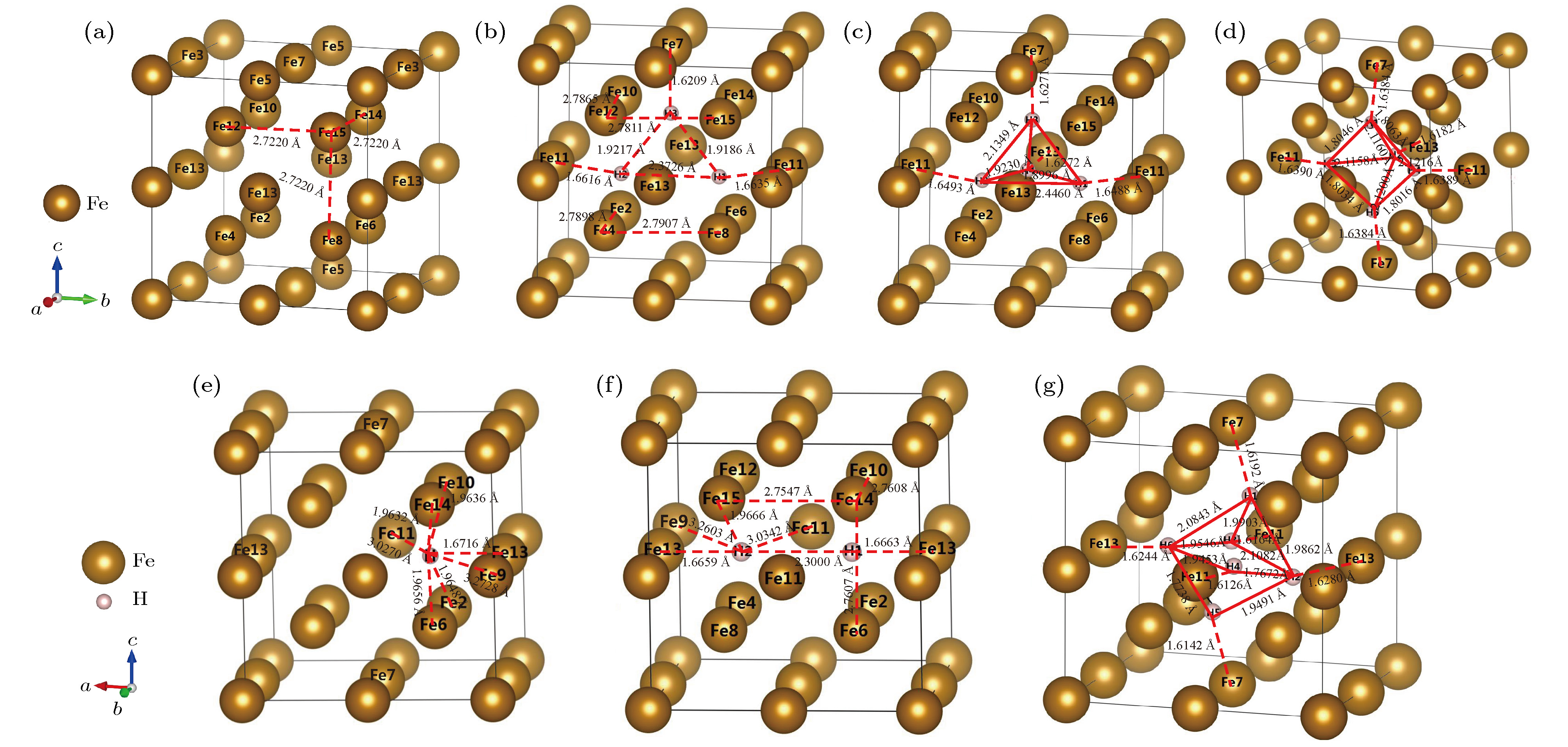
图 4 α-Fe+(nH-Vac)的晶体结构 (a) α-Fe+Vac; (b) α-Fe+(3H-Vac); (c) α-Fe+(4H-Vac); (d) α-Fe+(5H-Vac); (e) α-Fe+(1H-Vac); (f) α-Fe+(2H-Vac); (g) α-Fe+(6H-Vac)
Figure 4. Crystalline structure of α-Fe+(nH-Vac): (a) α-Fe+Vac; (b)α-Fe+(3H-Vac); (c) α-Fe+(4H-Vac); (d) α-Fe+(5H-Vac); (e) α-Fe+(1H-Vac); (f) α-Fe+(2H-Vac); (g) α-Fe+(6H-Vac).
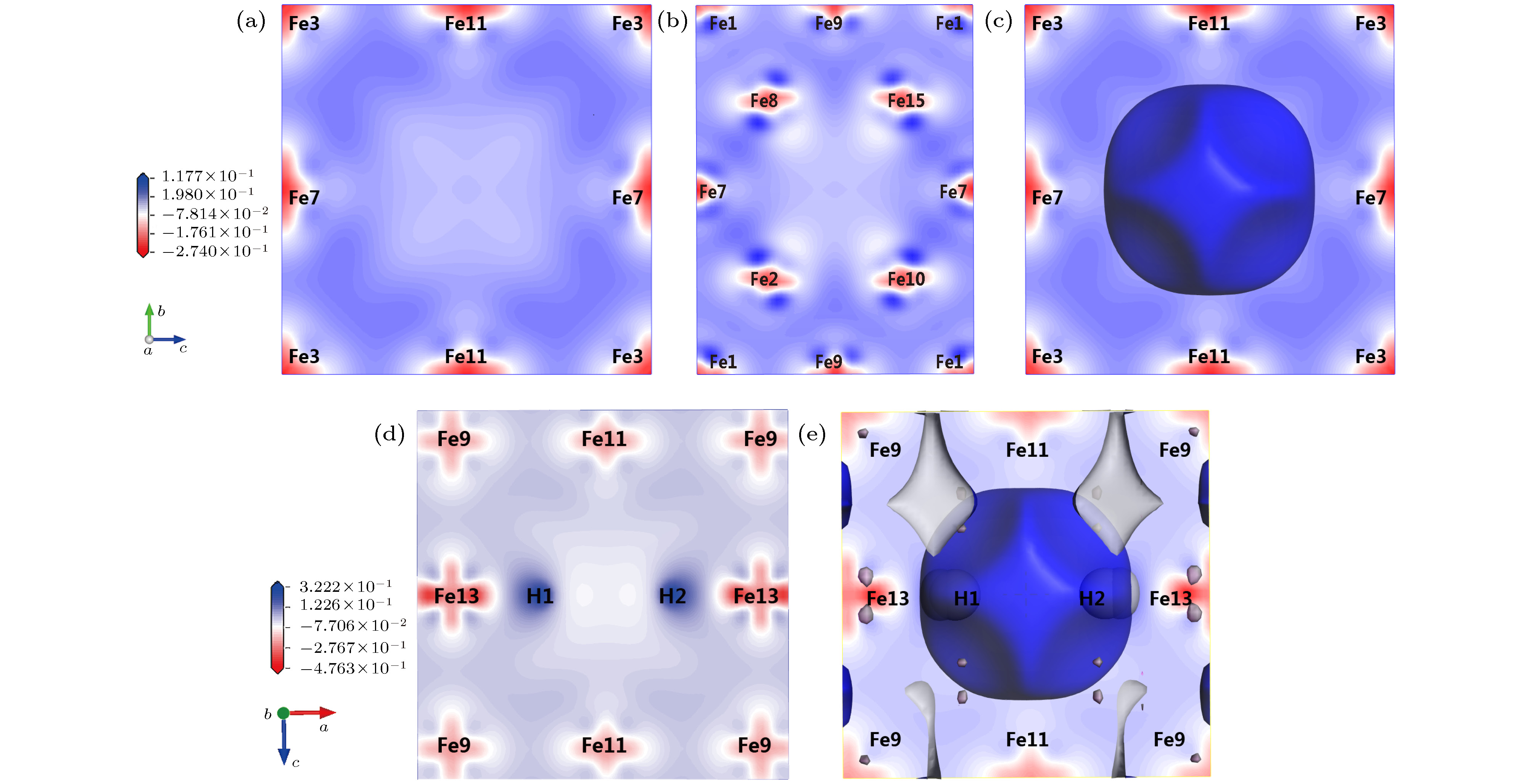
图 5 α-Fe+(nH-Vac)的等电荷面和差分电荷密度 (a) α-Fe+Vac ((100)面); (b) α-Fe+Vac ((110)面); (c) α-Fe+Vac等电荷面; (d) α-Fe+(2H-Vac) ((010)面); (e) α-Fe+(2H-Vac)等电荷面
Figure 5. Isoelectronic density surface and electron density difference of α-Fe+(nH-Vac): (a) Electron density difference of α-Fe+Vac in surface (100); (b) electron density difference of α-Fe+Vac in surface (110); (c) isoelectric density surface of α-Fe+Vac; (d) electron density difference of α-Fe+(2H-Vac) in surface (010); (e) isoelectric density surface of α-Fe+(2H-Vac).
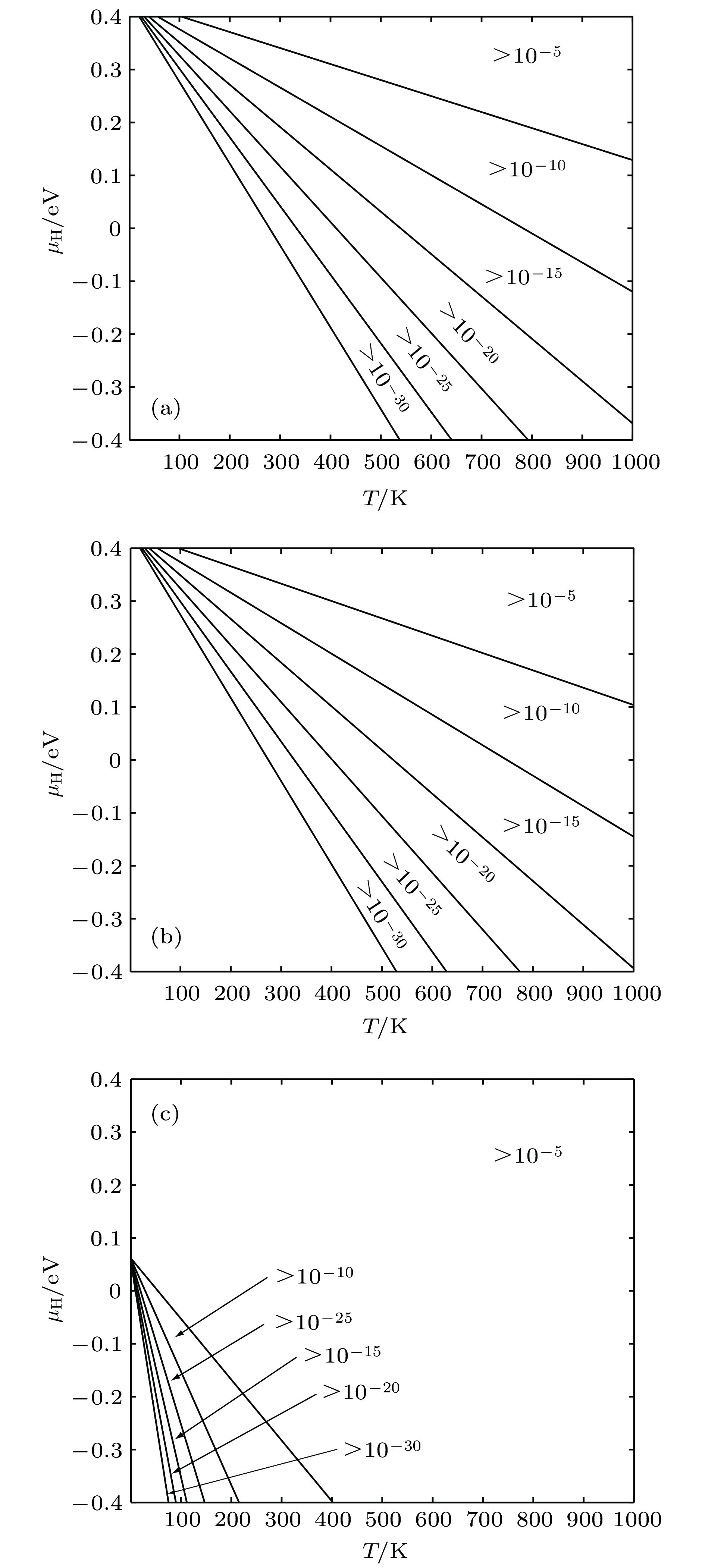
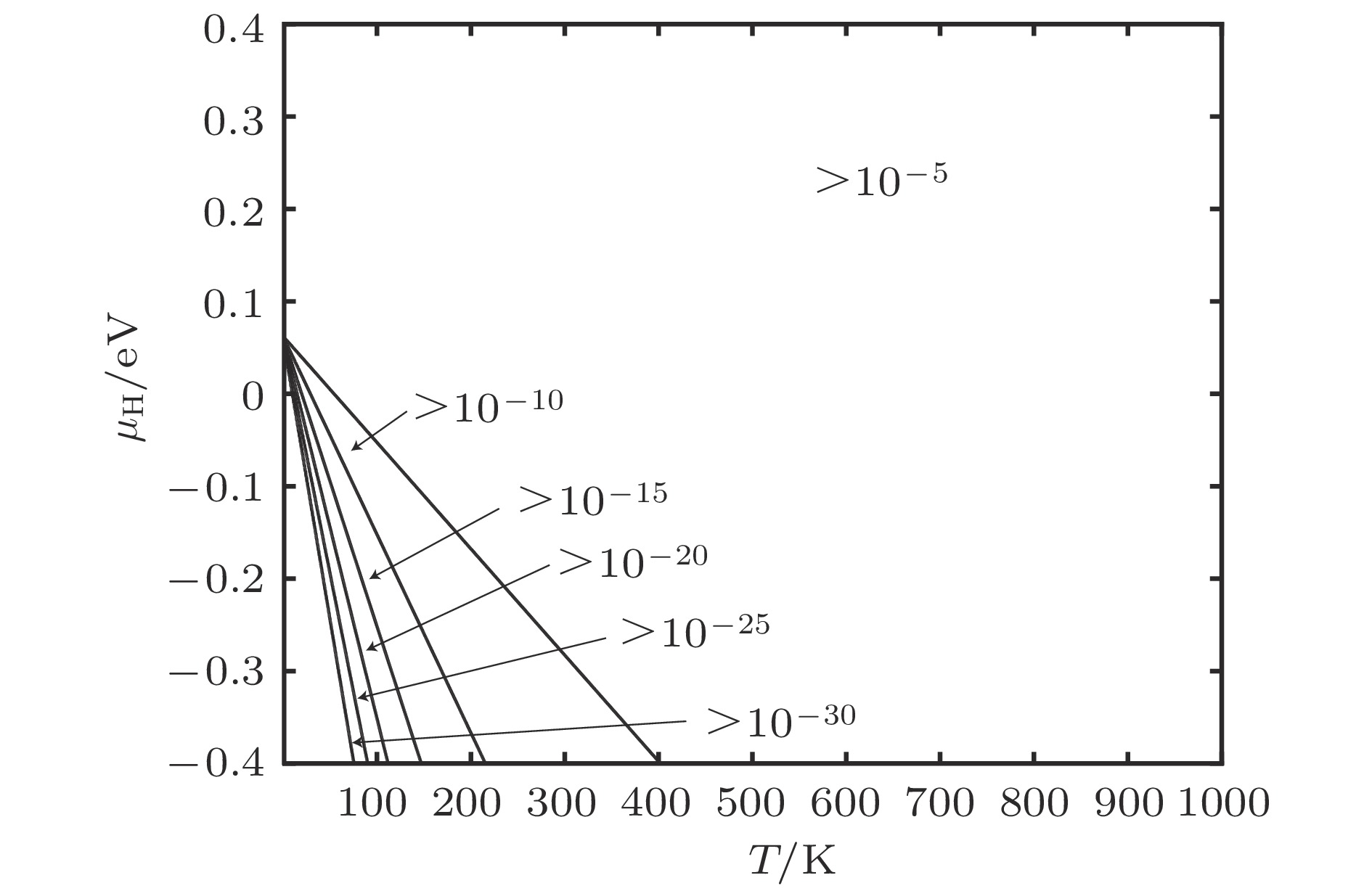
图 8 缺陷随T和μH变化情况 (a) 空位平衡浓度; (b) 空位内H平衡浓度; (c) 间隙H平衡浓度
Figure 8. Equilibrium concentration of defects for different temperature and H chemical potentials: (a) Equilibrium concentration of vacancies; (b) equilibrium concentration of H occupying in vacancies; (c) equilibrium concentration of H occupying in interstitial positions.
表 1 α-Fe+H的晶格常数和晶体结构
Table 1. Lattice parameters and crystalline structure of α-Fe+H.
晶体类型 a/Å b/Å c/Å α/(°) β/(°) γ/(°) V/Å 空间群 α-Fe+H(T-site) 5.6843 5.6612 5.6804 89.999 89.999 90.000 5.6843 115(P-4m2) α-Fe+H(O-site) 5.8019 5.6112 5.6112 90.000 90.000 90.000 5.8019 123(P4/mmm) 表 2 α-Fe和α-Fe+H结合能、形成能和溶解热
Table 2. Binding energy, formation energy, and heat of solution of α-Fe and α-Fe+H.
晶体类型 ${E_{{\rm{crystal}}}}$/eV ${E_{{\rm{ZP}}}}$/eV ${E_{{\rm{bind}}}}$/eV $E_{{\rm{form}}}$/eV $\Delta H_{{ {\rm{sol} } } }^{\rm{H} }$/eV α-Fe+H(T-site) –13861.050 0.246 5.182 0.390 0.390 α-Fe –13845.344 — 5.530 — 表 3 α-Fe+H (T-site)晶体原子轨道布居
Table 3. Atomic orbital population of α-Fe+H (T-site).
晶体类型 原子 轨道电荷占据数 总布居 净布居 s p d α-Fe-H(T-site) H 1.34 0 0 1.34 –0.34 Fe2, Fe4 0.62 0.66 6.65 7.93 0.07 Fe3, Fe11 0.62 0.67 6.65 7.94 0.06 Fe12, Fe10 0.65 0.72 6.62 7.99 0.01 Fe1, Fe9, Fe13, Fe14, Fe16 0.65 0.72 6.62 7.99 0.01 Fe8, Fe6 0.65 0.74 6.61 8.01 –0.01 α-Fe Fe 0.68 0.70 6.62 8.00 0 自由态 H 1.00 0 0 1.00 0 表 4 α-Fe-H (T-site)晶体键布居
Table 4. Bond population of α-Fe+H (T-site).
晶体类型 原子对 距离/Å 键布居 α-Fe-H (T-site) Fe2-H 1.6494 0.16 Fe3-H 1.6507 0.16 Fe2-Fe3 2.5558 –0.09 Fe2-Fe4 2.7286 –0.14 Fe8-Fe11 2.4783 0.17 Fe3-Fe12 2.4471 0.18 Fe8-Fe16 2.8401 0.05 Fe7-Fe8 2.4601 0.15 α-Fe-H (O-site) Fe2-Fe4 2.6287 –0.28 α-Fe Fe-Fe 2.4400 0.14 Fe-Fe 2.8174 0.06 表 5 α-Fe+(nH-Vac)的晶格常数和晶体结构
Table 5. Lattice parameters and crystalline structure of α-Fe+(nH-Vac).
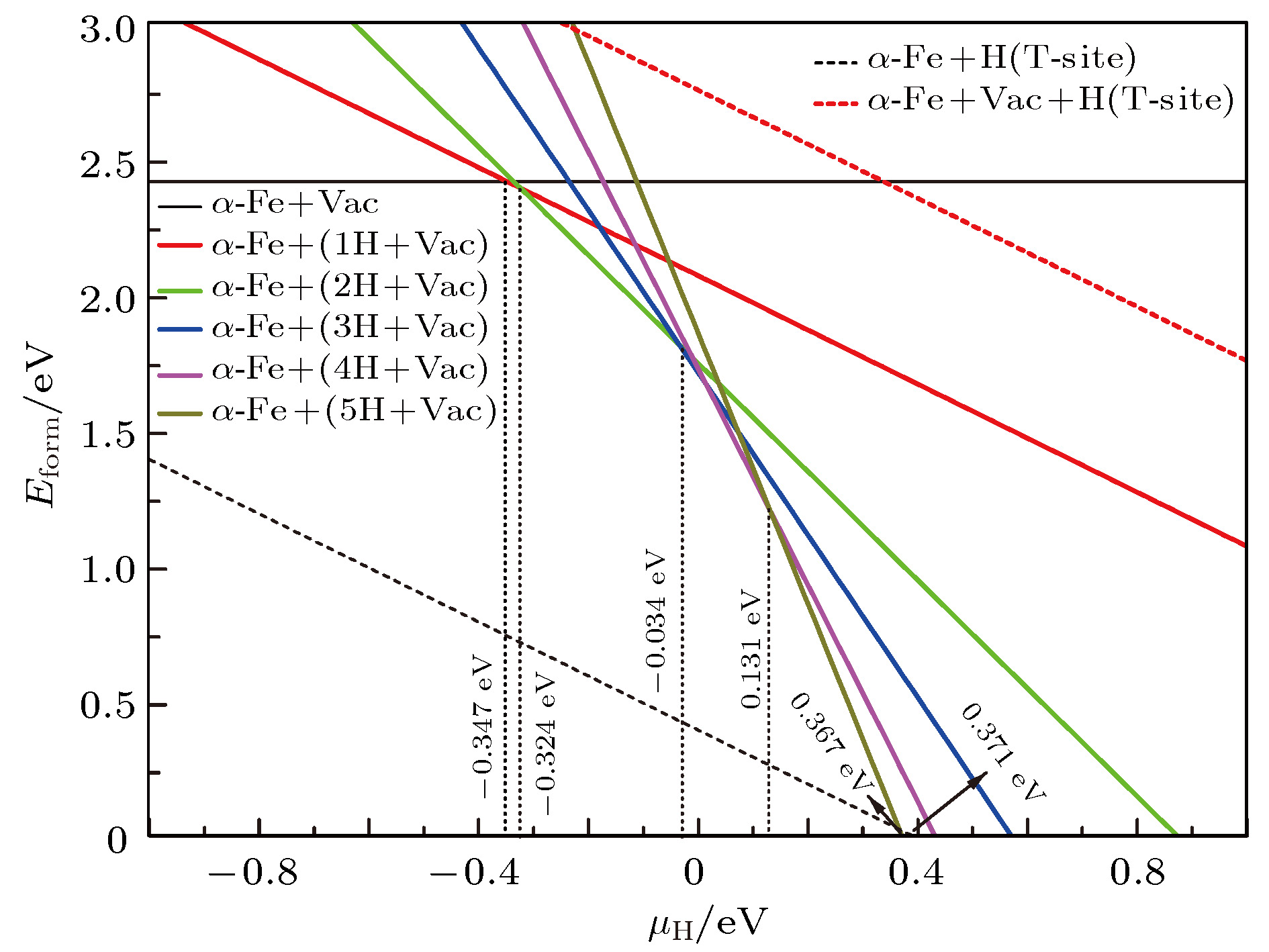
晶体类型 a/Å b/Å c/Å α/(°) β/(°) γ/(°) V/Å 空间群 α-Fe+Vac 5.6033 5.6033 5.6033 90.000 90.000 90.000 175.923 221${\rm{(}}Pm\overline {{\rm{3}}m} )$ α-Fe+(Vac-1H) 5.6321 5.6103 5.6103 90.000 90.001 89.999 177.270 99${\rm{(}}P{\rm{4}}MM)$ α-Fe+(Vac-2H) 5.6285 5.6285 5.6484 90.000 90.000 90.000 178.940 123(P4/MMM) α-Fe+(Vac-3H) 5.6297 5.6598 5.6853 90.004 90.011 90.002 181.154 25(PMM2) α-Fe+(Vac-4H) 5.6727 5.6943 5.6723 89.966 90.540 89.973 183.221 38(AMM2) α-Fe+(Vac-5H) 5.6905 5.7086 5.7093 90.000 90.004 90.002 185.467 99${\rm{(}}P{\rm{4}}MM)$ α-Fe+(Vac-6H) 5.7407 5.7270 5.7208 89.433 89.691 89.692 188.064 5(C2) 表 6 α-Fe+(nH-Vac)的结合能、形成能和溶解热
Table 6. Binding energy, formation energy, and heat of solution of α-Fe+(nH-Vac).
晶体类型 ${E_{{\rm{crystal}}}}$/eV ${E_{{\rm{ZP}}}}$/eV ${E_{{\rm{bind}}}}$/eV $E_{{\rm{form}}}$/eV $\Delta H_{_{ {\rm{sol} } } }^{\rm{H} }$/eV α–Fe+Vac –12977.593 — 5.369 2.416 — α–Fe+(Vac-1H) –12993.933 0.141 5.055 1.928 –0.347 α–Fe+(Vac-2H) –13010.262 0.295 4.777 1.450 –0.324 α–Fe+(Vac-3H) –13026.330 0.478 4.513 1.234 –0.034 α–Fe+(Vac-4H) –13042.359 0.670 4.275 1.056 0.014 α–Fe+(Vac-5H) –13058.299 0.889 4.055 0.968 0.131 α–Fe+(Vac-6H) –13073.995 1.149 3.842 1.123 0.438 表 7 α-Fe+(nH-Vac)对H原子的陷阱能
Table 7. Hydrogen trapping energy of α-Fe+(nH-Vac)
晶体类型 $E_{{\rm{trap}}}^{\rm{H}}$/eV 不考虑${E_{{\rm{ZP}}}}$ 考虑${E_{{\rm{ZP}}}}$ α-Fe+(Vac-1H) 0.633 0.778 α-Fe+(Vac-2H) 0.623 0.627 α-Fe+(Vac-3H) 0.361 0.211 α-Fe+(Vac-4H) 0.322 –0.011 α-Fe+(Vac-5H) 0.227 –0.297 α-Fe+(Vac-6H) –0.028 –0.772 表 8 α-Fe+(nH-Vac)晶体原子轨道布居
Table 8. Atomic orbital population of α-Fe+(nH-Vac).
晶体类型 原子 轨道电荷占据数 总布居 净布居 s p d α-Fe+Vac Fe7, Fe11, Fe13 0.74 0.72 6.68 8.13 –0.13 Fe3, Fe5, Fe9 0.68 0.73 6.63 8.04 –0.04 其余Fe原子 0.66 0.67 6.60 7.93 0.07 α-Fe+(Vac-2H) Fe13 0.71 0.71 6.73 8.15 –0.15 Fe9 0.66 0.70 6.62 7.98 0.02 Fe11 0.71 0.71 6.65 8.07 –0.07 α-Fe+Vac—α-Fe+(Vac-6H) H1 1.20—1.22 0 0 1.20—1.22 –0.20— –0.22 自由态 H 1.00 0 0 1.00 0 表 9 α-Fe+Vac+H(T-site)的结合能、形成能和溶解热
Table 9. Binding energy, formation energy, and heat of solution of α-Fe+Vac+H(T-site).
晶体类型 ${E_{{\rm{crystal}}}}$/eV ${E_{{\rm{ZP}}}}$/eV ${E_{{\rm{bind}}}}$/eV $E_{{\rm{form}}}$/eV $\Delta H_{{ {\rm{sol} } } }^{\rm{H} }$/eV α–Fe+Vac+H(T-site) –12993.354 — –12993.353 0.248—0.250 5.012 2.755—2.756 0.339—0.340 表 10 H平衡溶解度计算值和实验值
Table 10. Calculated and test value of equilibrium concentration of H atom.
温度/K μH/eV cH/% 计算值 实验值 298.15 –0.239 2.08 × 10–2 4.41 × 10–2
2.88 × 10–2, 其中晶格溶H占总扩散H含量的43%[2] -
[1] 张文钺 1999 焊接冶金学(基本原理)(北京: 机械工业出版社) 第241—244页
Zhang W Y 2012 Welding Metallurgy (Fundamental Principle) (Beijing: China Machine Press) pp241–244 (in Chinese)
[2] 张敬强 2015 博士学位论文 (哈尔滨: 哈尔滨工业大学)
Zhang J Q 2015 Ph.D Dissertation (Harbin: Harbin Institute of Technology) (in Chinese)
[3] Tateyama Y, Ohno T 2003 ISIJ Internation 43 573
 Google Scholar
Google Scholar
[4] Geng W T, Wan L, Du J P, Ogata S 2017 Scripta Materialia 134 105
 Google Scholar
Google Scholar
[5] MonasterioP R, Lau T T, Yip S, van Vliet K J 2009 Phys. Rev. Lett. 103 085501
 Google Scholar
Google Scholar
[6] Nagumo M, Nakamura M, Taika K 2001 Metall. Mater. Trans. A 32 339
 Google Scholar
Google Scholar
[7] Först C J, Slycke J, van Vliet K J, Yip S 2006 Phys. Rev. Lett. 96 175501
 Google Scholar
Google Scholar
[8] Fukai Y 1983 Jpn. J. Appl. Phys. 22 207
 Google Scholar
Google Scholar
[9] 张凤春, 李春福, 文平, 罗强, 冉曾令 2014 63 227101
 Google Scholar
Google Scholar
ZhangF C, Li C F, Wen P, Luo Q, Ran Z L 2014 Acta Phys. Sin. 63 227101
 Google Scholar
Google Scholar
[10] Du Y A, Ismer L, Rogal J, Hickel T, Neugebauer J, Drautz R 2011 Phys. Rev. B 84 144121
 Google Scholar
Google Scholar
[11] Nazarov R, Hickel T, Neugebauer J 2014 Phys. Rev. B 89 144108
 Google Scholar
Google Scholar
[12] Nazarov R, Hickel T, Neugebauer J 2012 Phys. Rev. B 85 144118
 Google Scholar
Google Scholar
[13] Hickel T, Grabowski B, Körmann F, Neugebauer J 2011 J. Phys. Condens. Mater. 24 053202
 Google Scholar
Google Scholar
[14] Ramasubramaniam A, Itakura M, Carter EA 2009 Phys. Rev. B 79 174101
 Google Scholar
Google Scholar
[15] Payne M C, Teter M P, Allan D C, Arias T A, Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
 Google Scholar
Google Scholar
[16] Milman V, Winkler B, White JA, Pickard C J, Payne M C, Akhmataskaya E V, Nobes R H 2000 Int. J. Quantum Chem. 77 895
 Google Scholar
Google Scholar
[17] Perdew JP, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[18] Methfessel M, Paxton A T 1989 Phys. Rev. B 40 3616
 Google Scholar
Google Scholar
[19] Acet M, Zähres H, Wassermann E F, Pepperhoff W 1994 Phys. Rev. B 49 6012
 Google Scholar
Google Scholar
[20] Hayward E, Fu C C 2013 Phys. Rev. B 87 174103
 Google Scholar
Google Scholar
[21] Baroni S, de Gironcoli S, Dal Corso A C, Giannozzi P 2001 Rev. Mod. Phys. 73 515
 Google Scholar
Google Scholar
[22] Bozzolo G, Ferrante J 1992 Phys. Rev. B 46 8600
 Google Scholar
Google Scholar
[23] Bhatia M A, Groh S, Solanki K N 2014 J. Appl. Phys. 116 064302
 Google Scholar
Google Scholar
[24] Tateyama Y, Ohno T 2003 Phys. Rev. B 67 174105
 Google Scholar
Google Scholar
[25] Mclellan R B, Harkins C G 1975 Mater. Sci. Eng. 18 5
 Google Scholar
Google Scholar
[26] Lynch SP 2013 Metall. Mater. Trans. A 44 1209
 Google Scholar
Google Scholar
[27] Lu G, Kaxiras E 2005 Phys. Rev. Lett. 94 155501
 Google Scholar
Google Scholar
[28] Ismer L, Hickel T, Neugebauer J 2010 Phys. Rev. B 81 094111
 Google Scholar
Google Scholar
[29] Korzhavyi P A, Abrikosov I A, Johansson B, Ruban A V, Skriver H L 1999 Phys. Rev. B 59 11693
 Google Scholar
Google Scholar
[30] Nazarov R, Hickel T, Neugebauer J 2010 Phys. Rev. B 82 224104
 Google Scholar
Google Scholar
[31] Speight J G 2005 Lange’s Handbook of Chemistry (16th Ed.) (New York: The McGraw-Hill Companies, Inc) pp1299−1309
Catalog
Metrics
- Abstract views: 13460
- PDF Downloads: 214
- Cited By: 0















 DownLoad:
DownLoad: