-
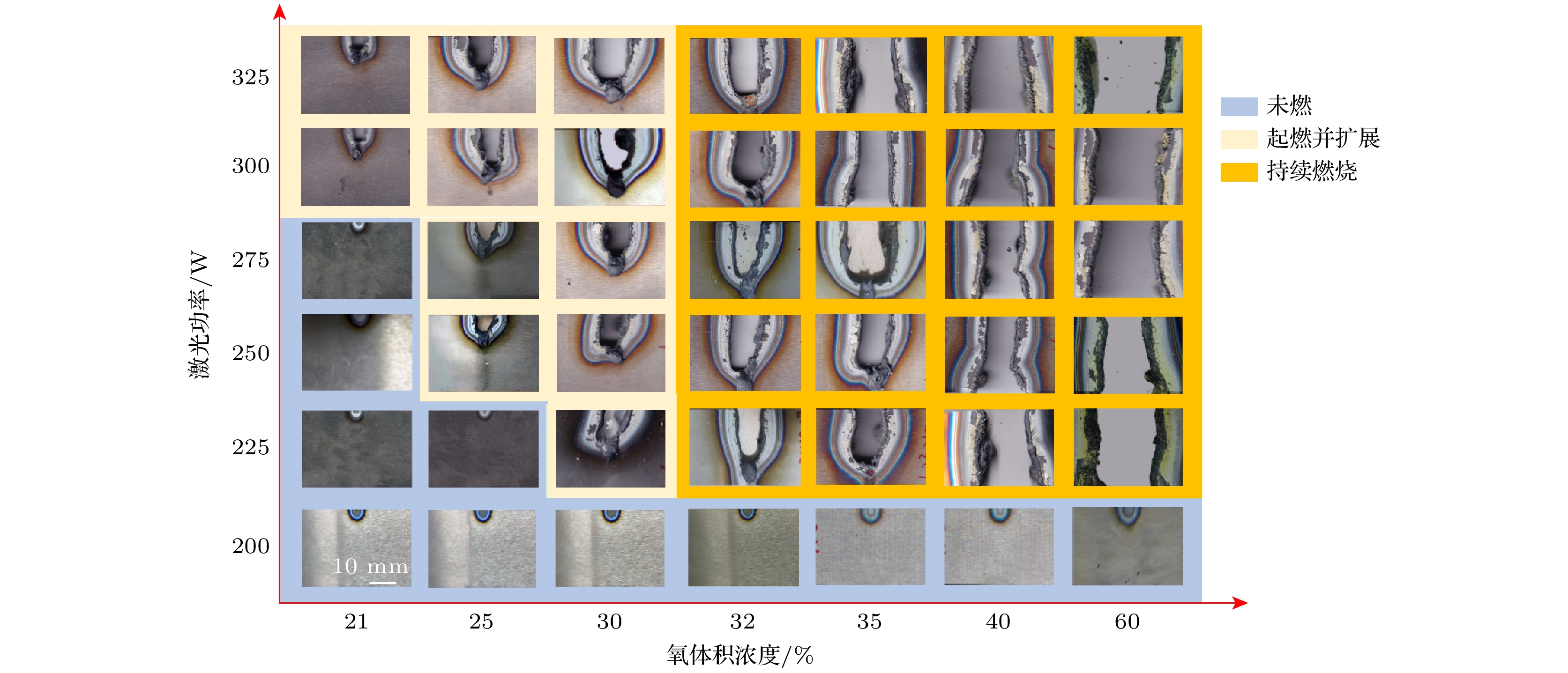
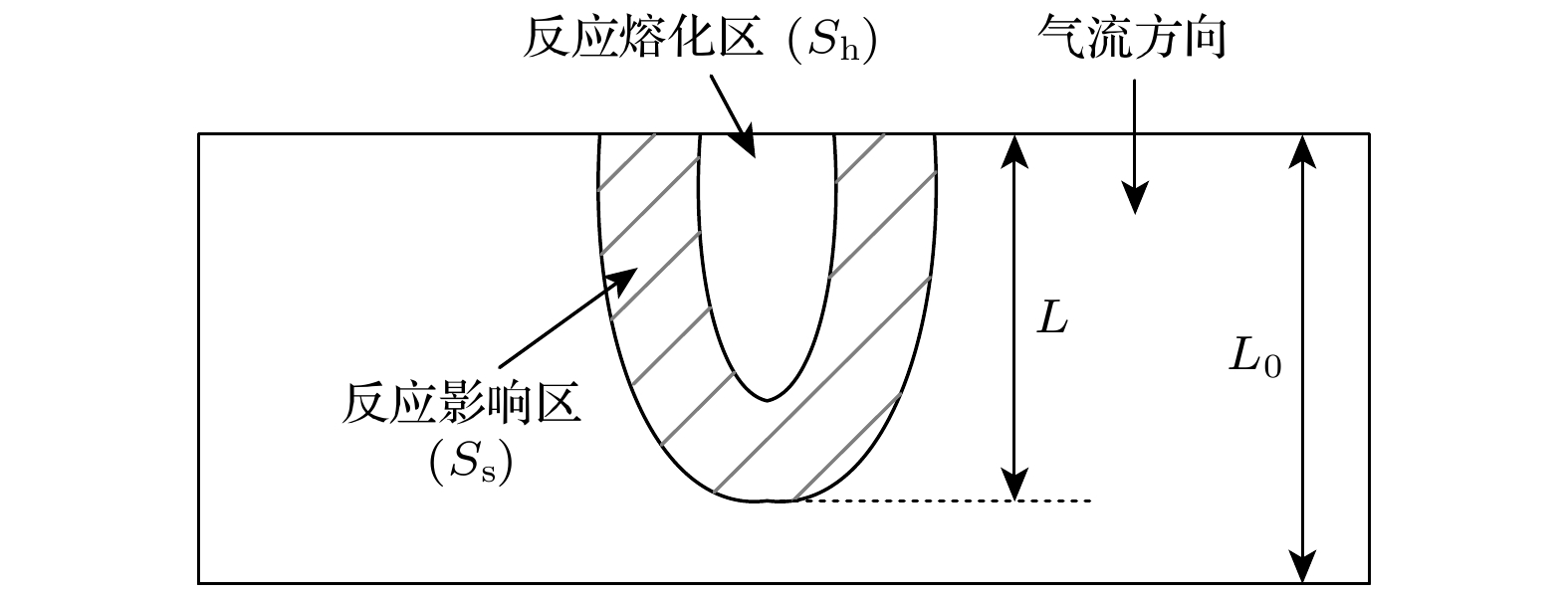
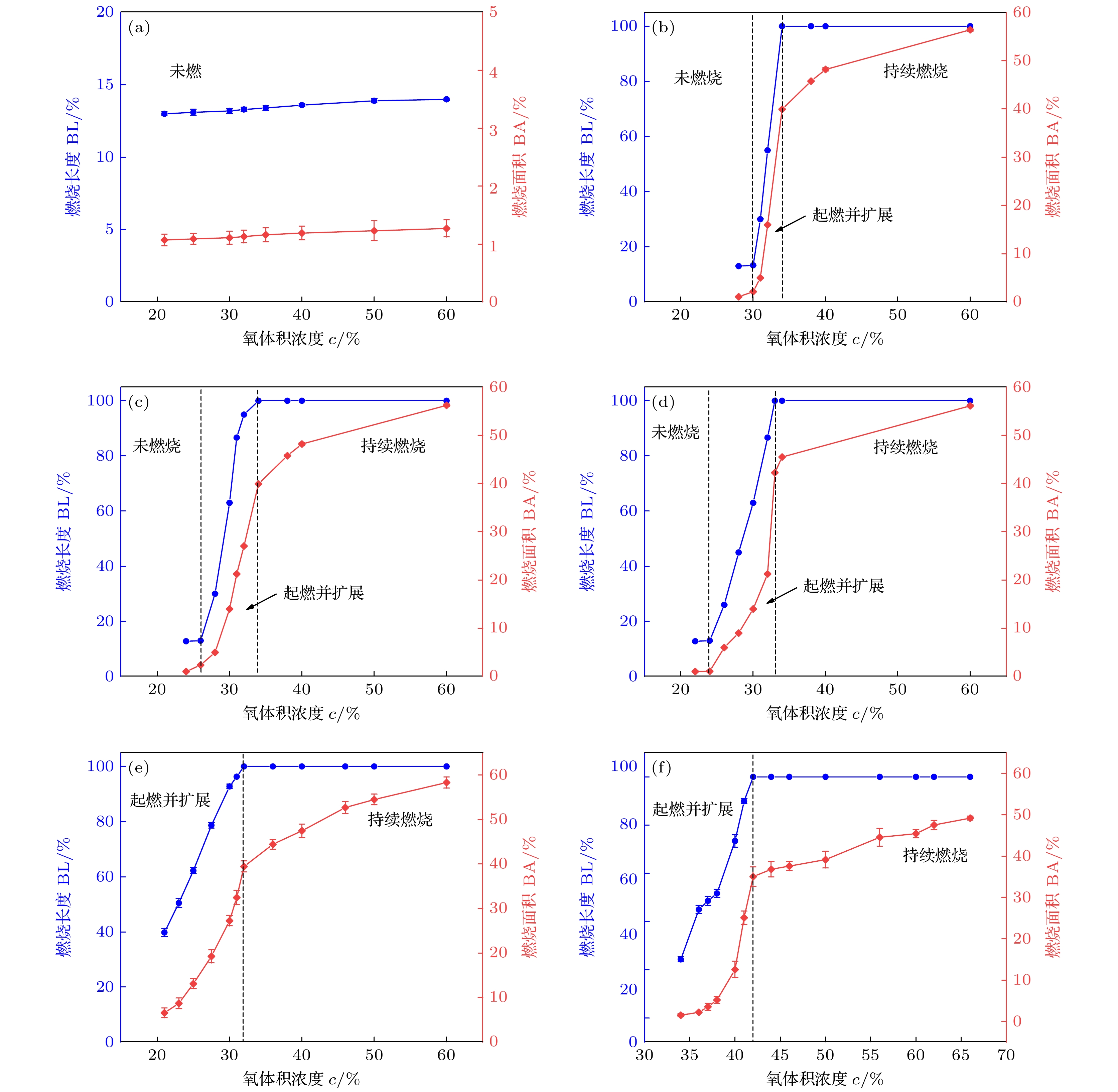
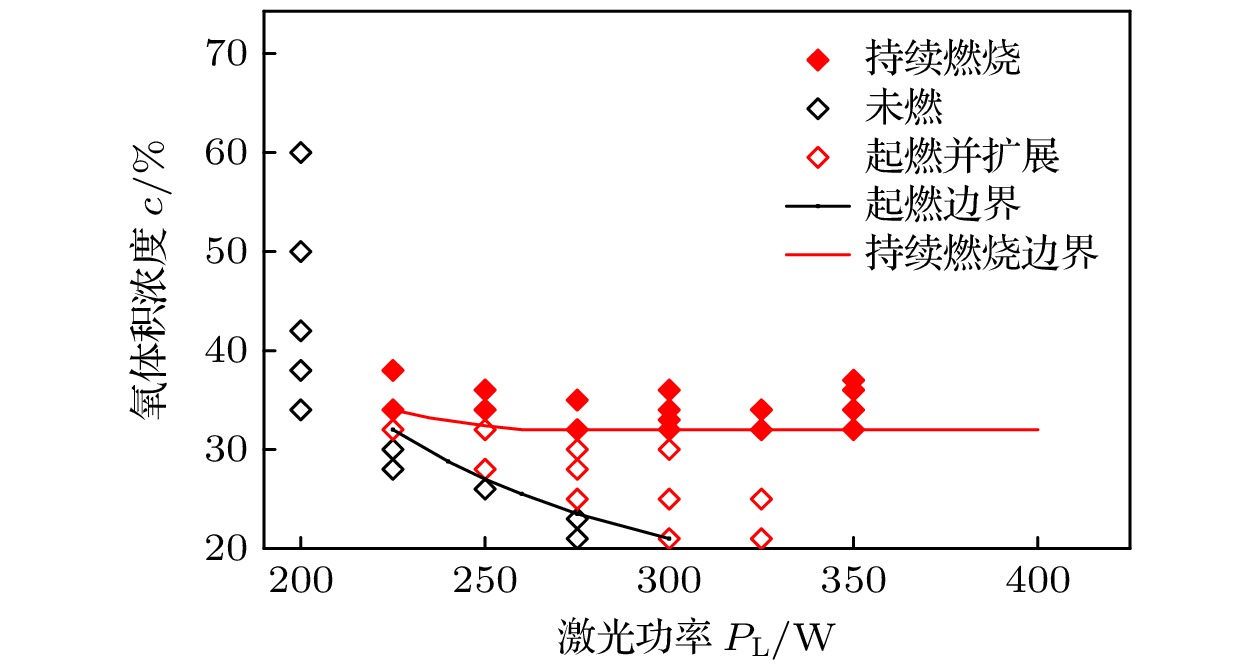
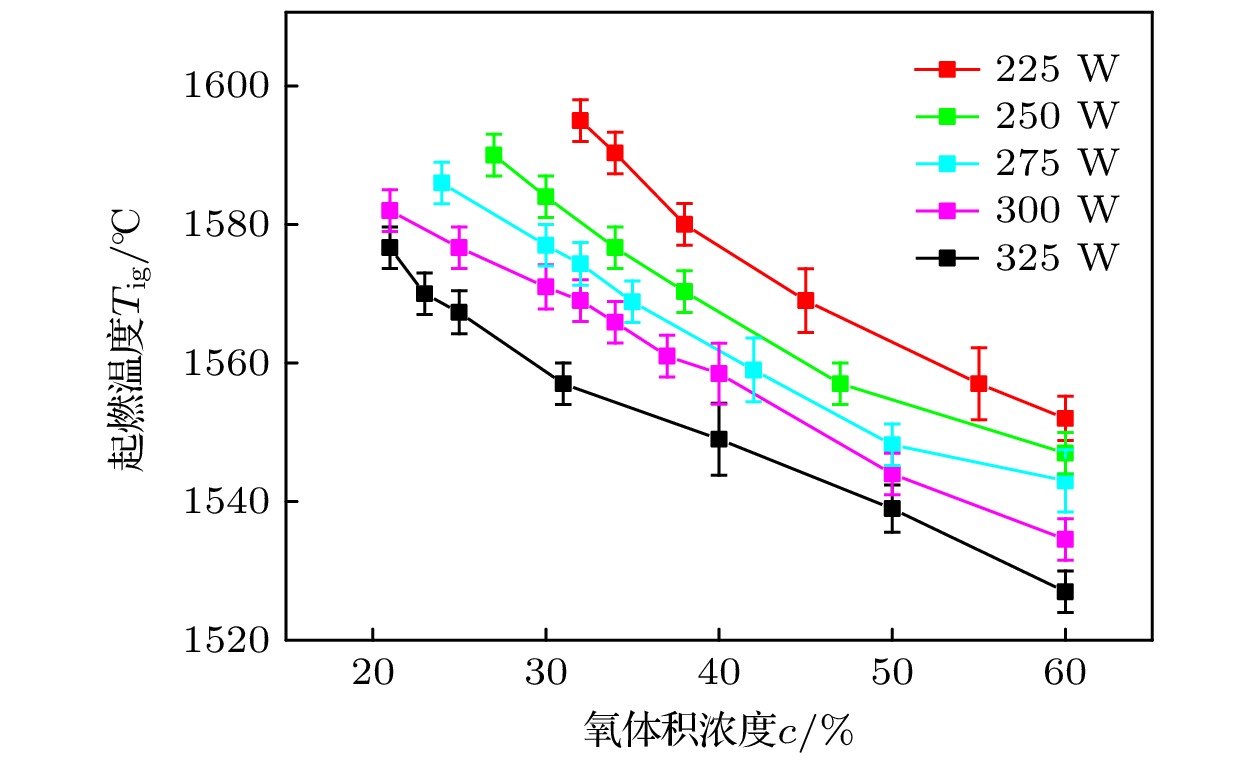
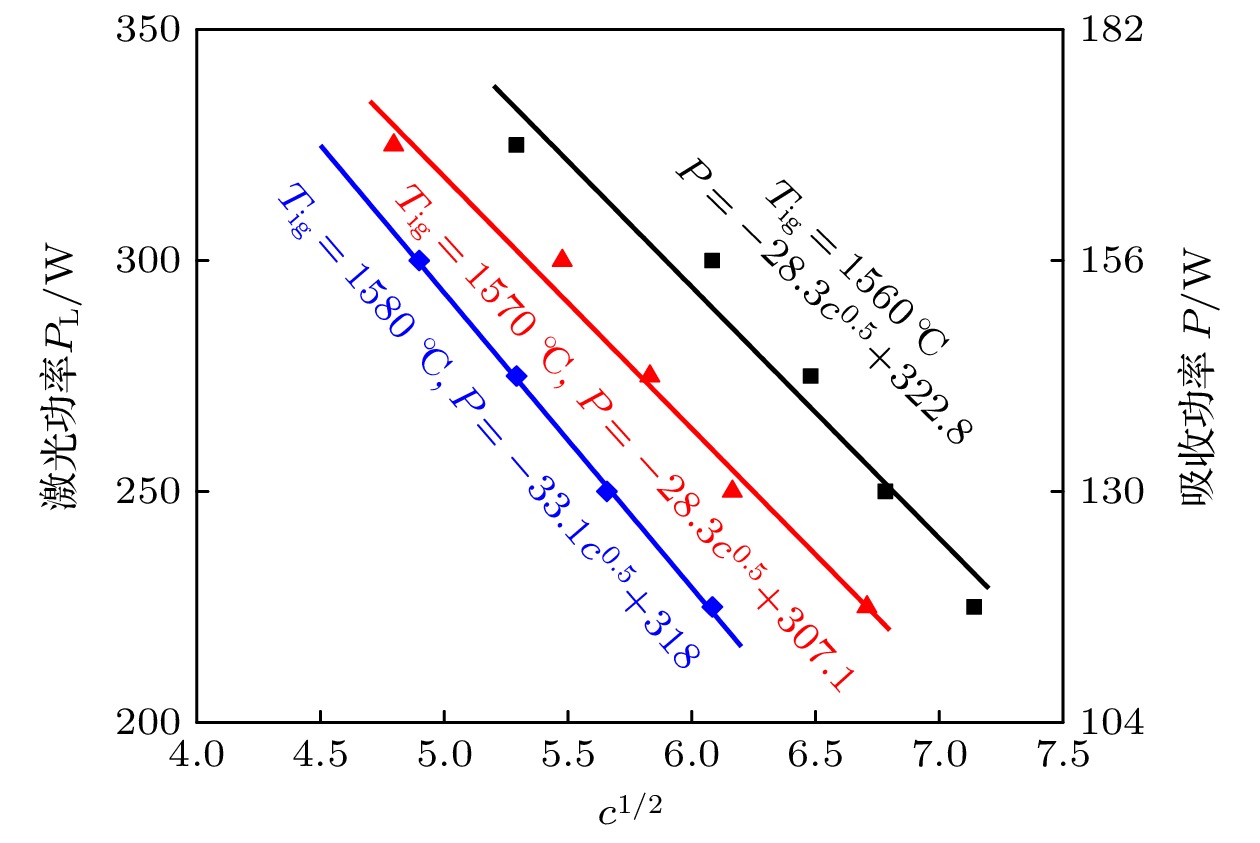
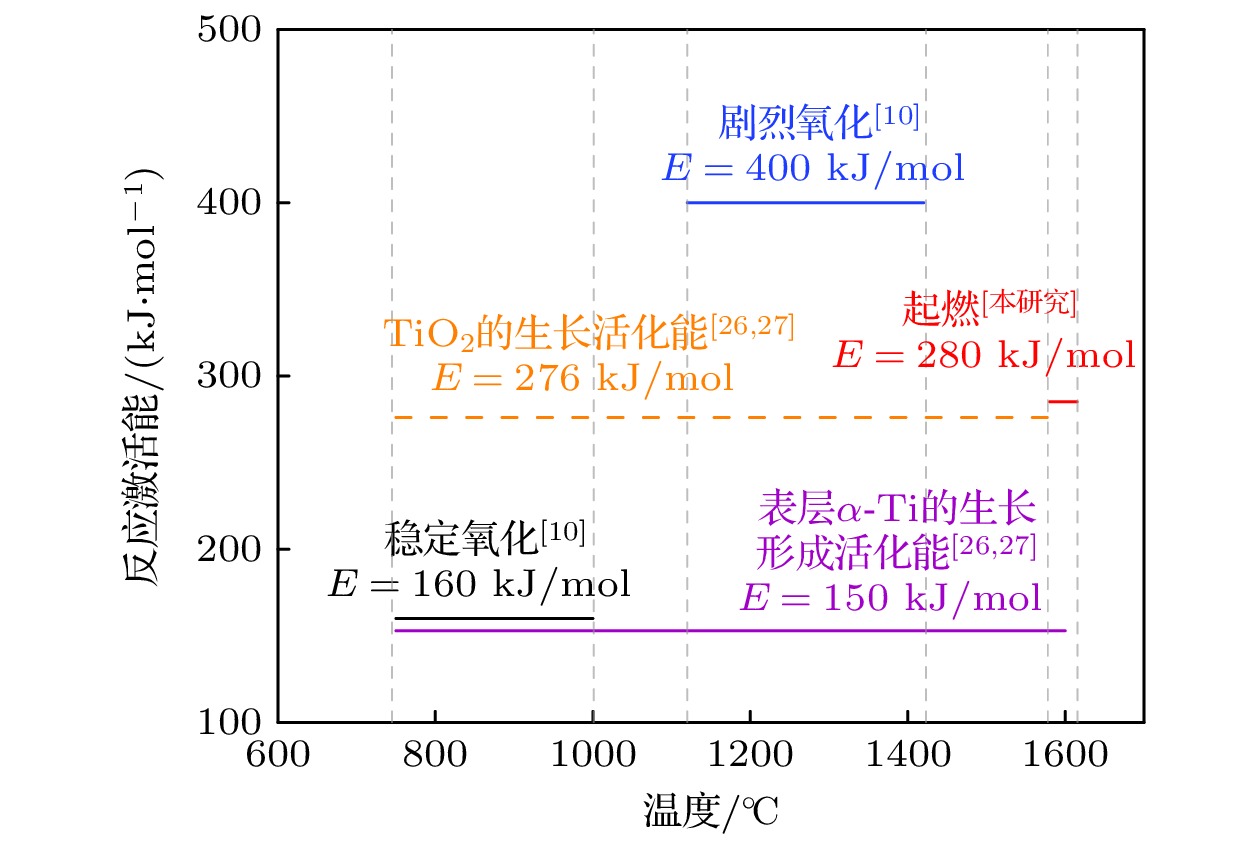
采用激光氧浓度实验方法, 研究激光功率和氧浓度对近α型高温钛合金(TA19合金)燃烧状态的影响, 发现合金的起燃温度随激光功率和氧浓度的增加而降低, 在200—325 W的激光功率以及21%—60%的氧体积浓度条件下, TA19合金的起燃温度为1527—1595 ℃, 低于合金熔点. 通过组织表征揭示保护性氧化层的失效形式, 并结合氧化层应力失效模型分析起燃机理: 1520 ℃以上TiO的高蒸气压特性导致表层TiO2下方形成孔隙缺陷, 加速TiO2层的热应力失效; 且起燃时需要同时满足临界温度条件和瞬时温度变化率条件. 在此基础上, 将保护性氧化层失效机理与能量方程结合以构建起燃模型; 根据实验数据拟合计算得到TA19合金在起燃阶段的反应激活能约为280 kJ/mol, 并得出起燃温度随激光功率和氧浓度变化的函数关系为
$ 1.2 \times {10^{10}}{{\mathrm{exp}}\Big({\dfrac{{ - 280000}}{{R{T_{{\text{ig}}}}}}}}\Big) {c^{\frac{1}{2}}} + 0.52{P_{\text{L}}} - 315 = 0 $ . 该结果对航空发动机复杂气流条件下的阻燃性能研究及其他类型钛合金的起燃温度预测提供理论参考.The risk of titanium fire increases significantly with the development of future aero-engine, however, the burning mechanisms of titanium alloys remain uncertain. Therefore, the ignition behavior and mechanism of near α high-temperature titanium alloy are studied in this work by an integrated experiment method, including laser-oxygen concentration ignition method, infrared temperature measurement and observation of molten metal by high-speed camera. Based on this, the ignition boundary curve is determined and the ignition temperature of the alloy is found to decrease from 1595 to 1527 ℃ with the laser power increasing from 200 to 325 W and oxygen concentration increasing from 21% to 60%. The ignition microstructure is characterized by FIB and TEM to study the evolution of reaction products. Pores are found to form beneath the TiO2 surface layer, which can be attributed to the instablity of TiO. The failure mechanism of protective oxide layer is further analyzed according to the thermal stress caused oxide layer damage model. When the temperature approaches the ignition temperature, which is below the melting point, the high vapor pressure of TiO leads to the formation of porous defects beneath the TiO2 surface, thus accelerating the fracture and failure of the TiO2 layer under thermal stress. It is revealed that critical conditions of temperature and instantaneous temperature change rate are needed to realize ignition. Based on this, an ignition model is further constructed to discuss the relationship among ignition temperature, laser power and oxgyen concentration. According to the experimental data fitting, the reaction activation energy of TA19 alloy during the ignition stage is calculated to be about 280 kJ/mol, and the function for calculating ignition temperature is given as follows:$ 1.2 \times {10^{10}}{{\mathrm{e}}^{\frac{{ - 280000}}{{R{T_{{\text{ig}}}}}}}}{c^{\frac{1}{2}}} + $ $ 0.52{P_{\mathrm{L}}} - 315 = 0 $ . This provides a theoretical reference for predicting the ignition temperatures of near α high temperature titanium alloy and other types of titanium alloys under complex airflow conditions in aircraft engines.-
Keywords:
- near α high temperature titanium alloy /
- protective oxide layer /
- ignition temperature /
- ignition mechanism
[1] 弭光宝, 谭勇, 陈航, 李培杰, 张学军 2024 航空材料学报 44 15
 Google Scholar
Google Scholar
Mi G B, Tan Y, Chen H, Li P J, Zhang X J 2024 J. Aeronaut. Mater. 44 15
 Google Scholar
Google Scholar
[2] Cai J M, Mi G B, Gao F, Huang H, Cao J X, Huang X, Cao C X 2016 J. Mater. Eng. 44 1
[3] Leyens C, Kocian K, Hausmann J, Kaysser W A 2003 Aerosp. Sci. Technol. 7 201
 Google Scholar
Google Scholar
[4] Wolf J S, Moyle D D, Pruitt A B, Bader J H 1976 J. Electrochem. Soc. 123 C251
[5] Leyens C, Peters M, Kaysser W A 1996 Mater. Sci. Technol. 15 1326
[6] Mi G B, Huang X, Li P J, Cao J X, Huang X, Cao C X 2012 Trans. Nonferrous Met. Soc. China 22 2409
 Google Scholar
Google Scholar
[7] Zhao Y Q, Zhou L, Deng J 1999 Rare Met. Mater. Eng. 28 77
[8] 弭光宝, 黄旭, 曹京霞, 王宝, 曹春晓 2016 65 056103
 Google Scholar
Google Scholar
Mi G B, Huang X, Cao J X, Wang B, Cao C X 2016 Acta Phys. Sin. 65 056103
 Google Scholar
Google Scholar
[9] Shao L, Xie G L, Liu X H, Wu Y, Tan Q, Xie L, Xin S W, Hao F, Yu J B, Xue W L, Feng K 2022 Corros. Sci. 194 109957
 Google Scholar
Google Scholar
[10] Ouyang P X, Mi G B, Cao J X, Huang X, He L J, Li P J 2018 Mater. Today Commun. 16 364
 Google Scholar
Google Scholar
[11] Rozenband V I, Vaganova N I 1992 Combust. Flame 88 113
 Google Scholar
Google Scholar
[12] 弭光宝, 黄旭, 曹京霞, 曹春晓 2012 航空材料学报 32 25
 Google Scholar
Google Scholar
Mi G B, Huang X, Cao J X, Cao C X 2012 J. Aeronaut. Mater. 32 25
 Google Scholar
Google Scholar
[13] Shafirovich E, Teoh S K, Varma A 2008 Combust. Flame 152 262
 Google Scholar
Google Scholar
[14] 吴明宇, 弭光宝, 李培杰, 黄旭 2023 72 166102
 Google Scholar
Google Scholar
Wu M Y, Mi G B, Li P J, Huang X 2023 Acta Phys. Sin. 72 166102
 Google Scholar
Google Scholar
[15] 梁贤烨, 弭光宝, 李培杰, 黄旭, 曹春晓 2020 65 216101
 Google Scholar
Google Scholar
Liang X Y, Mi G B, Li P J, Huang X, Cao C X 2020 Acta Phys. Sin. 65 216101
 Google Scholar
Google Scholar
[16] Shao L, Xie G L, Liu X H, Wu Y, Yu J B, Feng K, Xue W L 2021 Corros. Sci. 190 109641
 Google Scholar
Google Scholar
[17] Bolobov V I, Podlevskikh N A 2007 Combust. Explos. Shock Waves 43 405
 Google Scholar
Google Scholar
[18] Titanium Combustion Research Program and user's Manual for Deck CCD 1152-0.0, Glickstein M R http://apps.dtic.mil/ [2023-12-4]
[19] 弭光宝, 陈航 中国专利, ZL201711188505.5 [2017-11-23]]
Mi G B, Chen H Chinese Patent ZL201711188505.5 [2017-11-23]
[20] 黄伯云, 李成功, 石力开 2006 中国材料工程大典 (北京: 化学工业出版社) 第550页
Huang B Y, Li C G, Shi L K 2006 China Materials Engineering Cannon (Beijing: Chemistry Industry Press) p550
[21] Evans H E, Lobb R C 1984 Corros. Sci. 24 209
 Google Scholar
Google Scholar
[22] Evans H E, Lobb R C 1994 Mater. High Temp. 12 219
 Google Scholar
Google Scholar
[23] Park Y S, Butt D P 1998 Oxid. Met. 51 383
[24] 杨德钧, 沈卓身1999 金属腐蚀学(第一版) (北京: 冶金工艺出版社) 第11页
Yang D J, Shen Z S 1999 Metal Corrosion (Vol. 1) (Beijing: Metallurgical Industry Press) p11
[25] Bohle M, Etling D, Muller U, Sreenivasan K R S, Riedel U, Warnatz J 2004 Prandtl’s Essentials of Fluid Mechanics (Heidelberg: Springer) (2nd Ed.) p428
[26] Liu Z, Welsch G 1988 Metall. Trans. A 19 1121
 Google Scholar
Google Scholar
[27] Gaddam R, Sefer B, Pederson R, Antti M L 2015 Mater. Charact. 99 166
 Google Scholar
Google Scholar
-
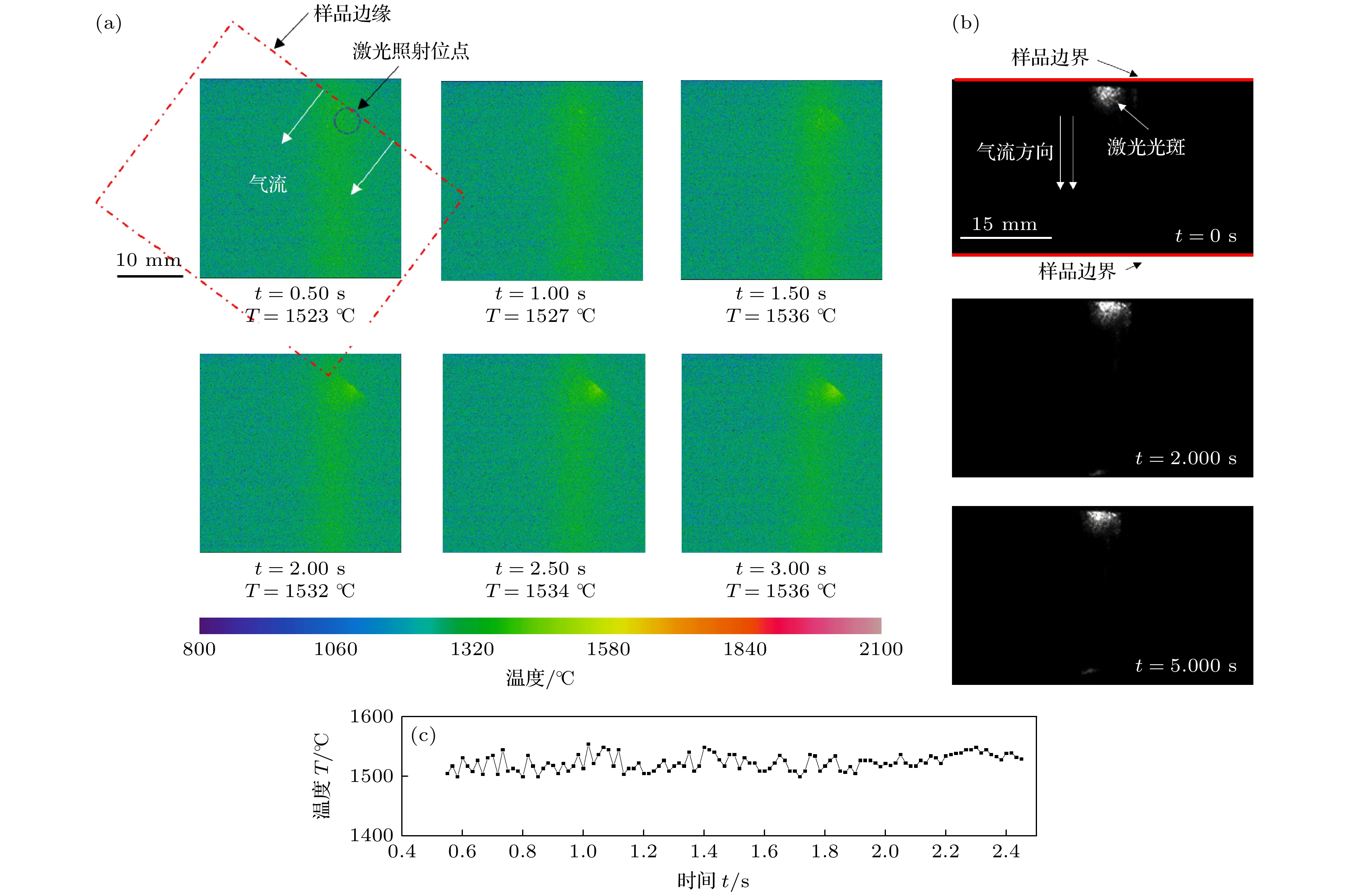
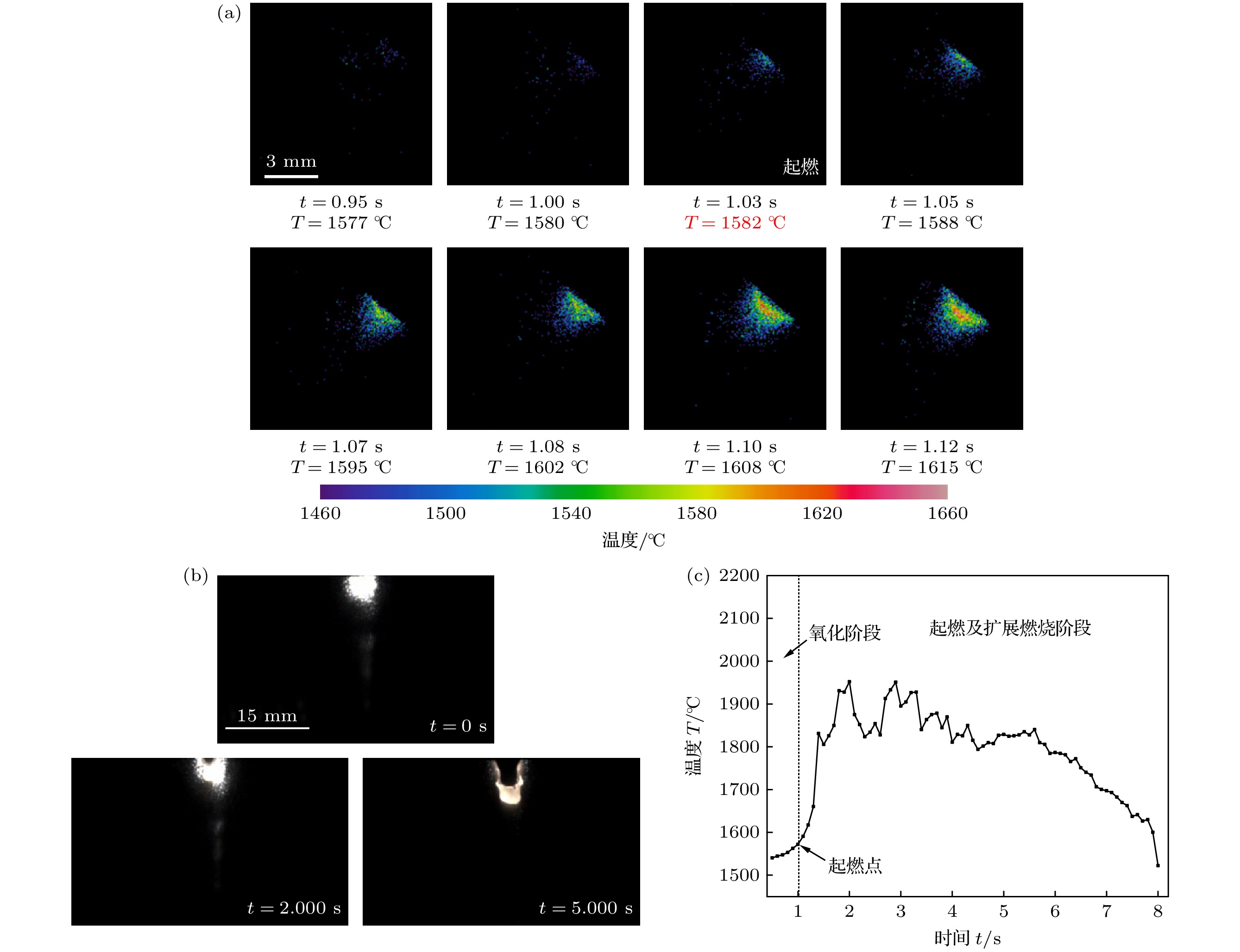
图 5 未燃烧TA19合金的温度及熔体特征 (a) 温度分布随时间的变化关系; (b) 熔体形成规律随时间的变化关系; (c) 试样最高温度随时间的变化规律
Fig. 5. The temperature and melt characteristics of unburnt TA19 alloy: (a) The relationship between temperature distribution and time; (b) the relationship between the formation of melt and time; (c) the variation of the highest temperature with time.
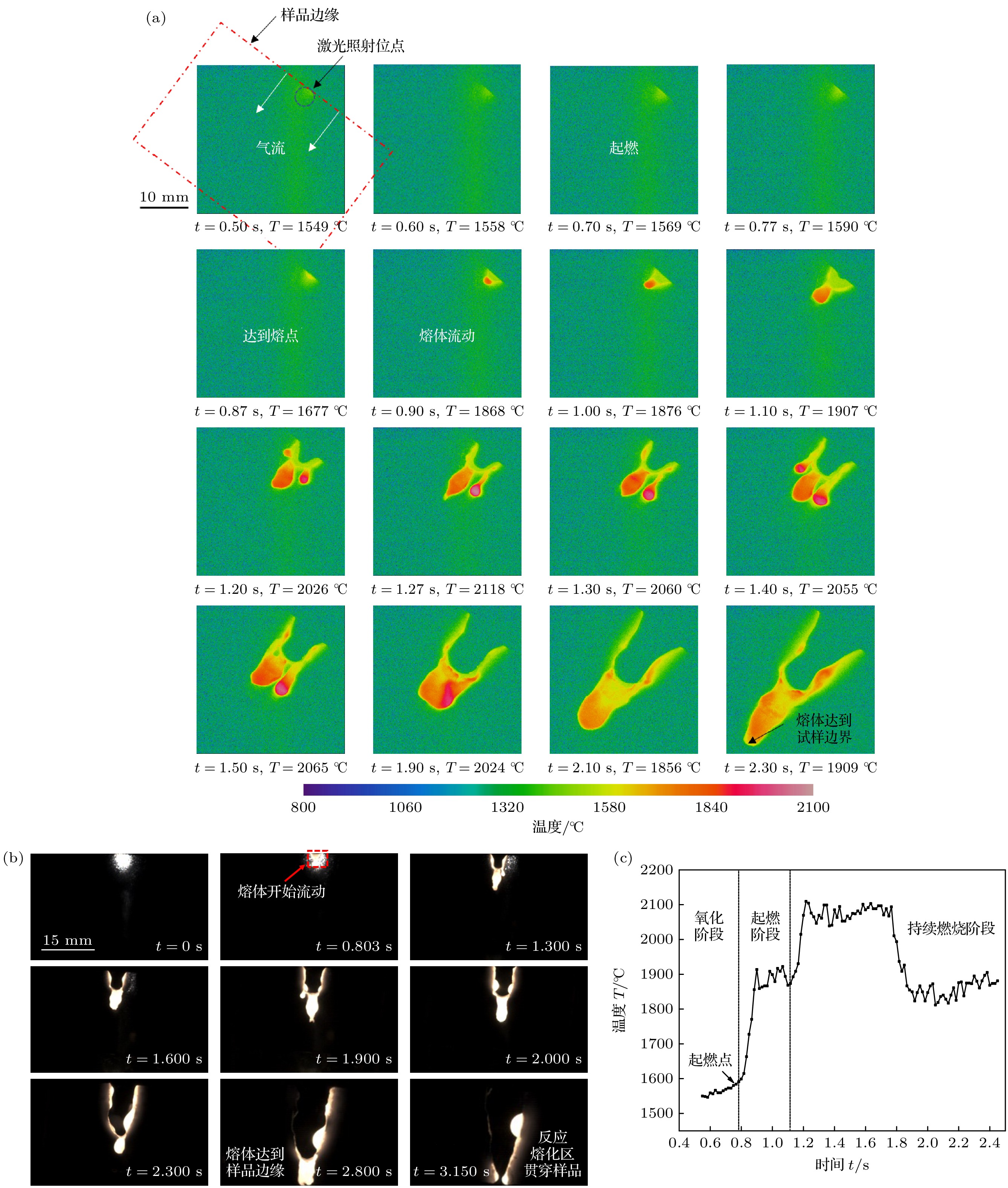
图 6 起燃并扩展TA19合金的温度及熔体特征 (a) 起燃前后的温度分布随时间的变化关系; (b) 熔体形成规律随时间的变化关系; (c) 试样最高温度随时间的变化规律
Fig. 6. The temperature and melt characteristics of TA19 alloy with ignition and extended combustion: (a) The relationship between temperature distribution and time; (b) the relationship between the formation of melt and time; (c) the variation of the highest temperature with time.
图 7 持续燃烧TA19合金的温度特征 (a) 温度分布随时间的变化关系; (b) 熔体形成规律随时间的变化关系; (c) 试样最高温度随时间的变化规律
Fig. 7. The temperature and melt characteristics of TA19 alloy with sustained burning: (a) The relationship between temperature distribution and time; (b) the relationship between the formation of melt and time; (c) the variation of the highest temperature with time.
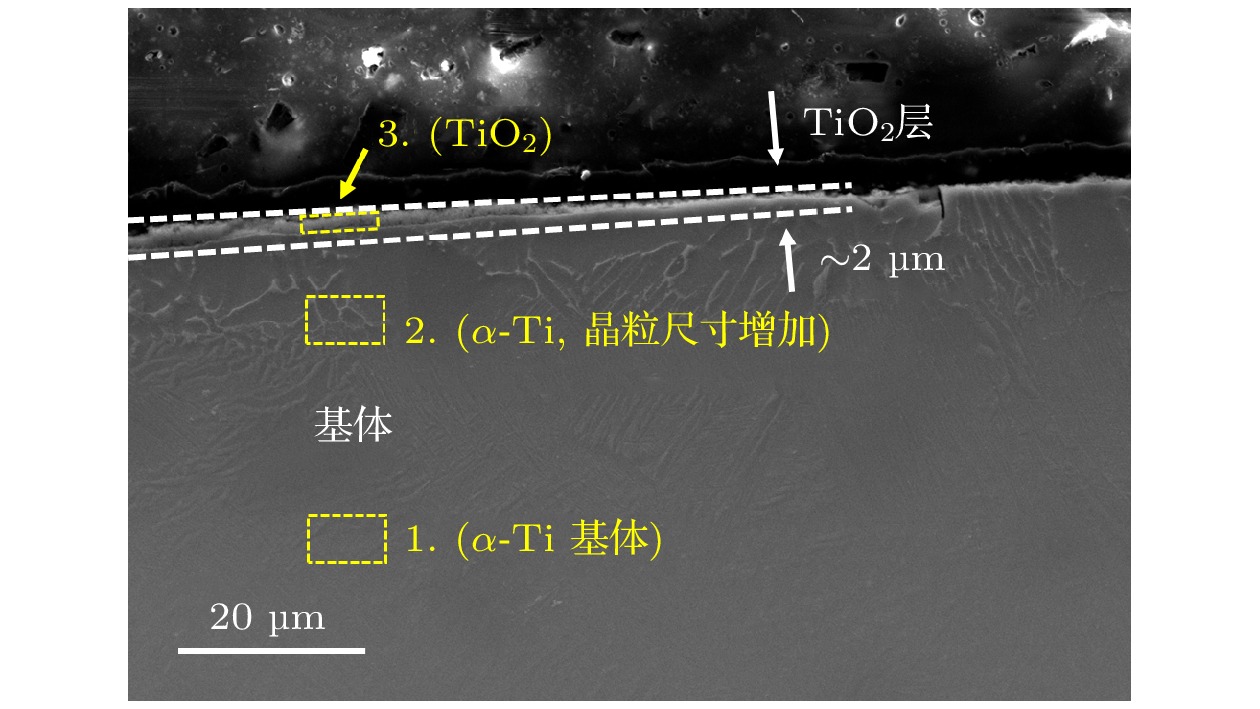
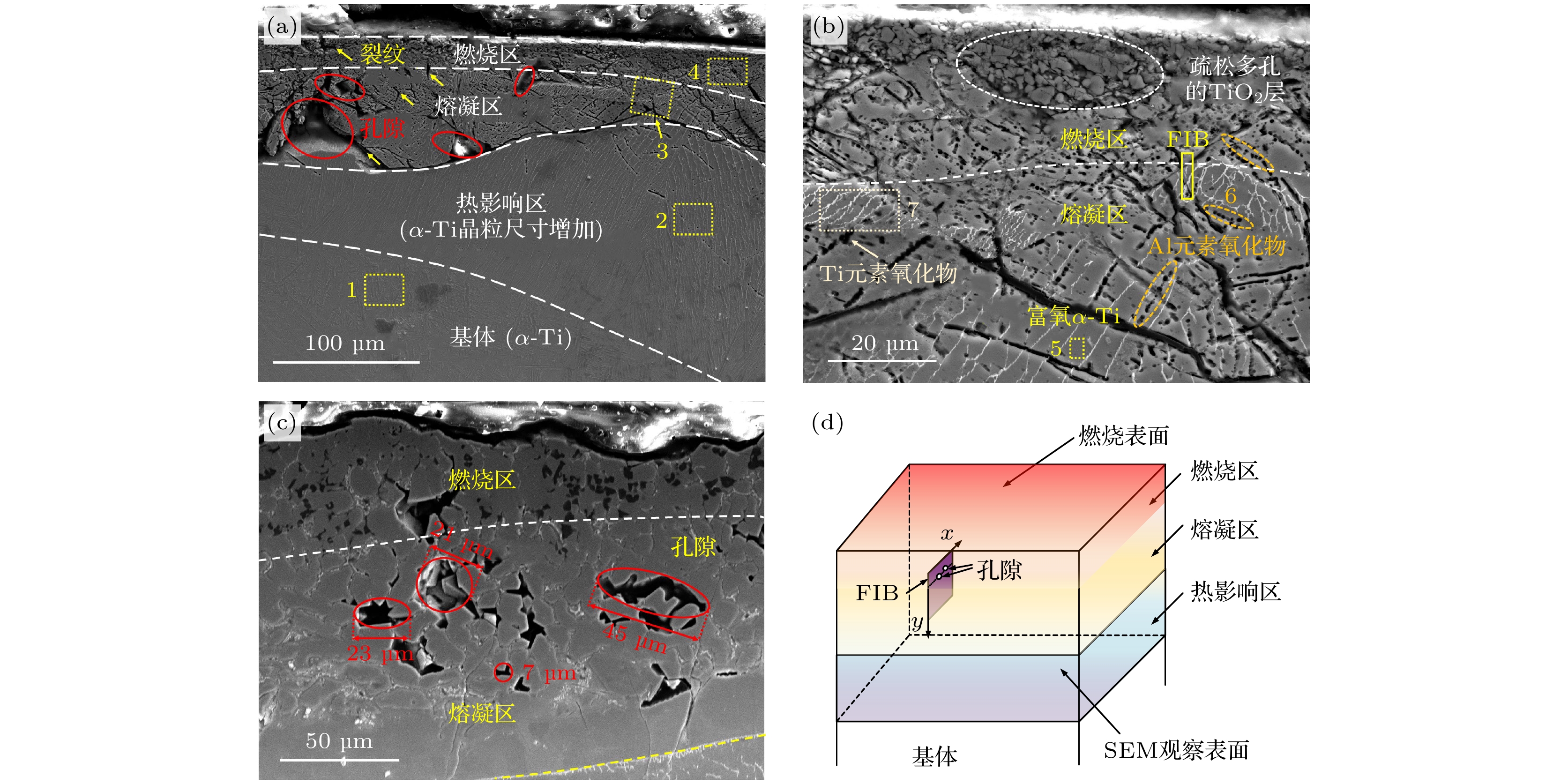
图 11 TA19合金起燃组织的SEM形貌 (a) 300 W激光功率和21%氧体积浓度条件形成的起燃组织整体形貌; (b) 图(a)熔凝区放大形貌; (c) 300 W激光功率和25%氧体积浓度条件形成的起燃组织形貌; (d) FIB取样位置示意图
Fig. 11. SEM microstructure of ignited TA19 alloy: (a) Overall microstructure formed under 300 W laser power and 21% oxygen volume concentration; (b) enlarged view of Figure (a); (c) microstructure formed under 300 W laser power and 25% oxygen volume concentration; (d) schematic diagram of FIB sampling location.
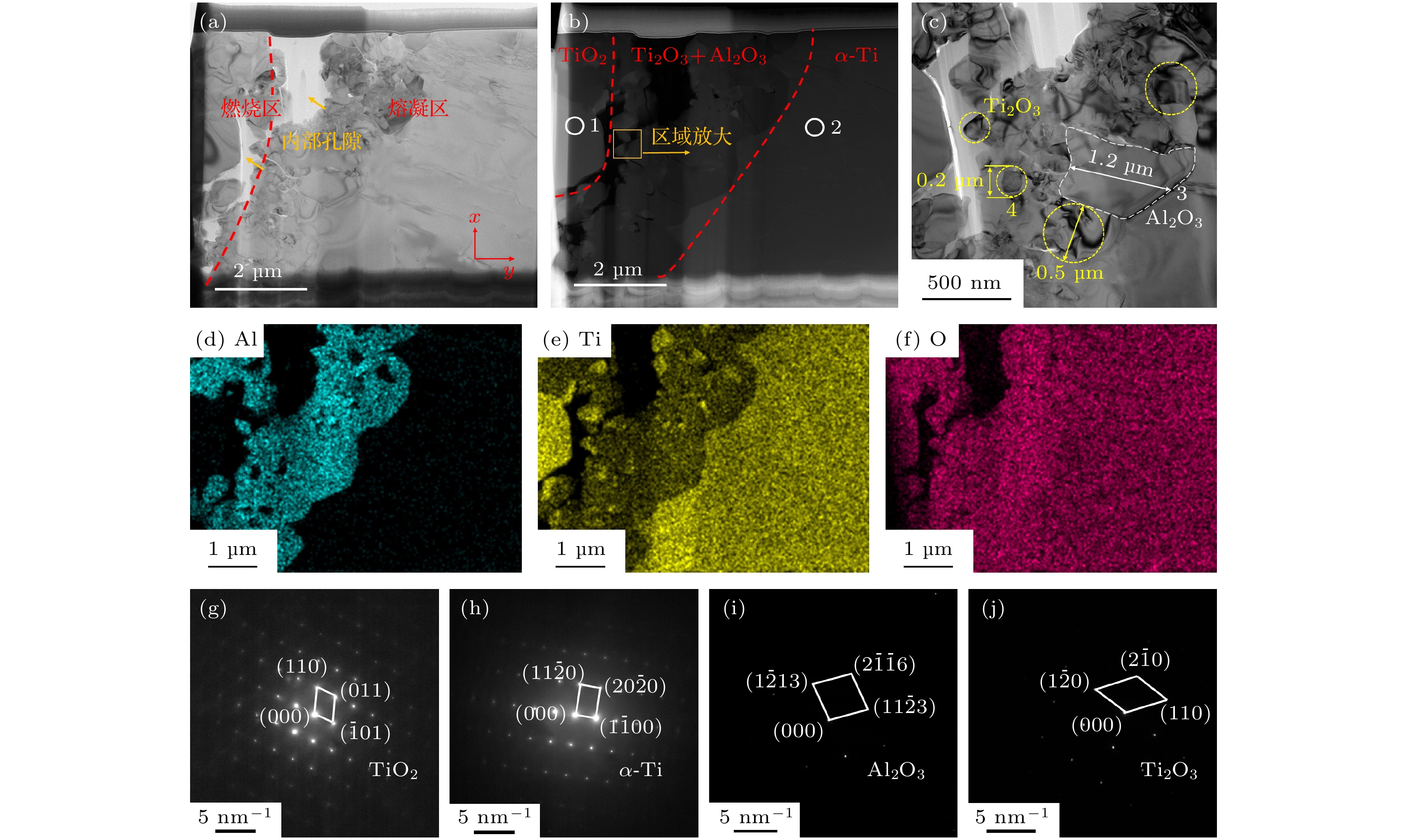
图 12 TA19合金起燃组织燃烧区/熔凝区界面的TEM表征结果 (a) 明场像; (b) 暗场像; (c) Ti2O3 + Al2O3混合区域放大图像; (d) Al元素分布; (e) Ti元素分布; (f) O元素分布; (g) 位置1的SAED结果; (h) 位置2的SAED结果; (i) 位置3的SAED结果; (j) 位置4的SAED结果
Fig. 12. TEM characterization results of the combustion zone/melting zone interface of ignited TA19 alloy: (a) Bright field image; (b) dark field image; (c) enlarged view of Ti2O3 + Al2O3 mixed region; (d) Al element distribution; (e) Ti element distribution; (f) O element distribution; (g) SAED pattern at position 1; (h) SAED pattern at position 2; (i) SAED pattern at position 3; (j) SAED pattern at position 4.
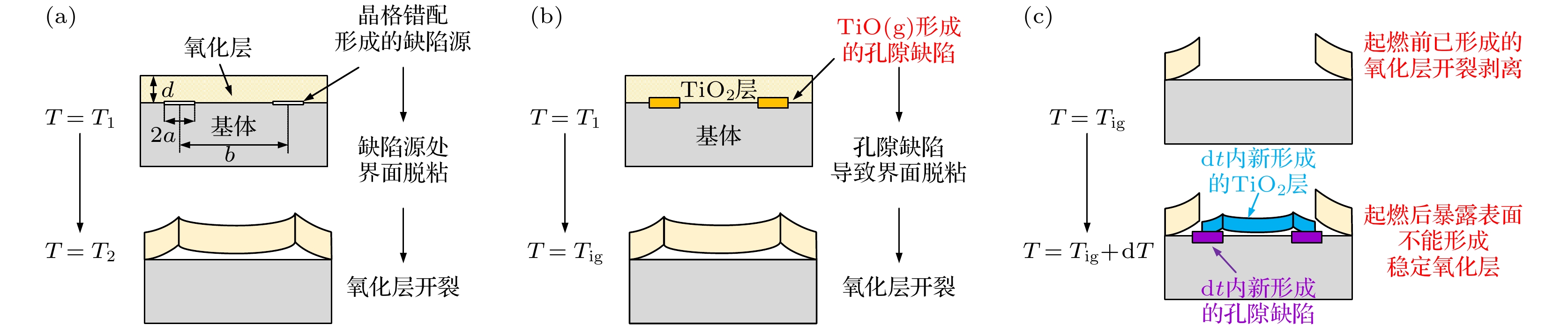
图 13 钛合金起燃过程的保护性氧化层应力失效模型 (a) Evans和Lobb[21,22]提出的基本模型; (b) 起燃前形成的氧化层在孔隙缺陷影响下发生开裂; (c) 起燃后新形成的氧化层不能保持结构稳定
Fig. 13. Stress failure model of oxide layer during the ignition of titanium alloy: (a) Basic model proposed by Evans and Lobb[21,22]; (b) the oxide layer formed before ignition cracks under the influence of pore defects; (c) the newly formed oxide layer after ignition cannot maintain stability.
表 1 TA19合金起燃组织的元素分布
Table 1. Element distribution in the ignited TA19 alloy.
元素原子数含量/% 位置1 位置2 位置3 位置4 位置5 位置6 位置7 Ti 80.5 70.2 42.7 32.7 59.8 8.3 42.5 Al 10.3 8.9 18.2 2.2 5.4 29.2 1.2 Zr 2.1 3.2 0.7 0.7 0.3 0.2 0.2 Mo 1.2 1.5 0.2 0.2 0.3 0.3 0.3 Sn 1.1 2.1 0.1 0.1 0.2 0.2 0.2 O 4.8 14.1 38.1 64.1 34.0 59.6 55.6 表 2 TA19合金起燃点瞬时温度变化率的实验测量值
Table 2. Measured results of instantaneous temperature change rate of TA19 alloy at ignition temperature.
激光功率PL/W 氧浓度c/% 起燃点瞬时温度
变化率/(℃·s–1)225 34 305 225 38 305 250 32 300 250 34 300 250 38 310 275 32 300 275 35 325 300 25 330 300 30 310 300 32 295 300 34 305 300 40 295 300 50 300 300 60 300 325 21 330 325 60 320 -
[1] 弭光宝, 谭勇, 陈航, 李培杰, 张学军 2024 航空材料学报 44 15
 Google Scholar
Google Scholar
Mi G B, Tan Y, Chen H, Li P J, Zhang X J 2024 J. Aeronaut. Mater. 44 15
 Google Scholar
Google Scholar
[2] Cai J M, Mi G B, Gao F, Huang H, Cao J X, Huang X, Cao C X 2016 J. Mater. Eng. 44 1
[3] Leyens C, Kocian K, Hausmann J, Kaysser W A 2003 Aerosp. Sci. Technol. 7 201
 Google Scholar
Google Scholar
[4] Wolf J S, Moyle D D, Pruitt A B, Bader J H 1976 J. Electrochem. Soc. 123 C251
[5] Leyens C, Peters M, Kaysser W A 1996 Mater. Sci. Technol. 15 1326
[6] Mi G B, Huang X, Li P J, Cao J X, Huang X, Cao C X 2012 Trans. Nonferrous Met. Soc. China 22 2409
 Google Scholar
Google Scholar
[7] Zhao Y Q, Zhou L, Deng J 1999 Rare Met. Mater. Eng. 28 77
[8] 弭光宝, 黄旭, 曹京霞, 王宝, 曹春晓 2016 65 056103
 Google Scholar
Google Scholar
Mi G B, Huang X, Cao J X, Wang B, Cao C X 2016 Acta Phys. Sin. 65 056103
 Google Scholar
Google Scholar
[9] Shao L, Xie G L, Liu X H, Wu Y, Tan Q, Xie L, Xin S W, Hao F, Yu J B, Xue W L, Feng K 2022 Corros. Sci. 194 109957
 Google Scholar
Google Scholar
[10] Ouyang P X, Mi G B, Cao J X, Huang X, He L J, Li P J 2018 Mater. Today Commun. 16 364
 Google Scholar
Google Scholar
[11] Rozenband V I, Vaganova N I 1992 Combust. Flame 88 113
 Google Scholar
Google Scholar
[12] 弭光宝, 黄旭, 曹京霞, 曹春晓 2012 航空材料学报 32 25
 Google Scholar
Google Scholar
Mi G B, Huang X, Cao J X, Cao C X 2012 J. Aeronaut. Mater. 32 25
 Google Scholar
Google Scholar
[13] Shafirovich E, Teoh S K, Varma A 2008 Combust. Flame 152 262
 Google Scholar
Google Scholar
[14] 吴明宇, 弭光宝, 李培杰, 黄旭 2023 72 166102
 Google Scholar
Google Scholar
Wu M Y, Mi G B, Li P J, Huang X 2023 Acta Phys. Sin. 72 166102
 Google Scholar
Google Scholar
[15] 梁贤烨, 弭光宝, 李培杰, 黄旭, 曹春晓 2020 65 216101
 Google Scholar
Google Scholar
Liang X Y, Mi G B, Li P J, Huang X, Cao C X 2020 Acta Phys. Sin. 65 216101
 Google Scholar
Google Scholar
[16] Shao L, Xie G L, Liu X H, Wu Y, Yu J B, Feng K, Xue W L 2021 Corros. Sci. 190 109641
 Google Scholar
Google Scholar
[17] Bolobov V I, Podlevskikh N A 2007 Combust. Explos. Shock Waves 43 405
 Google Scholar
Google Scholar
[18] Titanium Combustion Research Program and user's Manual for Deck CCD 1152-0.0, Glickstein M R http://apps.dtic.mil/ [2023-12-4]
[19] 弭光宝, 陈航 中国专利, ZL201711188505.5 [2017-11-23]]
Mi G B, Chen H Chinese Patent ZL201711188505.5 [2017-11-23]
[20] 黄伯云, 李成功, 石力开 2006 中国材料工程大典 (北京: 化学工业出版社) 第550页
Huang B Y, Li C G, Shi L K 2006 China Materials Engineering Cannon (Beijing: Chemistry Industry Press) p550
[21] Evans H E, Lobb R C 1984 Corros. Sci. 24 209
 Google Scholar
Google Scholar
[22] Evans H E, Lobb R C 1994 Mater. High Temp. 12 219
 Google Scholar
Google Scholar
[23] Park Y S, Butt D P 1998 Oxid. Met. 51 383
[24] 杨德钧, 沈卓身1999 金属腐蚀学(第一版) (北京: 冶金工艺出版社) 第11页
Yang D J, Shen Z S 1999 Metal Corrosion (Vol. 1) (Beijing: Metallurgical Industry Press) p11
[25] Bohle M, Etling D, Muller U, Sreenivasan K R S, Riedel U, Warnatz J 2004 Prandtl’s Essentials of Fluid Mechanics (Heidelberg: Springer) (2nd Ed.) p428
[26] Liu Z, Welsch G 1988 Metall. Trans. A 19 1121
 Google Scholar
Google Scholar
[27] Gaddam R, Sefer B, Pederson R, Antti M L 2015 Mater. Charact. 99 166
 Google Scholar
Google Scholar
计量
- 文章访问数: 3658
- PDF下载量: 360
- 被引次数: 0
















 下载:
下载: