-
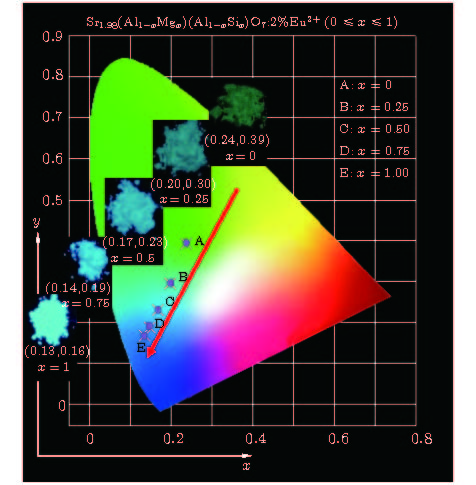
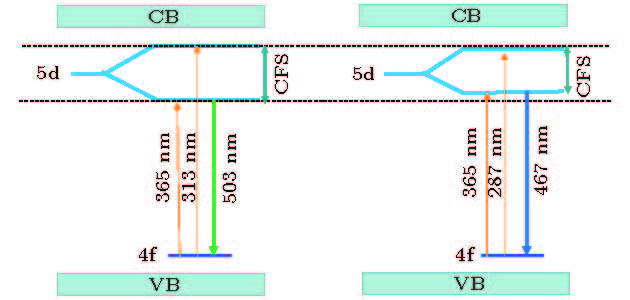
A series of Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+ phosphors is prepared by the high-temperature solid-state reaction method, the crystal structures and luminescent properties of the prepared phosphors are investigated by measuring the X-ray diffraction, luminescent spectra and optical microscope. The isomorphic compounds of Sr2Al2SiO7 and Sr2MgSi2O7 contain tetrahedra including [MgO4], [SiO4] and [AlO4]. Although the valences of the [MgO4]6–, [SiO4]4– and [AlO4]5– groups are different, the charge imbalance occurs when the [MgO4]6– and [SiO4]4– substitutes of [AlO4]5– and [AlO4]5–, respectively. While the groups are co-substituted, the charge imbalance disappears. And the larger volume of [MgO4] and the smaller volume of [SiO4] together replaces the similar volume of [AlO4], resulting in the decrease of [(Si/Al)O4] and increase of [(Mg/Al)O4]. Moreover, the decrease of unit cell parameters c and the increase of a and V due to the increased replacement of Mg2+ (0.57 Å for CN = 4) by Al3+(0.39 Å for CN = 4) and Si4+ (0.26 Å for CN = 4) by Al3+ (0.39 Å for CN = 4) cause the ambient temperature to change, the crystal field splitting of the Eu2+ cation to be weakened, and the emission spectra to be blue-shifted from 503 nm to 467 nm, which are closely related to the local coordination environment of the Eu2+, in addition, this reveals that the emission color of this series of phosphors can be tuned from green with color coordinate (0.2384, 0.3919) to blue (0.1342, 0.1673) by adjusting the chemical compositions via the [MgO4]6– and [SiO4]4– groups’ co-substitution for [AlO4]5–. The full width at half maximumof emission band is 120 nm when x = 0, the photoluminescence emission width decreases monotonically from 89 to 50 nm as x is increased from 0.25 to 1. In other words, the full width at half maximum of emission band exhibits a decreasing trend. The internal quantum efficiency is enhanced with increasing x in Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+ phosphors. These results verify that the groups’ substitutions are enhanced with polyhedron changing in the solid solutions and contribute largely to the luminescence properties of the phosphor.
-
Keywords:
- Sr2(Al1–xMgx)(Al1–xSi1+x)O7 /
- photoluminescence /
- co-substitution /
- coordinating polyhedron
[1] Chen M Y, Xia Z G, Molokeev M S, Wang T, Liu Q L 2017 Chem. Mater. 29 1430
 Google Scholar
Google Scholar
[2] Li S X, Wang L, Zhu Q Q, Tang D M, Liu X J, Cheng G F, Lu L, Taked T, Hirosaki N, Huang Z R, Xie R J 2016 J. Mater. Chem. C 4 11219
 Google Scholar
Google Scholar
[3] Li S X, Wang L, Tang M M, Cho Y J, Liu X J, Zhou X T, Lu L, Zhang L, Taked T, Hirosaki N, Xie R J 2018 Chem. Mater. 30 494
 Google Scholar
Google Scholar
[4] Xia Z G, Liu G K, Wen J G, Mei Z G, Balasubramanian M, Molokeev M S, Peng L C, Gu L, Miller D J, Liu Q L, Poeppelmeier K R 2016 J. Am. Chem. Soc. 138 1158
 Google Scholar
Google Scholar
[5] Xia Z G, Poeppelmeier K R 2017 Accounts Chem. Res. 50 1222
 Google Scholar
Google Scholar
[6] Ji H P, Huang Z H, Xia Z G, Molokeev M S, Atuchin V V, Fang M H, Liu Y G 2015 J. Phys. Chem. 119 2038
 Google Scholar
Google Scholar
[7] Xia Z G, Liu Q 2016 Prog. Mater. Sci. 84 59
 Google Scholar
Google Scholar
[8] Ye S, Xiao F, Pan Y X, Ma Y Y, Zhang Q Y 2011 Mat. Sci. Eng. R. 71 1
[9] Shang M M, Liang S S, Qu N R, Lian H Z, Lin J 2017 Chem. Mater. 29 1813
 Google Scholar
Google Scholar
[10] Dubey S, Deshmukh P, Satapathy S, Singh M K, Gupta P K 2016 Luminesence 32 839
 Google Scholar
Google Scholar
[11] 赵永旺, 苏全帅, 张超, 安胜利 2016 稀土 37 85
 Google Scholar
Google Scholar
Zhao Y W, Su Q S, Zhang C, An S L 2016 Chinese Rare Earths 37 85
 Google Scholar
Google Scholar
[12] 赵永旺, 张超, 赵文广, 安胜利 2017 稀土 38 87
Zhao Y W, Zhang C, Zhao G W, An S L 2017 Chinese Rare Earths 38 87
[13] Shuang Y M, Zhu F L, Wang J D 2008 J. Func. Mater. 39 1078
[14] Xia Z G, Ma C G, Molokeev M S, Liu Q L, Rickert K, Poeppelmeier K R 2015 J. Am. Chem. Soc. 137 12494
 Google Scholar
Google Scholar
[15] Lu F C, Bai L J, Dang W, Yang Z P, Lin P 2015 ECS J. Solid State Sc. 4 27
 Google Scholar
Google Scholar
[16] Tam T T H, Hung N Y, Lien N D K, Kien N D T, Huy P T 2016 Sci. Adv. Mater. 1 204
 Google Scholar
Google Scholar
[17] 梁敬魁 2011 粉末衍射法测定晶体结构(上册)(北京:科学出版社) 第78页
Liang J K 2003 Determination of Crystal Structure by Powder Diffraction (Vol. 1) (Beijing: Science Press) p78 (in Chinese)
[18] Denault K A, George N C, Paden S R, Brinkley S, Mikhailovsky A A, Neuefeind J, DenBaars S P, Seshadri R J 2012 Mater. Chem. 22 18204
 Google Scholar
Google Scholar
[19] Denault K A, Brgoch J, Gaultois M W, Mikhailovsky A, Petry R, Winkler H, DenBaars S P, Seshadri R 2014 Chem. Mater. 26 2275
 Google Scholar
Google Scholar
[20] Guo Y, Park S H, Choi B C, Jeong J H, Kim J H 2018 J. Alloy. Compd. 742 159
 Google Scholar
Google Scholar
-
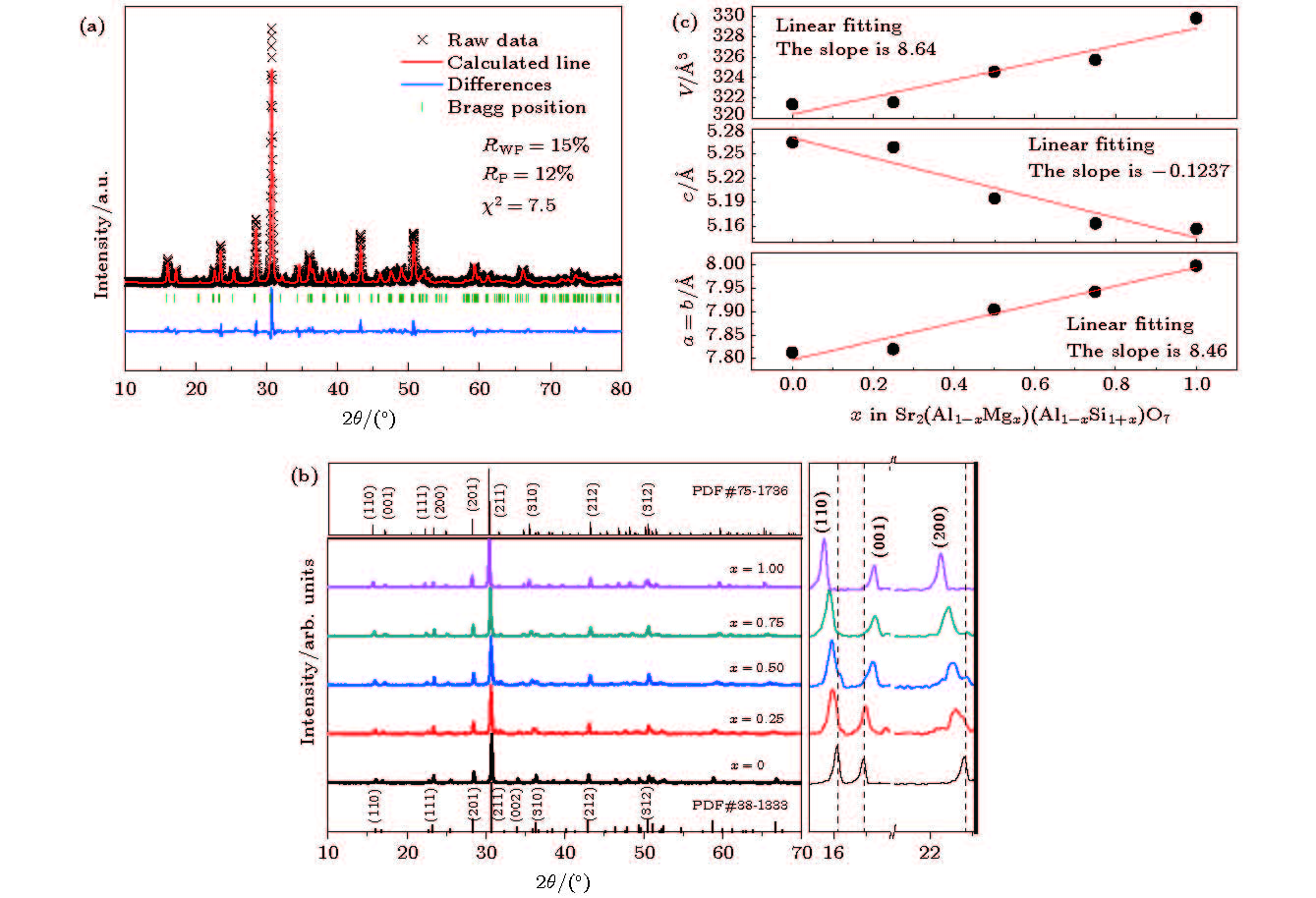
图 1 (a) 样品C的XRD精修图谱;(b) Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x
≤ 1)荧光粉的XRD图谱和2θ范围为15°—23°的峰位移动放大图;(c)晶胞参数随浓度x变化曲线图 Figure 1. (a) Rietveld refinement of C; (b) XRD patterns of the Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x
≤ 1), the right inset is the magnified XRD patterns for 2θ region form 15° to 23°; (c) give the cell parameters (a/b, c) and volume (V), respectively, as a function of x concentration 图 3 (a), (b)在波长365 nm激发下样品A, C, E的光学显微镜图像和Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x ≤ 1)荧光粉的归一化发射光谱
Figure 3. (a) Optical microscope image of A, C, E excited at a wavelength of 365 nm and (b) normalized emission spectra of Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x ≤ 1) phosphors under 365 nm UV light excitation
图 5 在荧光粉Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x ≤ 1)中, 随着x的增加, 多面体[(Si/Al)O4]的收缩和多面体[(Mg/Al)O4]的膨胀对发光中心多面体的扭曲
Figure 5. Increasing of x leads to polyhedral [(Si/Al)O4] shrinkage and polyhedral [(Mg/Al)O4] expansion distortion the luminescent center polyhedron of Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x ≤ 1) phosphors
表 1 Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0≤x ≤ 1)荧光粉发射波长的半高宽, 色坐标值和内量子效率
Table 1. The PL bands, color coordinate value, and internal quantum efficiency of Sr1.98(Al1–xMgx)(Al1–xSi1+x)O7: 2%Eu2+(0 ≤ x ≤ 1) phosphors
样品 发射光谱 色坐标 IQY λem/nm FWHM/nm x y A 503 120 0.2384 0.3919 4.3% B 472 89 0.1972 0.2951 6.1% C 470 61 0.1675 0.2312 7.3% D 468 54 0.1463 0.1873 9.0% E 468 50 0.1342 0.1673 23% -
[1] Chen M Y, Xia Z G, Molokeev M S, Wang T, Liu Q L 2017 Chem. Mater. 29 1430
 Google Scholar
Google Scholar
[2] Li S X, Wang L, Zhu Q Q, Tang D M, Liu X J, Cheng G F, Lu L, Taked T, Hirosaki N, Huang Z R, Xie R J 2016 J. Mater. Chem. C 4 11219
 Google Scholar
Google Scholar
[3] Li S X, Wang L, Tang M M, Cho Y J, Liu X J, Zhou X T, Lu L, Zhang L, Taked T, Hirosaki N, Xie R J 2018 Chem. Mater. 30 494
 Google Scholar
Google Scholar
[4] Xia Z G, Liu G K, Wen J G, Mei Z G, Balasubramanian M, Molokeev M S, Peng L C, Gu L, Miller D J, Liu Q L, Poeppelmeier K R 2016 J. Am. Chem. Soc. 138 1158
 Google Scholar
Google Scholar
[5] Xia Z G, Poeppelmeier K R 2017 Accounts Chem. Res. 50 1222
 Google Scholar
Google Scholar
[6] Ji H P, Huang Z H, Xia Z G, Molokeev M S, Atuchin V V, Fang M H, Liu Y G 2015 J. Phys. Chem. 119 2038
 Google Scholar
Google Scholar
[7] Xia Z G, Liu Q 2016 Prog. Mater. Sci. 84 59
 Google Scholar
Google Scholar
[8] Ye S, Xiao F, Pan Y X, Ma Y Y, Zhang Q Y 2011 Mat. Sci. Eng. R. 71 1
[9] Shang M M, Liang S S, Qu N R, Lian H Z, Lin J 2017 Chem. Mater. 29 1813
 Google Scholar
Google Scholar
[10] Dubey S, Deshmukh P, Satapathy S, Singh M K, Gupta P K 2016 Luminesence 32 839
 Google Scholar
Google Scholar
[11] 赵永旺, 苏全帅, 张超, 安胜利 2016 稀土 37 85
 Google Scholar
Google Scholar
Zhao Y W, Su Q S, Zhang C, An S L 2016 Chinese Rare Earths 37 85
 Google Scholar
Google Scholar
[12] 赵永旺, 张超, 赵文广, 安胜利 2017 稀土 38 87
Zhao Y W, Zhang C, Zhao G W, An S L 2017 Chinese Rare Earths 38 87
[13] Shuang Y M, Zhu F L, Wang J D 2008 J. Func. Mater. 39 1078
[14] Xia Z G, Ma C G, Molokeev M S, Liu Q L, Rickert K, Poeppelmeier K R 2015 J. Am. Chem. Soc. 137 12494
 Google Scholar
Google Scholar
[15] Lu F C, Bai L J, Dang W, Yang Z P, Lin P 2015 ECS J. Solid State Sc. 4 27
 Google Scholar
Google Scholar
[16] Tam T T H, Hung N Y, Lien N D K, Kien N D T, Huy P T 2016 Sci. Adv. Mater. 1 204
 Google Scholar
Google Scholar
[17] 梁敬魁 2011 粉末衍射法测定晶体结构(上册)(北京:科学出版社) 第78页
Liang J K 2003 Determination of Crystal Structure by Powder Diffraction (Vol. 1) (Beijing: Science Press) p78 (in Chinese)
[18] Denault K A, George N C, Paden S R, Brinkley S, Mikhailovsky A A, Neuefeind J, DenBaars S P, Seshadri R J 2012 Mater. Chem. 22 18204
 Google Scholar
Google Scholar
[19] Denault K A, Brgoch J, Gaultois M W, Mikhailovsky A, Petry R, Winkler H, DenBaars S P, Seshadri R 2014 Chem. Mater. 26 2275
 Google Scholar
Google Scholar
[20] Guo Y, Park S H, Choi B C, Jeong J H, Kim J H 2018 J. Alloy. Compd. 742 159
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11127
- PDF Downloads: 68
- Cited By: 0














 DownLoad:
DownLoad: