-
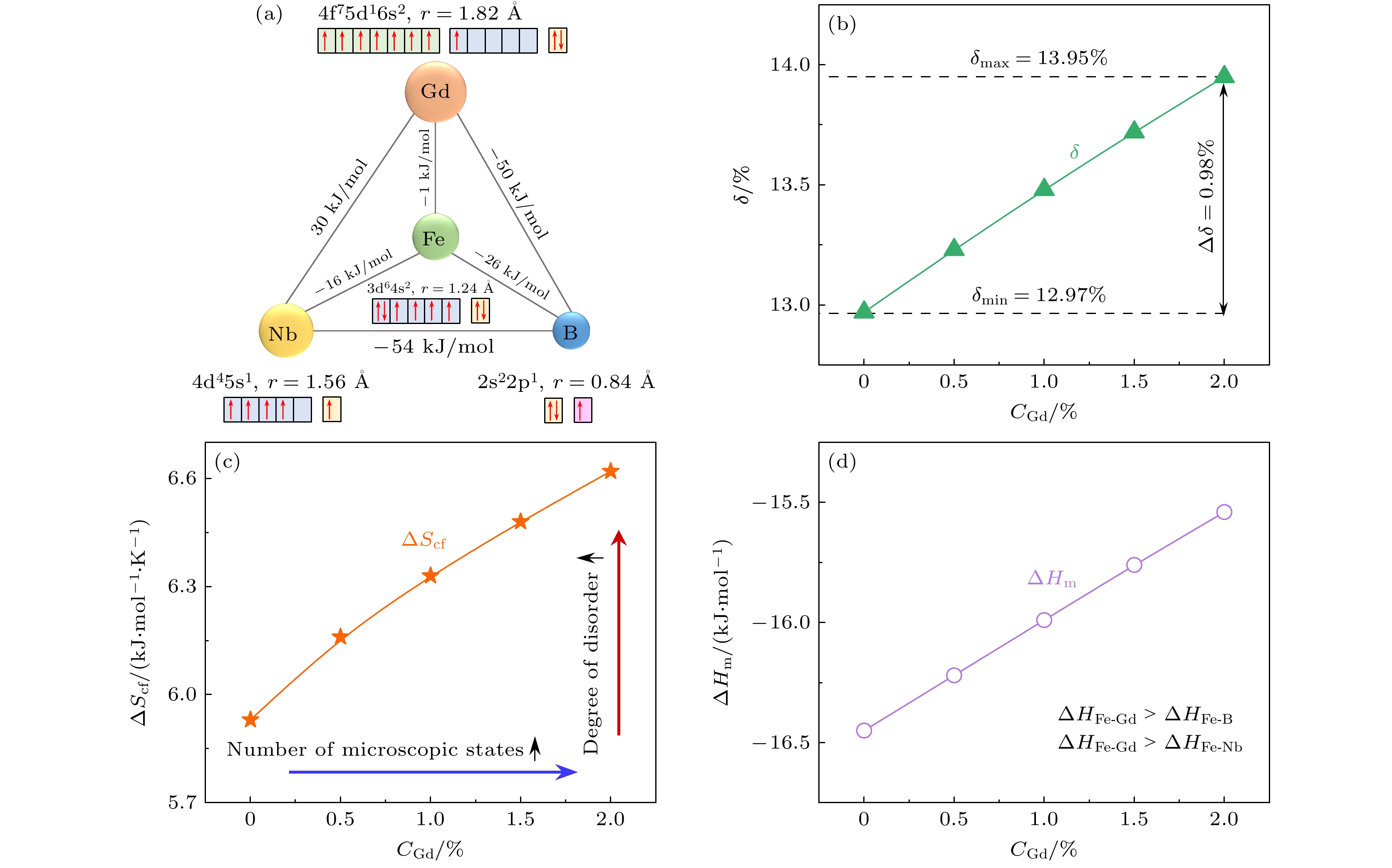
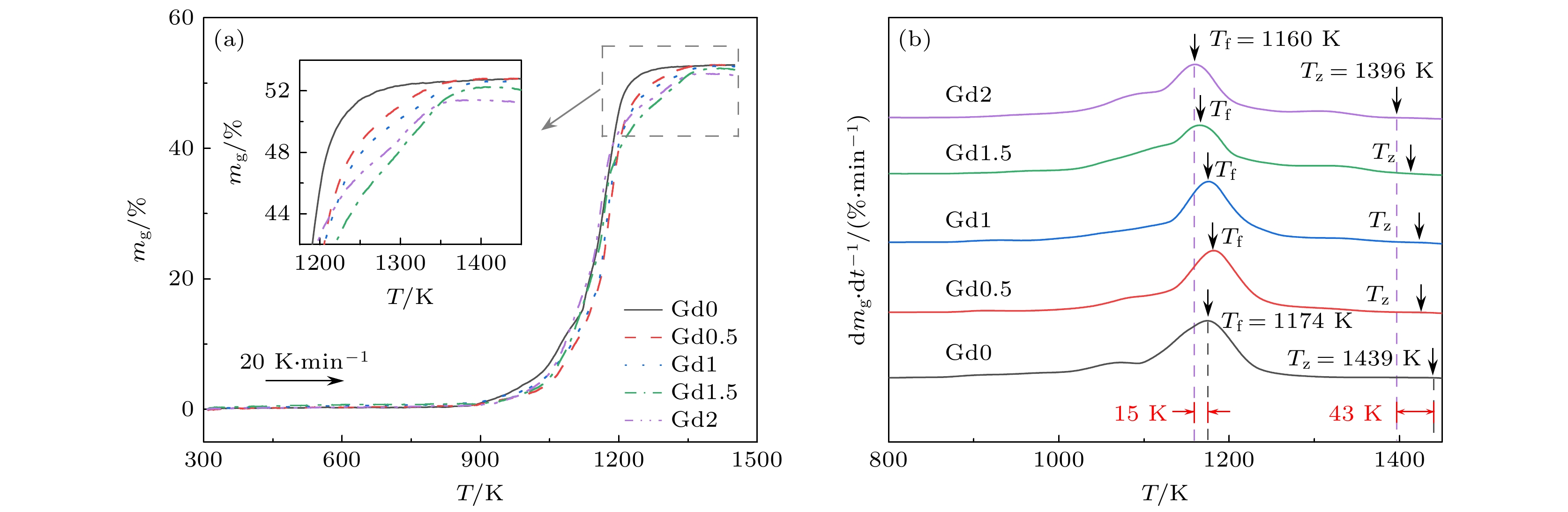
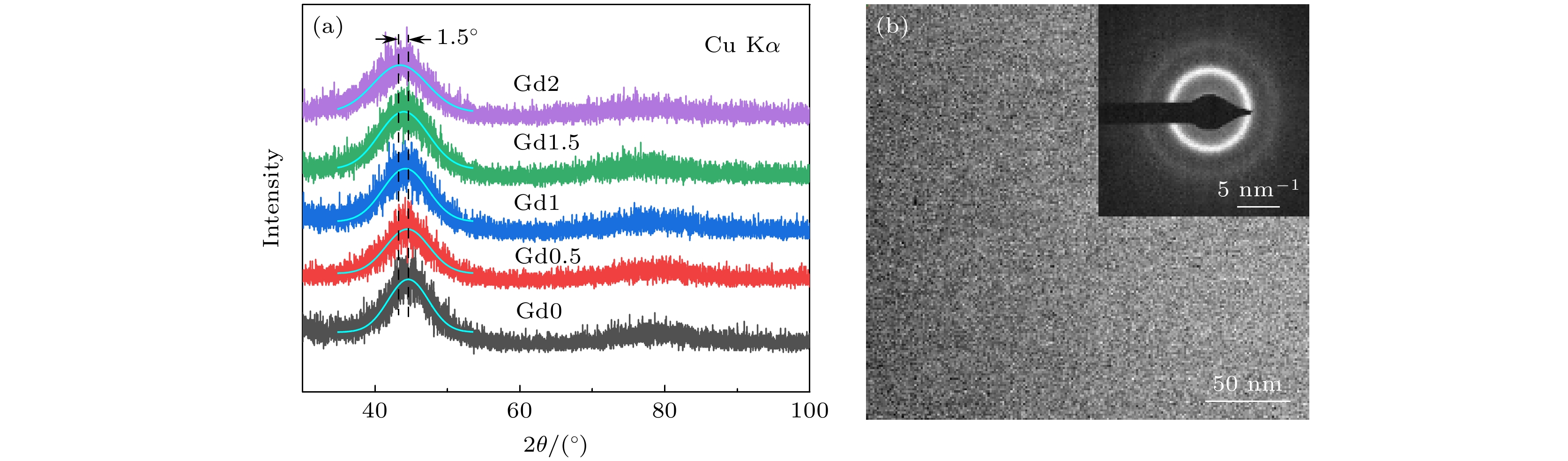
研究了Gd含量对(Fe73B22Nb5)100–xGdx (x = 0, 0.5, 1.0, 1.5, 2.0)合金非晶形成能力、热稳定性和磁学性能的影响规律, 并对比分析了非晶氧化机制. 通过添加Gd元素, 合金的原子尺寸差超过13%, 构型熵增大了30%, 提升了合金的非晶形成能力. 随着Gd含量的增大, 过冷液相区范围达到73 K, 热稳定性得到明显增强. Gd元素导致合金局部各向异性受到限制, 准位错偶极子型缺陷密度降低. 这有效减少了阻碍磁畴壁旋转的钉扎位点, 提高合金软磁性能. 此外, Gd元素使得非晶在氧化过程中对温度的变化更为敏感, 达到最大氧化速率的温度降低了15 K, 但是并未恶化其抗氧化性能. Gd原子受结合能影响向表层迁移, 形成的富Gd氧化物填充了表层缺陷, 占据了大量顶部空间, 合金表面附近的结构更加致密. 这种结构减少了氧原子通过微观组织界面进行扩散的通道, 有助于增强抗氧化性能.In this work, we use the rapid solidification technique to prepare five kinds of metallic glasses with different Gd content, and investigate in depth the influences of Gd content on the amorphous formation capability, thermal stability, and magnetic properties of (Fe73B22Nb5)100–xGdx (x = 0, 0.5, 1.0, 1.5, 2.0) alloys. By comparing the microstructural morphology and solute distribution of oxidation products before adding Gd and those after adding Gd, the amorphous oxidation mechanism is analyzed systematically. With the addition of Gd, the atomic size difference of the alloys exceeds 13%, and the configuration entropy increases from 7.27 kJ/(mol·K) to 9.44 kJ/(mol·K). The glass-forming ability of the alloy is significantly improved. The increase of Gd content can increase the glass transition temperature of the alloy to 864 K, and the undercooled liquid region can reach 73 K, significantly enhancing the thermal stability of the metallic glasses. The Gd limits the local anisotropy of the alloy and reduces the density of quasi-dislocation dipole defects. This can effectively reduce the pinning sites that hinder the rotation of magnetic domain walls, thereby improving the soft magnetic property. By comparing with the metallic glasses without Gd, only 2% (atomic percentage) Gd can reduce the coercivity by 8%. Moreover, the Gd makes the metallic glasses more sensitive to temperature variation in the oxidation process, and the temperature of the maximum oxidation rate is reduced by 15 K. However, their antioxidant performance does not deteriorate. The Gd atoms are influenced by binding energy and migrate to the surface, forming Gd-rich oxides. They fill surface defects and occupy a large part of the top space, leading to the structure becoming more compact near the surface. This structure reduces the channels for oxygen atoms to diffuse through the microstructure interface, which helps to improve antioxidant capability. This work provides a new approach for designing high performance Fe-based metallic glasses.
-
Keywords:
- rare earth element /
- metallic glass /
- rapid solidification /
- oxidation mechanism
[1] Inoue A, Zhang T, Takeuchi A 1997 Appl. Phys. Lett. 71 464
 Google Scholar
Google Scholar
[2] Dou L T, Liu H S, Hou L, Xue L, Yang W M, Zhao Y C, Chang C T, Shen B L 2014 J. Magn. Magn. Mater. 358 23
 Google Scholar
Google Scholar
[3] Xu S, Wang J R, Wang N R, Wang T, Han Z H, Wang Y 2021 Mater. Today Commun. 26 101906
 Google Scholar
Google Scholar
[4] Yoshizawa Y, Oguma S, Yamauchi K 1988 J. Appl. Phys. 64 6044
 Google Scholar
Google Scholar
[5] Torrens-Serra J, Bruna P, Rodriguez-Viejo J, Roth S, Clavaguera-Mora M T 2010 Intermetallics 18 773
 Google Scholar
Google Scholar
[6] Suzuki K, Makino A, Inoue A, Masumoto T 1991 J. Appl. Phys. 70 6232
 Google Scholar
Google Scholar
[7] Shen B L, Akiba M, Inoue A 2006 Phys. Rev. B 73 104204
 Google Scholar
Google Scholar
[8] Ramasamy P, Stoica M, Bera S, Calin M, Eckert J 2017 J. Alloys Compd. 707 78
 Google Scholar
Google Scholar
[9] 孙吉, 沈鹏飞, 尚其忠, 张鹏雁, 刘莉, 李明瑞, 侯龙, 李维火 2023 72 026101
 Google Scholar
Google Scholar
Sun J, Shen P F, Shang Q Z, Zhang P Y, Liu L, Li M R, Hou L, Li W H 2023 Acta Phys. Sin. 72 026101
 Google Scholar
Google Scholar
[10] Guo S F, Chen K C, Xie S H, Yu P, Huang Y J, Zhang H J 2013 J. Non Cryst. Solids 369 29
 Google Scholar
Google Scholar
[11] Tsai P H, Xiao A C, Li J B, Jang J S C, Chu J P, Huang J C 2014 J. Alloys Compd. 586 94
 Google Scholar
Google Scholar
[12] Wang S Y, Jiang W, Hu H D, Liu P F, Wu J L, Zhang B 2017 Prog. Nat. Sci. Mater. 27 503
 Google Scholar
Google Scholar
[13] Greer A L, Rutherford K L, Hutchings I M 2002 Int. Mater. Rev. 47 87
 Google Scholar
Google Scholar
[14] 张雅楠, 王有骏, 孔令体, 李金富 2012 61 157502
 Google Scholar
Google Scholar
Zhang Y N, Wang Y J, Kong L T, Li J F 2012 Acta Phys. Sin. 61 157502
 Google Scholar
Google Scholar
[15] 孟绍怡, 郝奇, 吕国建, 乔吉超 2023 72 076101
 Google Scholar
Google Scholar
Meng S Y, Hao Q, Lyu G J, Qiao J C 2023 Acta Phys. Sin. 72 076101
 Google Scholar
Google Scholar
[16] Shen J, Chen Q, Sun J, Fan H, Wang G 2005 Appl. Phys. Lett. 86 151907
 Google Scholar
Google Scholar
[17] Zhao Y B, Bai Y W, Ding Y J, Hu L N 2020 J. Non Cryst. Solids 537 120020
 Google Scholar
Google Scholar
[18] Styles M J, Sun W W, East D R, Kimpton J A, Gibson M A, Hutchinson C R 2016 Acta Mater. 117 170
 Google Scholar
Google Scholar
[19] Park J M, Park J S, Na J H, Kim D H 2006 Mater. Sci. Eng. , A 435 425
 Google Scholar
Google Scholar
[20] Pan S P, Qin J Y, Gu T K 2010 J. Appl. Phys. 107 033503
 Google Scholar
Google Scholar
[21] Liang X Y, Li Y H, Bao F, Zhu Z W, Zhang H F, Zhang W 2021 Intermetallics 132 107135
 Google Scholar
Google Scholar
[22] Zhao L Z, Tian H C, Zhong X C, Liu Z W, Greneche J M, Ramanujan R V 2020 J. Rare Earth 38 1317
 Google Scholar
Google Scholar
[23] Chrobak A, Nosenko V, Haneczok G, Boichyshyn L, Kotur B, Bajorek A, Zivotsky O, Hendrych A 2011 Mater. Chem. Phys. 130 603
 Google Scholar
Google Scholar
[24] Li X M, Wang Y, Yi J, Kong L T, Li J F 2019 J. Alloys Compd. 790 626
 Google Scholar
Google Scholar
[25] Zhang Y, Zhou Y J, Lin J P, Chen G L, Liaw P K 2008 Adv. Eng. Mater. 10 534
 Google Scholar
Google Scholar
[26] Inoue A 1997 Proc. Jpn. Acad. 73 19
 Google Scholar
Google Scholar
[27] Inoue A 2000 Acta Mater. 48 279
 Google Scholar
Google Scholar
[28] Dong Y, Wunderlich R, Biskupek J, Cao Q P, Wang X D, Zhang D X, Jiang J Z, Fecht H J 2017 Scripta Mater. 137 94
 Google Scholar
Google Scholar
[29] Huang X M, Chang C T, Chang Z Y, Wang X D, Cao Q P, Shen B L, Inoue A, Jiang J Z 2008 J. Alloys Compd. 460 708
 Google Scholar
Google Scholar
[30] Ruderman M A, Kittel C 1954 Phys. Rev. 96 99
 Google Scholar
Google Scholar
[31] Yano K 2000 J. Magn. Magn. Mater. 208 207
 Google Scholar
Google Scholar
[32] Tao S, Ma T Y, Jian H, Ahmad Z, Tong H, Yan M 2010 Mater. Sci. Eng., A 528 161
 Google Scholar
Google Scholar
[33] Jian H, Luo W, Tao S, Yan M 2010 J. Alloys Compd. 505 315
 Google Scholar
Google Scholar
[34] Bitoh T, Makino A, Inoue A 2003 Mater. Trans. 44 2020
 Google Scholar
Google Scholar
[35] Guo W M, Wu Y P, Zhang J F, Yuan W H 2016 Surf. Coat. Technol. 307 392
 Google Scholar
Google Scholar
[36] Koster U, Jastrow L, Meuris M 2007 Mater. Sci. Eng., A 449 165
 Google Scholar
Google Scholar
-
表 1 (Fe73B22Nb5)100–xGdx (x = 0, 0.5, 1.0, 1.5, 2.0)非晶合金的热物性参数
Table 1. Thermophysical parameters of (Fe73B22Nb5)100–xGdx (x = 0, 0.5, 1.0, 1.5, 2.0) metallic glasses.
Alloy Tg/K Tx/K Tp/K TS/K TL/K ∆T/K Trg γ Gd0 811 855 862 1409 1529 44 0.576 0.365 Gd0.5 826 875 885 1364 1521 49 0.606 0.377 Gd1 838 897 908 1362 1528 59 0.615 0.384 Gd1.5 863 933 941 1359 1504 70 0.635 0.398 Gd2 864 937 943 1357 1494 73 0.637 0.400 -
[1] Inoue A, Zhang T, Takeuchi A 1997 Appl. Phys. Lett. 71 464
 Google Scholar
Google Scholar
[2] Dou L T, Liu H S, Hou L, Xue L, Yang W M, Zhao Y C, Chang C T, Shen B L 2014 J. Magn. Magn. Mater. 358 23
 Google Scholar
Google Scholar
[3] Xu S, Wang J R, Wang N R, Wang T, Han Z H, Wang Y 2021 Mater. Today Commun. 26 101906
 Google Scholar
Google Scholar
[4] Yoshizawa Y, Oguma S, Yamauchi K 1988 J. Appl. Phys. 64 6044
 Google Scholar
Google Scholar
[5] Torrens-Serra J, Bruna P, Rodriguez-Viejo J, Roth S, Clavaguera-Mora M T 2010 Intermetallics 18 773
 Google Scholar
Google Scholar
[6] Suzuki K, Makino A, Inoue A, Masumoto T 1991 J. Appl. Phys. 70 6232
 Google Scholar
Google Scholar
[7] Shen B L, Akiba M, Inoue A 2006 Phys. Rev. B 73 104204
 Google Scholar
Google Scholar
[8] Ramasamy P, Stoica M, Bera S, Calin M, Eckert J 2017 J. Alloys Compd. 707 78
 Google Scholar
Google Scholar
[9] 孙吉, 沈鹏飞, 尚其忠, 张鹏雁, 刘莉, 李明瑞, 侯龙, 李维火 2023 72 026101
 Google Scholar
Google Scholar
Sun J, Shen P F, Shang Q Z, Zhang P Y, Liu L, Li M R, Hou L, Li W H 2023 Acta Phys. Sin. 72 026101
 Google Scholar
Google Scholar
[10] Guo S F, Chen K C, Xie S H, Yu P, Huang Y J, Zhang H J 2013 J. Non Cryst. Solids 369 29
 Google Scholar
Google Scholar
[11] Tsai P H, Xiao A C, Li J B, Jang J S C, Chu J P, Huang J C 2014 J. Alloys Compd. 586 94
 Google Scholar
Google Scholar
[12] Wang S Y, Jiang W, Hu H D, Liu P F, Wu J L, Zhang B 2017 Prog. Nat. Sci. Mater. 27 503
 Google Scholar
Google Scholar
[13] Greer A L, Rutherford K L, Hutchings I M 2002 Int. Mater. Rev. 47 87
 Google Scholar
Google Scholar
[14] 张雅楠, 王有骏, 孔令体, 李金富 2012 61 157502
 Google Scholar
Google Scholar
Zhang Y N, Wang Y J, Kong L T, Li J F 2012 Acta Phys. Sin. 61 157502
 Google Scholar
Google Scholar
[15] 孟绍怡, 郝奇, 吕国建, 乔吉超 2023 72 076101
 Google Scholar
Google Scholar
Meng S Y, Hao Q, Lyu G J, Qiao J C 2023 Acta Phys. Sin. 72 076101
 Google Scholar
Google Scholar
[16] Shen J, Chen Q, Sun J, Fan H, Wang G 2005 Appl. Phys. Lett. 86 151907
 Google Scholar
Google Scholar
[17] Zhao Y B, Bai Y W, Ding Y J, Hu L N 2020 J. Non Cryst. Solids 537 120020
 Google Scholar
Google Scholar
[18] Styles M J, Sun W W, East D R, Kimpton J A, Gibson M A, Hutchinson C R 2016 Acta Mater. 117 170
 Google Scholar
Google Scholar
[19] Park J M, Park J S, Na J H, Kim D H 2006 Mater. Sci. Eng. , A 435 425
 Google Scholar
Google Scholar
[20] Pan S P, Qin J Y, Gu T K 2010 J. Appl. Phys. 107 033503
 Google Scholar
Google Scholar
[21] Liang X Y, Li Y H, Bao F, Zhu Z W, Zhang H F, Zhang W 2021 Intermetallics 132 107135
 Google Scholar
Google Scholar
[22] Zhao L Z, Tian H C, Zhong X C, Liu Z W, Greneche J M, Ramanujan R V 2020 J. Rare Earth 38 1317
 Google Scholar
Google Scholar
[23] Chrobak A, Nosenko V, Haneczok G, Boichyshyn L, Kotur B, Bajorek A, Zivotsky O, Hendrych A 2011 Mater. Chem. Phys. 130 603
 Google Scholar
Google Scholar
[24] Li X M, Wang Y, Yi J, Kong L T, Li J F 2019 J. Alloys Compd. 790 626
 Google Scholar
Google Scholar
[25] Zhang Y, Zhou Y J, Lin J P, Chen G L, Liaw P K 2008 Adv. Eng. Mater. 10 534
 Google Scholar
Google Scholar
[26] Inoue A 1997 Proc. Jpn. Acad. 73 19
 Google Scholar
Google Scholar
[27] Inoue A 2000 Acta Mater. 48 279
 Google Scholar
Google Scholar
[28] Dong Y, Wunderlich R, Biskupek J, Cao Q P, Wang X D, Zhang D X, Jiang J Z, Fecht H J 2017 Scripta Mater. 137 94
 Google Scholar
Google Scholar
[29] Huang X M, Chang C T, Chang Z Y, Wang X D, Cao Q P, Shen B L, Inoue A, Jiang J Z 2008 J. Alloys Compd. 460 708
 Google Scholar
Google Scholar
[30] Ruderman M A, Kittel C 1954 Phys. Rev. 96 99
 Google Scholar
Google Scholar
[31] Yano K 2000 J. Magn. Magn. Mater. 208 207
 Google Scholar
Google Scholar
[32] Tao S, Ma T Y, Jian H, Ahmad Z, Tong H, Yan M 2010 Mater. Sci. Eng., A 528 161
 Google Scholar
Google Scholar
[33] Jian H, Luo W, Tao S, Yan M 2010 J. Alloys Compd. 505 315
 Google Scholar
Google Scholar
[34] Bitoh T, Makino A, Inoue A 2003 Mater. Trans. 44 2020
 Google Scholar
Google Scholar
[35] Guo W M, Wu Y P, Zhang J F, Yuan W H 2016 Surf. Coat. Technol. 307 392
 Google Scholar
Google Scholar
[36] Koster U, Jastrow L, Meuris M 2007 Mater. Sci. Eng., A 449 165
 Google Scholar
Google Scholar
计量
- 文章访问数: 4916
- PDF下载量: 76
- 被引次数: 0













 下载:
下载: