-
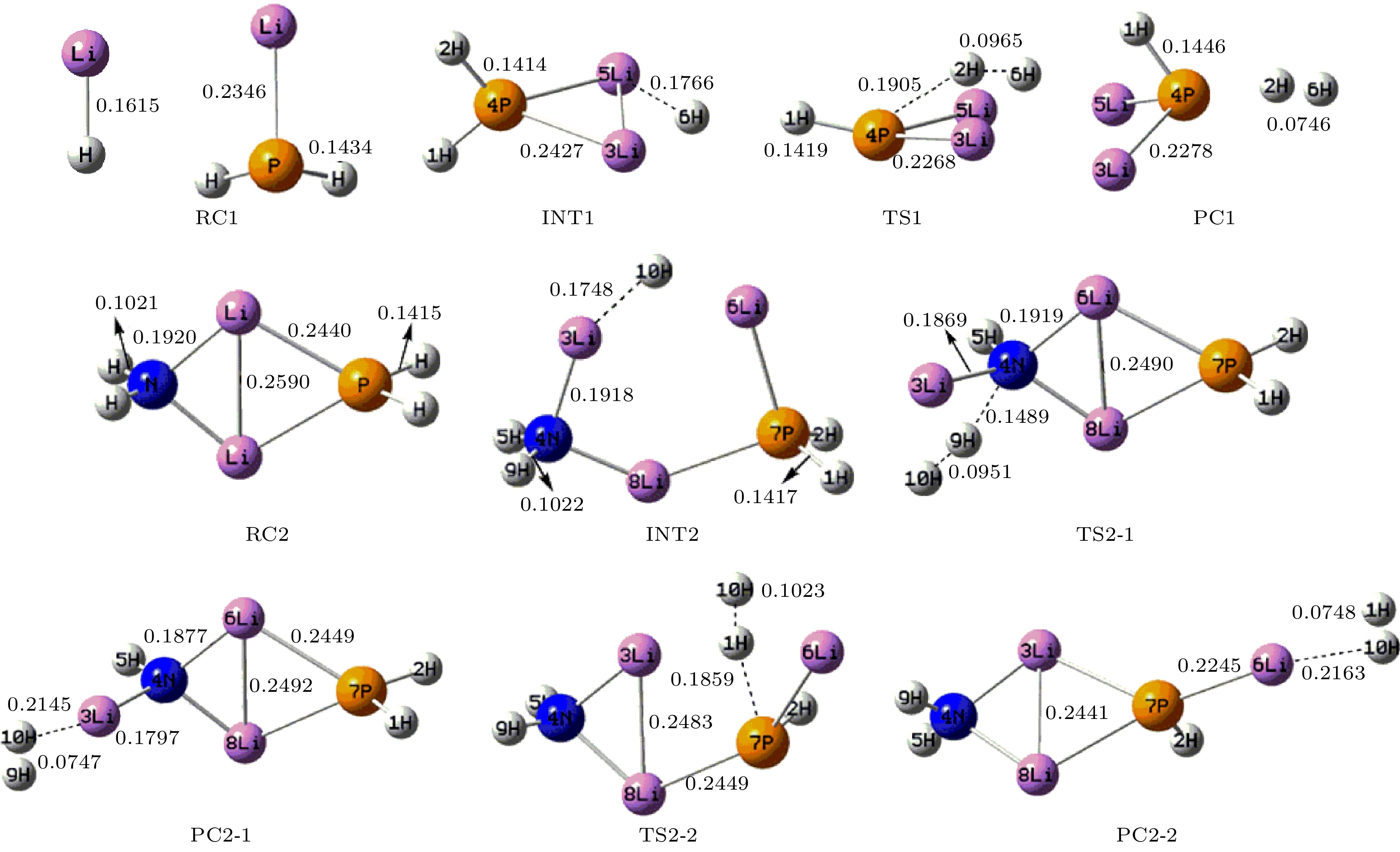
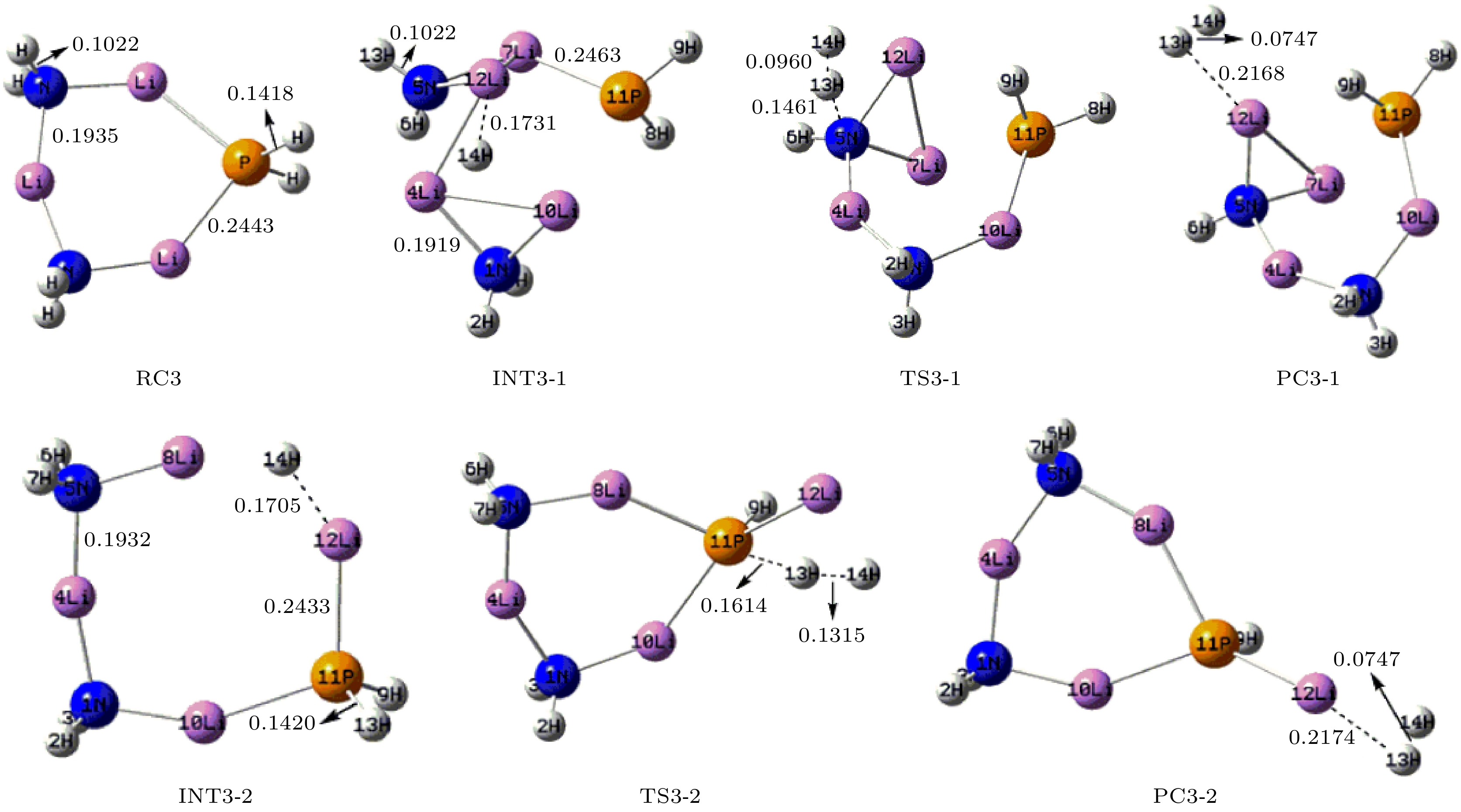
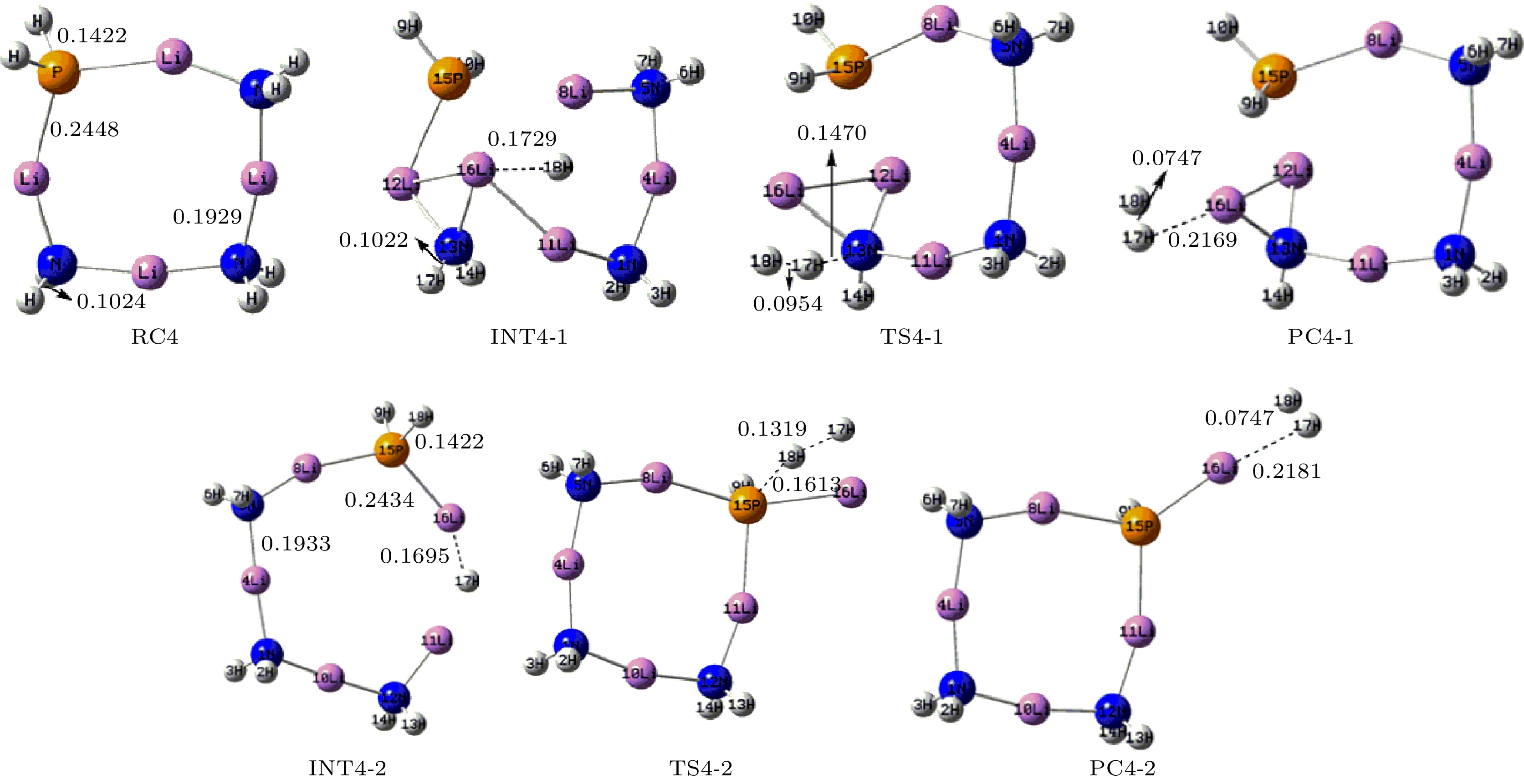
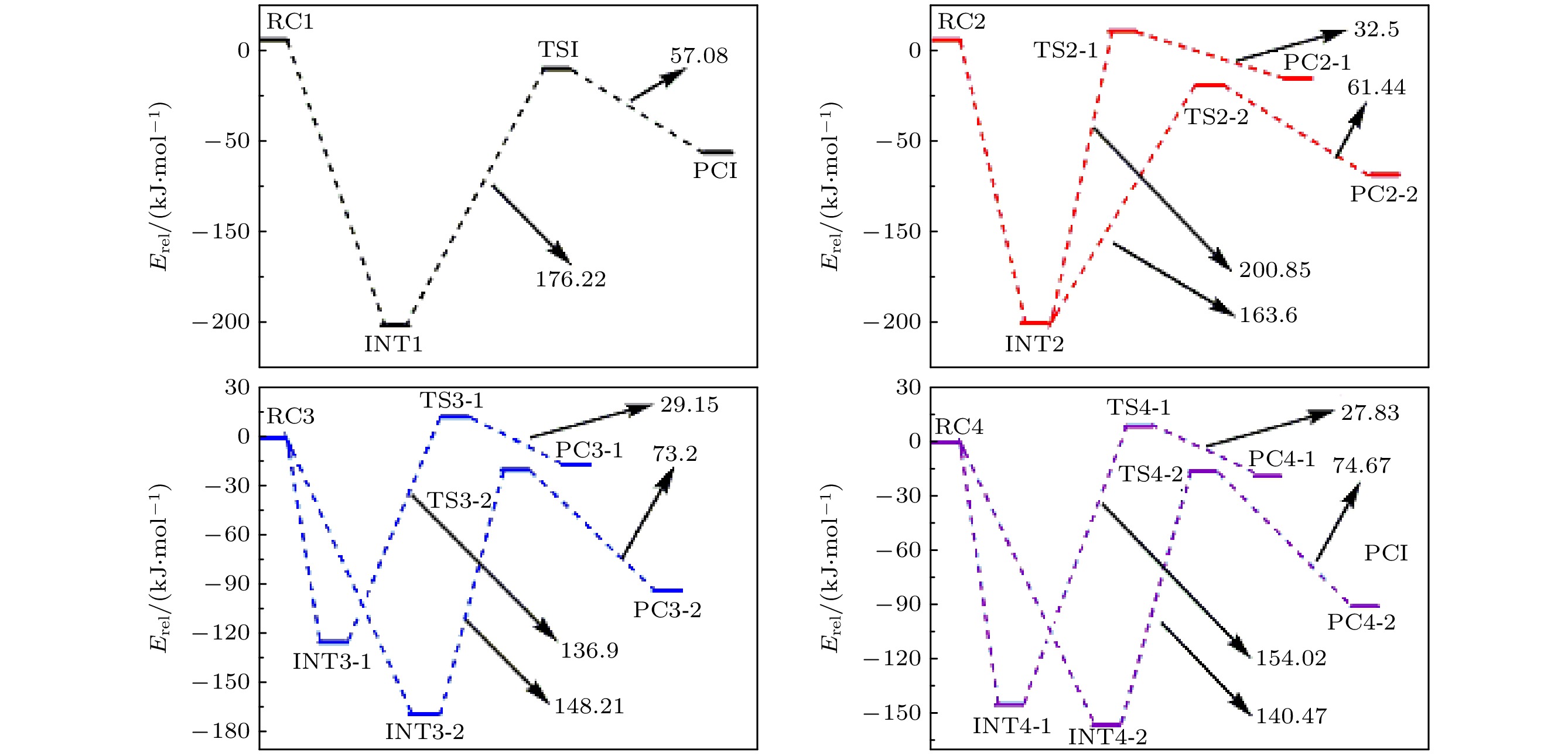
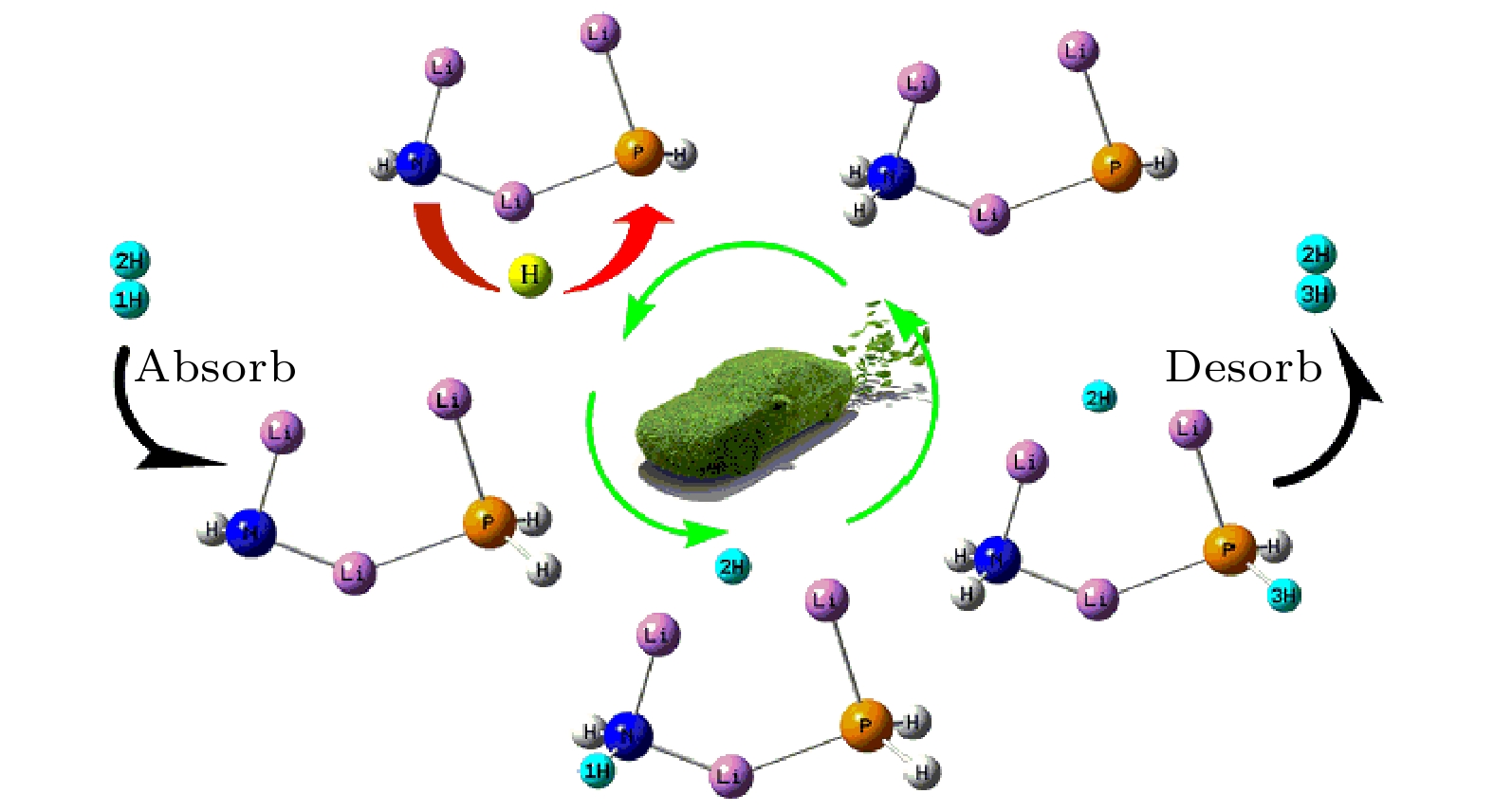
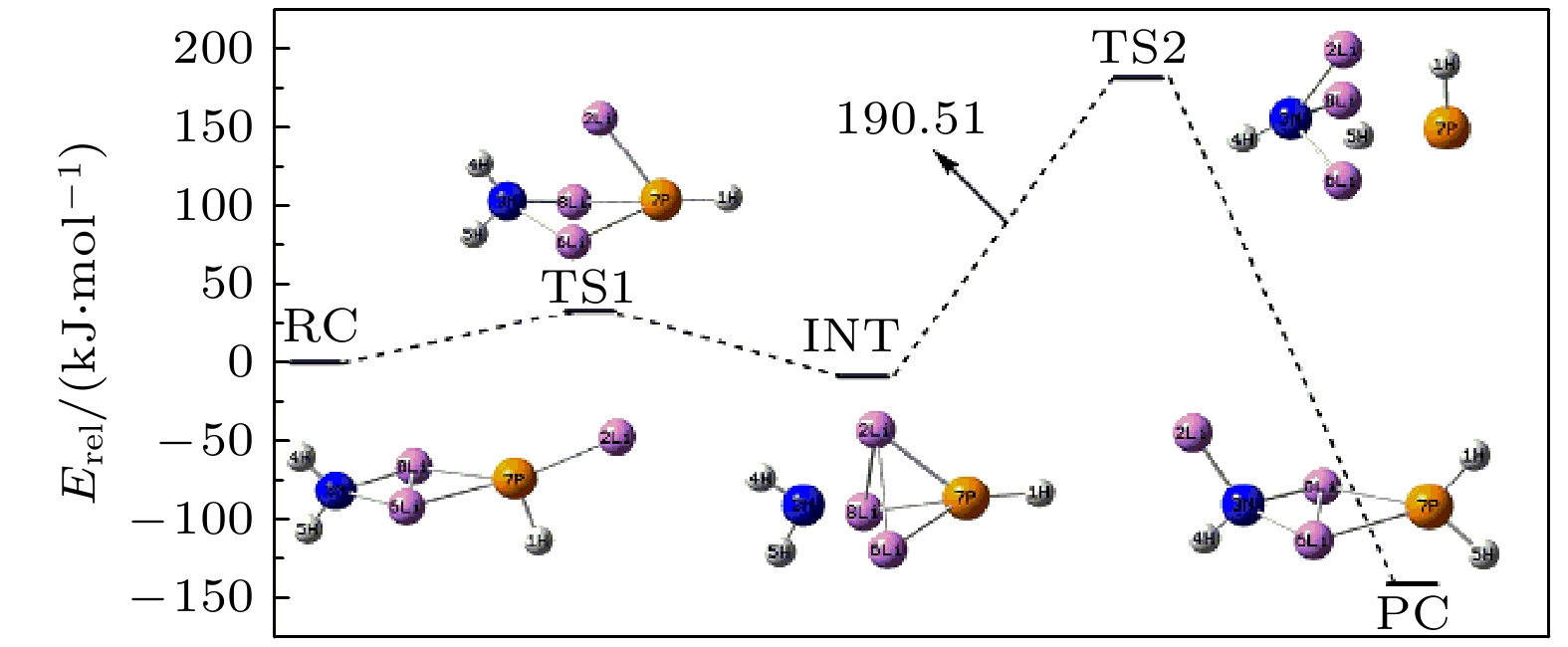
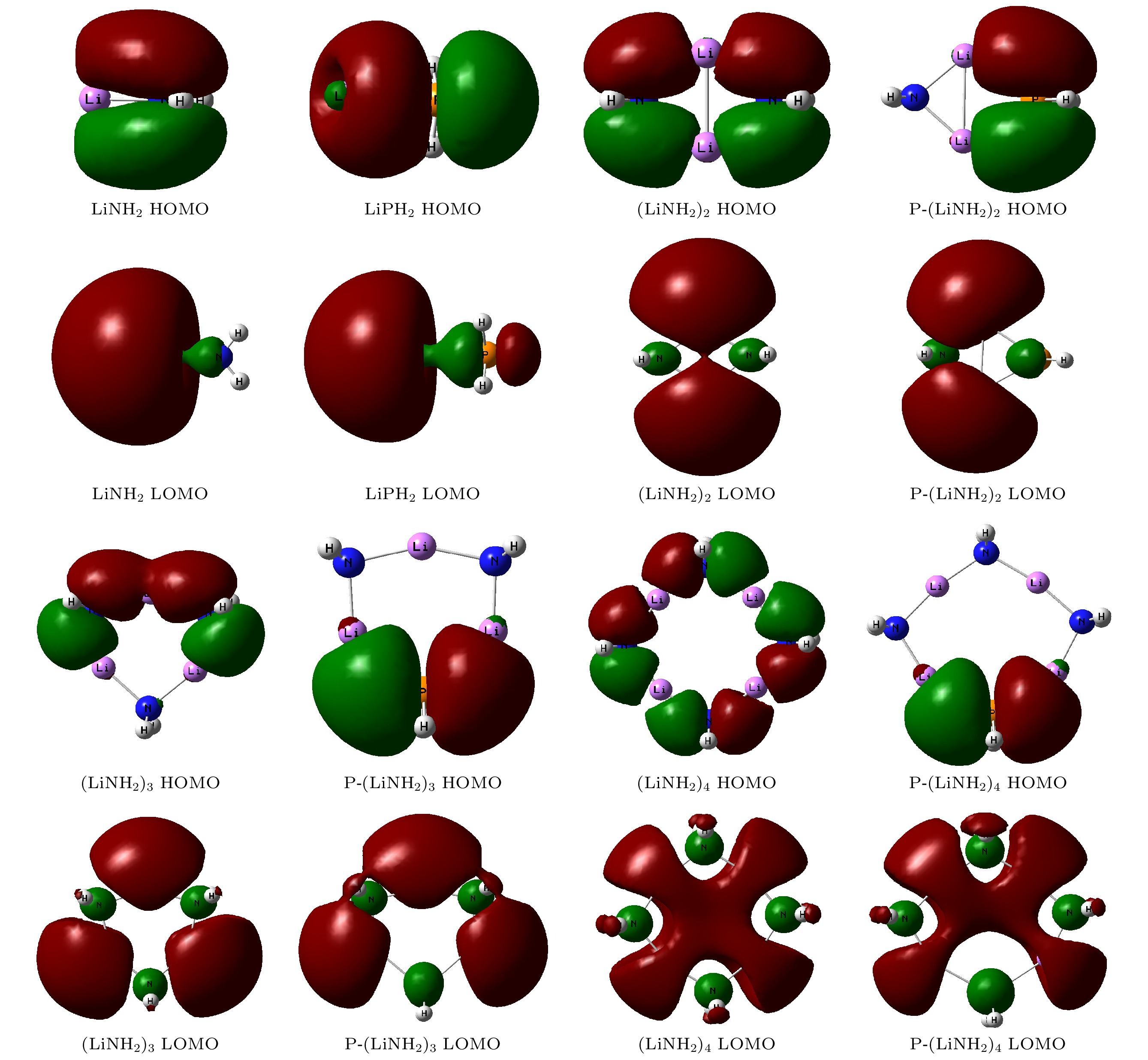
采用密度泛函理论, 对P掺杂的(LiNH2)n(n = 1—4)团簇与LiH的反应机理进行理论分析, 反应中的驻点进行了构型优化, 通过频率和内禀反应坐标的计算来判断各驻点及其相互连接关系的正确性. 计算了能够反映反应物的稳定性参数. 结果表明: P掺杂后对最低未占据轨道影响较小, 最高占据轨道分布有较大幅度向掺杂原子处的转移, 失电子区域集中在P原子处. 经P原子掺杂, 降低了氨基锂团簇的稳定性, 反应脱氢能垒有所降低. 反应更倾向于由—PH2官能团脱氢和在—NH2官能团处储氢. 在循环储放氢过程中, 实现氢在—NH2和—PH2官能团之间的有效转移, 可进一步改善该材料的储放氢的可逆性.Hydrogen energy is considered a clean energy with great development prospects. In the field of hydrogen energy applications, the solid-state chemical hydrogen storage method using hydrogen storage materials as media has received widespread attention due to its safety and high hydrogen storage density. In the research on metal-N-H system hydrogen storage materials, current studies focus on improving the kinetic conditions for hydrogen storage. In this study, the B3LYP hybrid functional method of density functional theory is used to investigate the reaction mechanism between P-doped LiNH2 clusters and LiH at a cluster level, and explore the effects of doping, in addition a new hydrogen storage mechanism called “secondary hydrogen transfer” is proposed. The full-geometry optimization of (LiNH2)n (n = 1–4) clusters and their P-doped clusters at the 6-31G(d,p) basis set level are carried out, and their corresponding most stable configurations are obtained. The distribution and stability of the frontier orbitals of the relevant reactants are calculated. Using the same method and basis set, the theoretical calculation and analysis of the reaction mechanism between P-doped (LiNH2)n (n = 1–4) clusters and LiH are conducted, including the configuration optimization of the stationary points in each reaction path, and the correctness of the connection between the stationary points is determined through frequency and intrinsic reaction coordinate calculations. The results show that P doping has a small effect on the lowest unoccupied molecular orbital, while the highest occupied molecular orbital has a significant transition towards the doping atom, and the electron-deficient region is concentrated at the P atom. P doping reduces the stability of the lithium amide clusters and enhances their ability to participate in chemical reactions and reaction activity, and the reaction dehydrogenation energy barrier decreases. The reaction dehydrogenation energy barrier between P-doped LiNH2 clusters and LiH is significantly lower than that between LiNH2 and LiH, which is consistent with the analysis of reactant stability. Additionally, it is found that the reaction between P-doped LiNH2 clusters and LiH tends to dehydrogenate through the —PH2 functional group and store hydrogen at the —NH2 functional group. Therefore, a new idea of “secondary hydrogen transfer” is proposed, in which effective transfer of hydrogen between —NH2 and —PH2 functional groups takes place during the cyclic hydrogen storage process, thus the reversibility of hydrogen storage is further improved and the hydrogen desorption energy barrier of the material is reduced.
[1] Schlapbach L, Zuttel A 2001 Nature 414 353
 Google Scholar
Google Scholar
[2] 马通祥, 高雷章, 胡蒙均, 胡丽文, 温良英, 扈玫珑 2018 功能材料 49 4001
 Google Scholar
Google Scholar
Ma T X, Gao L Z, Hu M J, Hu L W, Wen L Y, Hu M L 2018 J. Funct. Mater. 49 4001
 Google Scholar
Google Scholar
[3] Chen P, Xiong Z, Luo J, Lin J, Tan K L 2002 Nature 420 302
 Google Scholar
Google Scholar
[4] Zhang X, Sun Y, Xia G, Yu X 2022 J. Alloys Compd. 899 163254
 Google Scholar
Google Scholar
[5] Sitthiwet C, Plerdsranoy P, Utke O, Nijpanich S, Chanlek N, Eiamlamai P, Utke R 2022 J. Alloys Compd. 909 164673
 Google Scholar
Google Scholar
[6] Bai Z X, Zeng W, Tang B, Fan D H, Liu Q J, Jiang C L, Chang X H 2022 Int. J. Mod. Phys. B 36 2250074
 Google Scholar
Google Scholar
[7] Chen Y, Sun X, Zhang W K, Gan Y P, Xia Y, Zhang J, Huang H, Liang C, Pan H 2020 ACS Appl. Mater. Interfaces 12 15255
 Google Scholar
Google Scholar
[8] Li C, Li C, Fan M, Chen H, Shu K, Zhang Y, Gao M, Liu Y, Pan H 2019 J. Energy Chem. 35 37
 Google Scholar
Google Scholar
[9] Goshome K, Jain A, Miyaoka H, Yamamoto H, Kojima Y, Ichikawa T 2019 Molecules 24 1348
 Google Scholar
Google Scholar
[10] Shukla V, Bhatnagar A, Singh S, Soni P K, Verma S K, Yadav T P, Shaz M A, Srivastava O N 2019 Dalton Trans. 48 30
 Google Scholar
Google Scholar
[11] 马星宇, 王二锐, 邱树君, 褚海亮, 邹勇进, 向翠丽, 闫二虎, 徐芬, 孙立贤 2016 材料导报 30 206
 Google Scholar
Google Scholar
Ma X Y, Wang E R, Qiu S J, Chu H L, Zou Y J, Xiang C L, Yan E H, Xu F, Sun L J 2016 Mater. Rep. 30 206
 Google Scholar
Google Scholar
[12] Wang J, Liu T, Wu G, Li W, Liu Y, Araujo C M, Scheicher R H, Blomqvist A, Ahuja R, Xiong Z, Yang P, Gao M, Pan H, Chen P 2009 Angew. Chem. Int. Ed. 48 5828
 Google Scholar
Google Scholar
[13] Zhang J, Liu Y, Zhang X, Yang Y, Zhang Q, Jin T, Wang Y, Gao M, Sun L, Pan H 2016 Int. J. Hydrogen Energy 41 11264
 Google Scholar
Google Scholar
[14] Zhang B, Yuan J, Wu Y 2018 Int. J. Hydrogen Energy 44 19294
 Google Scholar
Google Scholar
[15] Qiu S, Gao W, Ma X, Chu H, Zou Y, Xiang C, Zhang H, Xu F, Sun L 2018 Int. J. Hydrogen Energy 43 13975
 Google Scholar
Google Scholar
[16] Zhu X L, Han S M, Zhao X, Li Y, Liu B Z 2014 Rare Met. 33 86
 Google Scholar
Google Scholar
[17] Rohit R, Rajesh K, Vivek S, Ashish B, Srivastava O 2017 Int. J. Hydrogen Energy 42 29350
 Google Scholar
Google Scholar
[18] Orimo S, Nakamori Y, Kitahara G, Miwa K, Ohba N, Noritake T, Towata 2004 Appl. Phys. A 79 1765
 Google Scholar
Google Scholar
[19] Zhang Y R, Dong B X, Zhao J, Teng Y L, Li Z W, Wang L 2017 Int. J. Hydrogen Energy 42 17149
 Google Scholar
Google Scholar
[20] 邵子霁 2019 博士学位论文 (长春: 吉林大学)
Shao Z J 2019 Ph. D. Dissertation (Changchun: Jilin University) (in Chinese)
[21] Lee C, Yang W, Parr R G 1988 Phys. Rev. B 37 785
 Google Scholar
Google Scholar
[22] Becke, Axel D 1993 J. Chem. Phys. 98 5648
 Google Scholar
Google Scholar
[23] 张陈俊, 王养丽, 陈朝康 2018 67 113101
 Google Scholar
Google Scholar
Zhang C J, Wang Y L, Chen C K 2018 Acta Phys. Sin. 67 113101
 Google Scholar
Google Scholar
[24] Ivakhnenko E, Malay V, Demidov O, Starikov A, Minkin V 2022 Tetrahedron 103 132575
 Google Scholar
Google Scholar
[25] Fukui K, Yonezawa T, Shingu H 1952 J. Chem. Phys. 20 722
 Google Scholar
Google Scholar
[26] Ichikawa T, Isobe S, Hanada N, Fujii H 2004 J. Alloys Compd. 365 271
 Google Scholar
Google Scholar
[27] Janot R, Eymery J B, Tarascon J M 2007 J. Power Sources 164 496
 Google Scholar
Google Scholar
-
表 1 反应物的动力学稳定性参数(单位: eV)
Table 1. Kinetic stability parameters of reactants (unit: eV).
Cluster ELOMO EHOMO ΔE EIP LiNH2 –1.143 –4.218 3.075 7.054 LiPH2 –1.633 –4.789 3.156 7.292 (LiNH2)2 –0.490 –5.306 4.816 7.607 P-(LiNH2)2 –0.707 –4.816 4.109 7.253 (LiNH2)3 –0.408 –5.714 5.306 7.678 P-(LiNH2)3 –0.490 –5.225 4.735 7.651 (LiNH2)4 –0.463 –5.823 5.360 7.548 P-(LiNH2)4 –0.490 –5.442 4.952 7.406 表 2 反应各驻点的能量、相对能量及部分振动频率
Table 2. Total energies and relative energies at the critical points of potential energy surface and vibrational frequencies.
Species Etotal/a.u. Erel/a.u. Erel/(kJ·mol–1) Frequency/cm–1 RC1 –358.15783 0 0 — — INT1 –358.23259 –0.07476 –196.28 177.1 277.4 TS1 –358.16547 –0.00764 –20.06 –740.0 163.7 PC1 –358.18721 –0.02938 –77.14 22.8 110.9 RC2 –421.73285 0 0 — — INT2 –421.80701 –0.07416 –194.71 91.7 111.7 TS2-1 –421.73051 0.00234 6.14 –924.0 60.1 PC2-1 –421.74289 –0.01004 –26.36 40.4 57.5 TS2-2 –421.7447 –0.01185 –31.11 –841.0 54.0 PC2-2 –421.7681 –0.03525 –92.55 22.0 34.2 RC3 –485.30464 0 0 — — INT3-1 –485.35191 –0.04727 –124.11 77.2 87.3 TS3-1 –485.29977 0.00487 12.79 –959.3 78.8 PC3-1 –485.31087 –0.00623 –16.36 44.2 58.8 INT3-2 –485.3685 –0.06386 –167.66 38.6 64.5 TS3-2 –485.31205 –0.00741 –19.45 –935.4 37.8 PC3-2 –485.33993 –0.03529 –92.65 26.7 31.0 RC4 –548.86487 0 0 — — INT4-1 –548.91997 –0.0551 –144.67 41.9 61.6 TS4-1 –548.86131 0.00356 9.35 –927.3 28.9 PC4-1 –548.87191 –0.00704 –18.48 29.6 45.7 INT4-2 –548.92434 –0.05947 –156.14 7.0 33.8 TS4-2 –548.87084 –0.00597 –15.67 –922.3 19.8 PC4-2 –548.89928 –0.03441 –90.34 17.7 30.4 -
[1] Schlapbach L, Zuttel A 2001 Nature 414 353
 Google Scholar
Google Scholar
[2] 马通祥, 高雷章, 胡蒙均, 胡丽文, 温良英, 扈玫珑 2018 功能材料 49 4001
 Google Scholar
Google Scholar
Ma T X, Gao L Z, Hu M J, Hu L W, Wen L Y, Hu M L 2018 J. Funct. Mater. 49 4001
 Google Scholar
Google Scholar
[3] Chen P, Xiong Z, Luo J, Lin J, Tan K L 2002 Nature 420 302
 Google Scholar
Google Scholar
[4] Zhang X, Sun Y, Xia G, Yu X 2022 J. Alloys Compd. 899 163254
 Google Scholar
Google Scholar
[5] Sitthiwet C, Plerdsranoy P, Utke O, Nijpanich S, Chanlek N, Eiamlamai P, Utke R 2022 J. Alloys Compd. 909 164673
 Google Scholar
Google Scholar
[6] Bai Z X, Zeng W, Tang B, Fan D H, Liu Q J, Jiang C L, Chang X H 2022 Int. J. Mod. Phys. B 36 2250074
 Google Scholar
Google Scholar
[7] Chen Y, Sun X, Zhang W K, Gan Y P, Xia Y, Zhang J, Huang H, Liang C, Pan H 2020 ACS Appl. Mater. Interfaces 12 15255
 Google Scholar
Google Scholar
[8] Li C, Li C, Fan M, Chen H, Shu K, Zhang Y, Gao M, Liu Y, Pan H 2019 J. Energy Chem. 35 37
 Google Scholar
Google Scholar
[9] Goshome K, Jain A, Miyaoka H, Yamamoto H, Kojima Y, Ichikawa T 2019 Molecules 24 1348
 Google Scholar
Google Scholar
[10] Shukla V, Bhatnagar A, Singh S, Soni P K, Verma S K, Yadav T P, Shaz M A, Srivastava O N 2019 Dalton Trans. 48 30
 Google Scholar
Google Scholar
[11] 马星宇, 王二锐, 邱树君, 褚海亮, 邹勇进, 向翠丽, 闫二虎, 徐芬, 孙立贤 2016 材料导报 30 206
 Google Scholar
Google Scholar
Ma X Y, Wang E R, Qiu S J, Chu H L, Zou Y J, Xiang C L, Yan E H, Xu F, Sun L J 2016 Mater. Rep. 30 206
 Google Scholar
Google Scholar
[12] Wang J, Liu T, Wu G, Li W, Liu Y, Araujo C M, Scheicher R H, Blomqvist A, Ahuja R, Xiong Z, Yang P, Gao M, Pan H, Chen P 2009 Angew. Chem. Int. Ed. 48 5828
 Google Scholar
Google Scholar
[13] Zhang J, Liu Y, Zhang X, Yang Y, Zhang Q, Jin T, Wang Y, Gao M, Sun L, Pan H 2016 Int. J. Hydrogen Energy 41 11264
 Google Scholar
Google Scholar
[14] Zhang B, Yuan J, Wu Y 2018 Int. J. Hydrogen Energy 44 19294
 Google Scholar
Google Scholar
[15] Qiu S, Gao W, Ma X, Chu H, Zou Y, Xiang C, Zhang H, Xu F, Sun L 2018 Int. J. Hydrogen Energy 43 13975
 Google Scholar
Google Scholar
[16] Zhu X L, Han S M, Zhao X, Li Y, Liu B Z 2014 Rare Met. 33 86
 Google Scholar
Google Scholar
[17] Rohit R, Rajesh K, Vivek S, Ashish B, Srivastava O 2017 Int. J. Hydrogen Energy 42 29350
 Google Scholar
Google Scholar
[18] Orimo S, Nakamori Y, Kitahara G, Miwa K, Ohba N, Noritake T, Towata 2004 Appl. Phys. A 79 1765
 Google Scholar
Google Scholar
[19] Zhang Y R, Dong B X, Zhao J, Teng Y L, Li Z W, Wang L 2017 Int. J. Hydrogen Energy 42 17149
 Google Scholar
Google Scholar
[20] 邵子霁 2019 博士学位论文 (长春: 吉林大学)
Shao Z J 2019 Ph. D. Dissertation (Changchun: Jilin University) (in Chinese)
[21] Lee C, Yang W, Parr R G 1988 Phys. Rev. B 37 785
 Google Scholar
Google Scholar
[22] Becke, Axel D 1993 J. Chem. Phys. 98 5648
 Google Scholar
Google Scholar
[23] 张陈俊, 王养丽, 陈朝康 2018 67 113101
 Google Scholar
Google Scholar
Zhang C J, Wang Y L, Chen C K 2018 Acta Phys. Sin. 67 113101
 Google Scholar
Google Scholar
[24] Ivakhnenko E, Malay V, Demidov O, Starikov A, Minkin V 2022 Tetrahedron 103 132575
 Google Scholar
Google Scholar
[25] Fukui K, Yonezawa T, Shingu H 1952 J. Chem. Phys. 20 722
 Google Scholar
Google Scholar
[26] Ichikawa T, Isobe S, Hanada N, Fujii H 2004 J. Alloys Compd. 365 271
 Google Scholar
Google Scholar
[27] Janot R, Eymery J B, Tarascon J M 2007 J. Power Sources 164 496
 Google Scholar
Google Scholar
计量
- 文章访问数: 5041
- PDF下载量: 89
- 被引次数: 0













 下载:
下载: