-
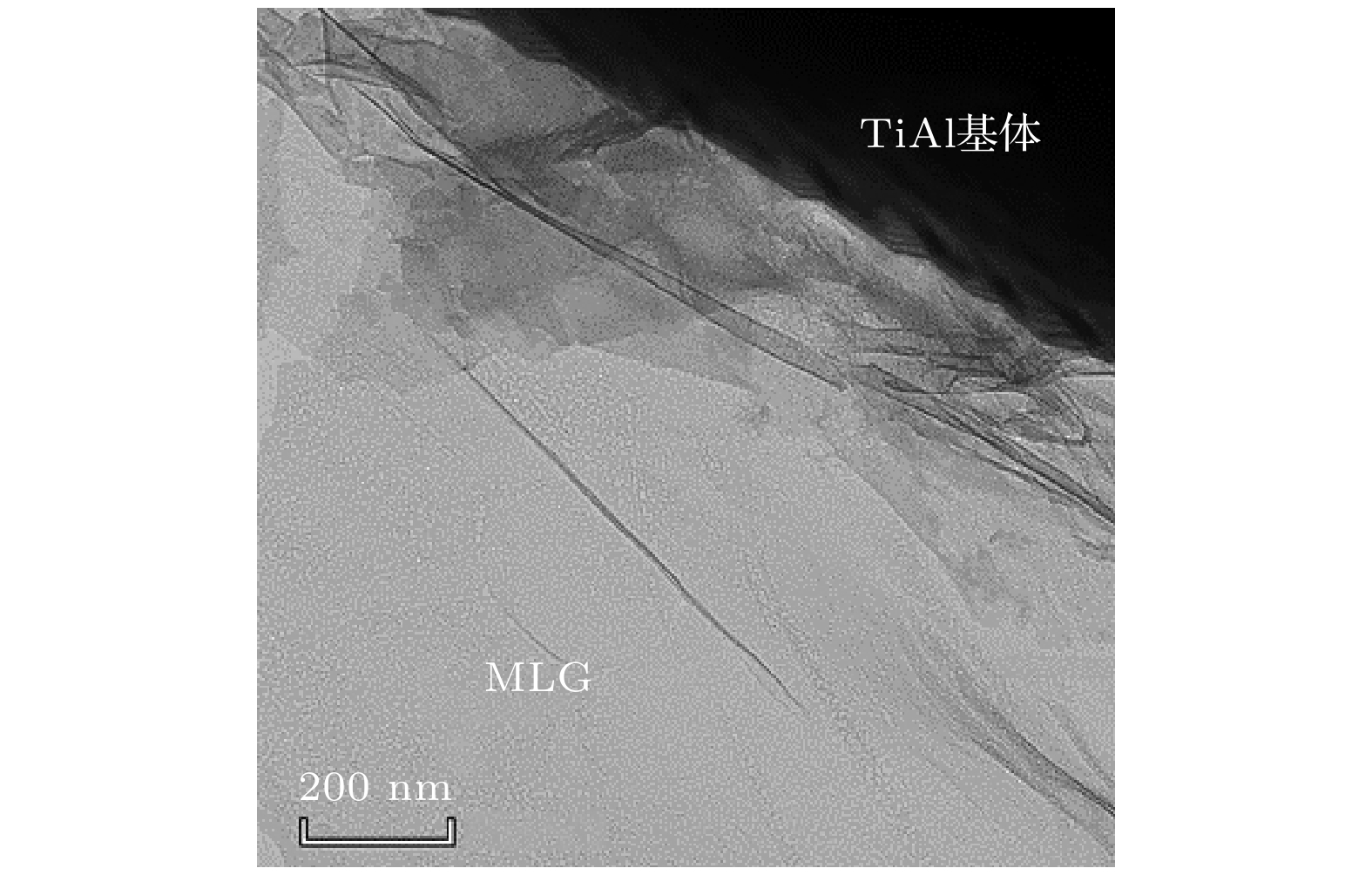
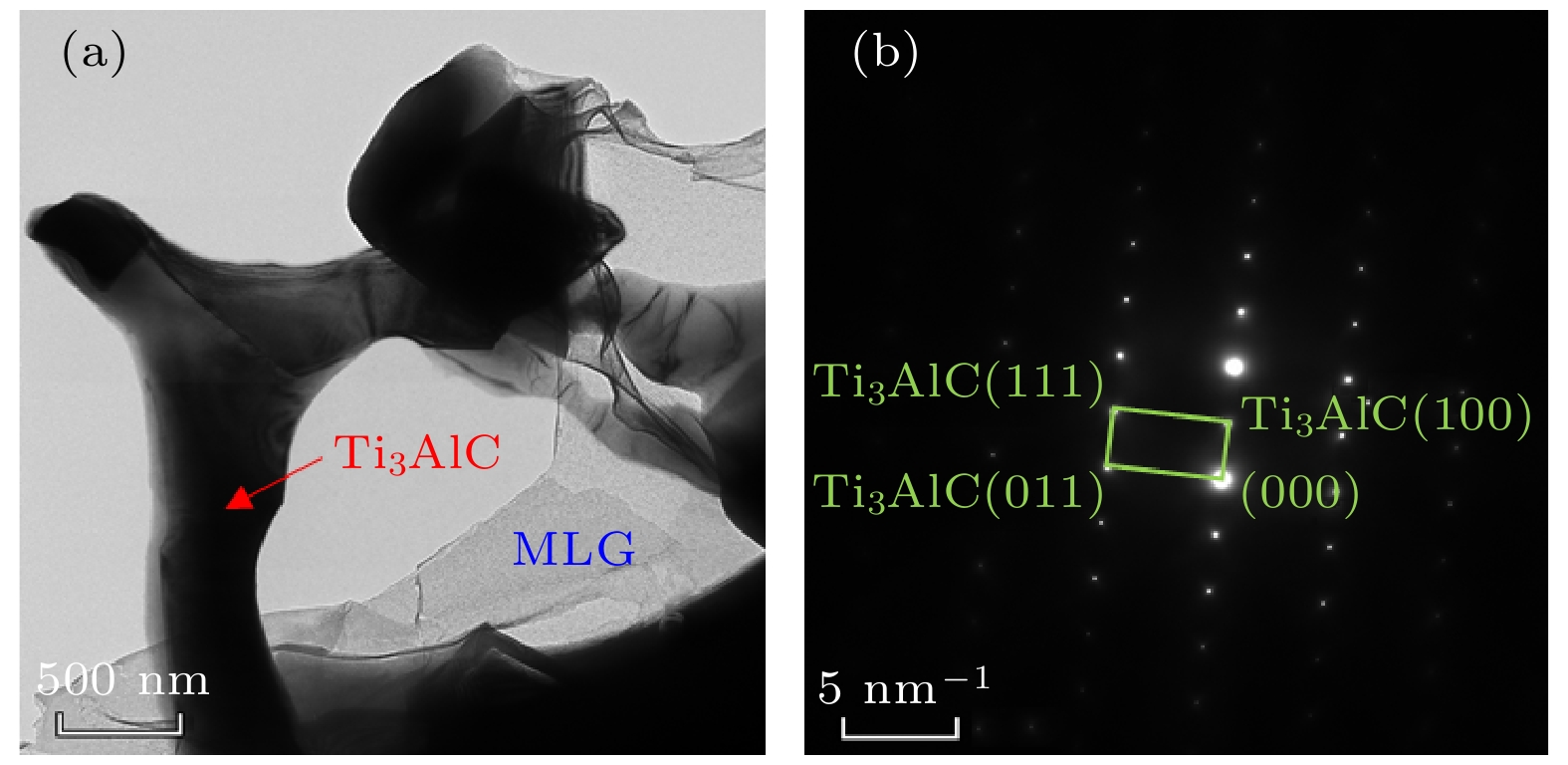
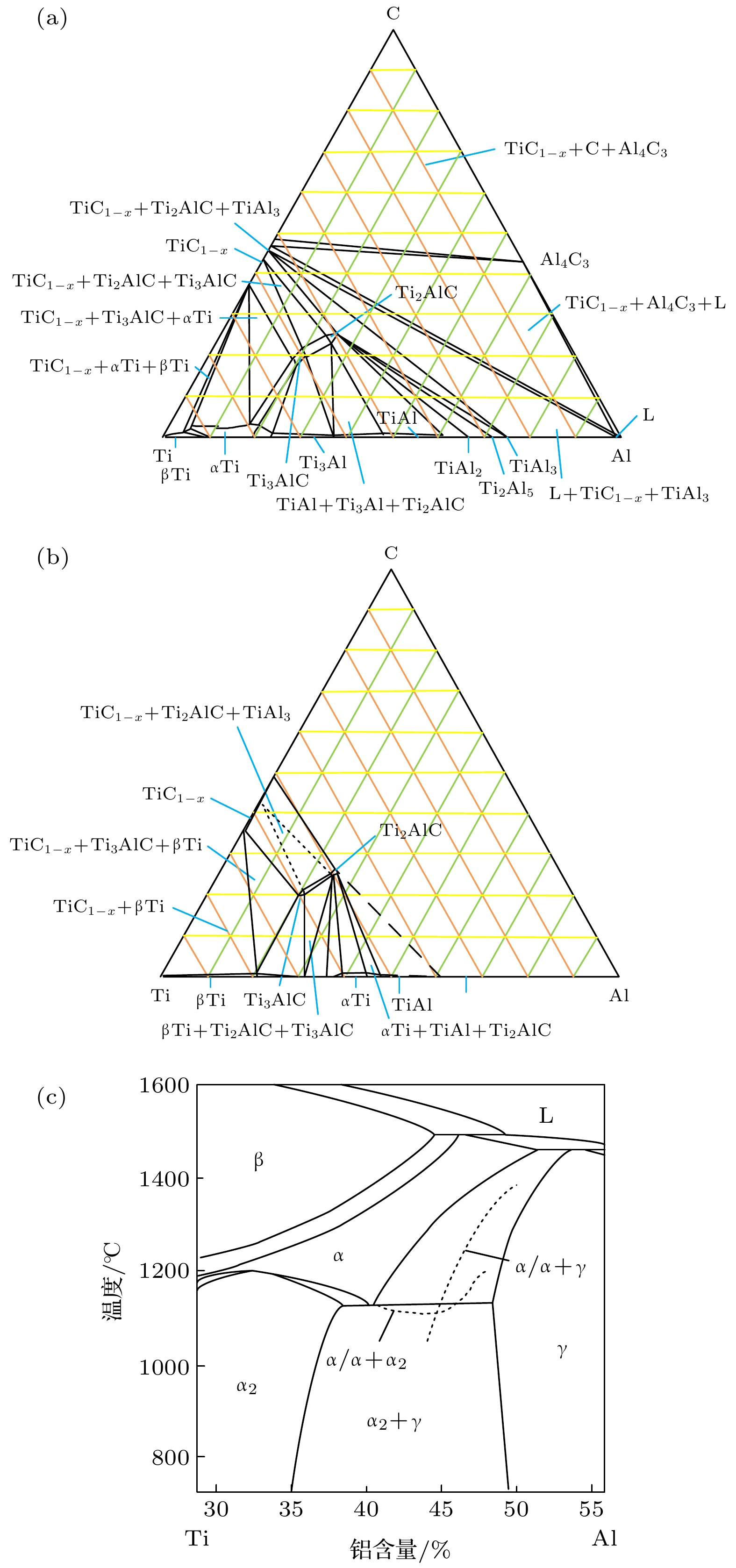
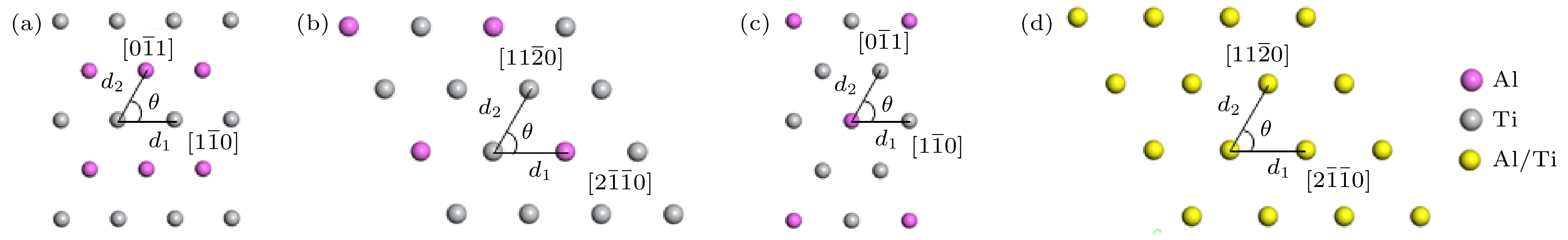
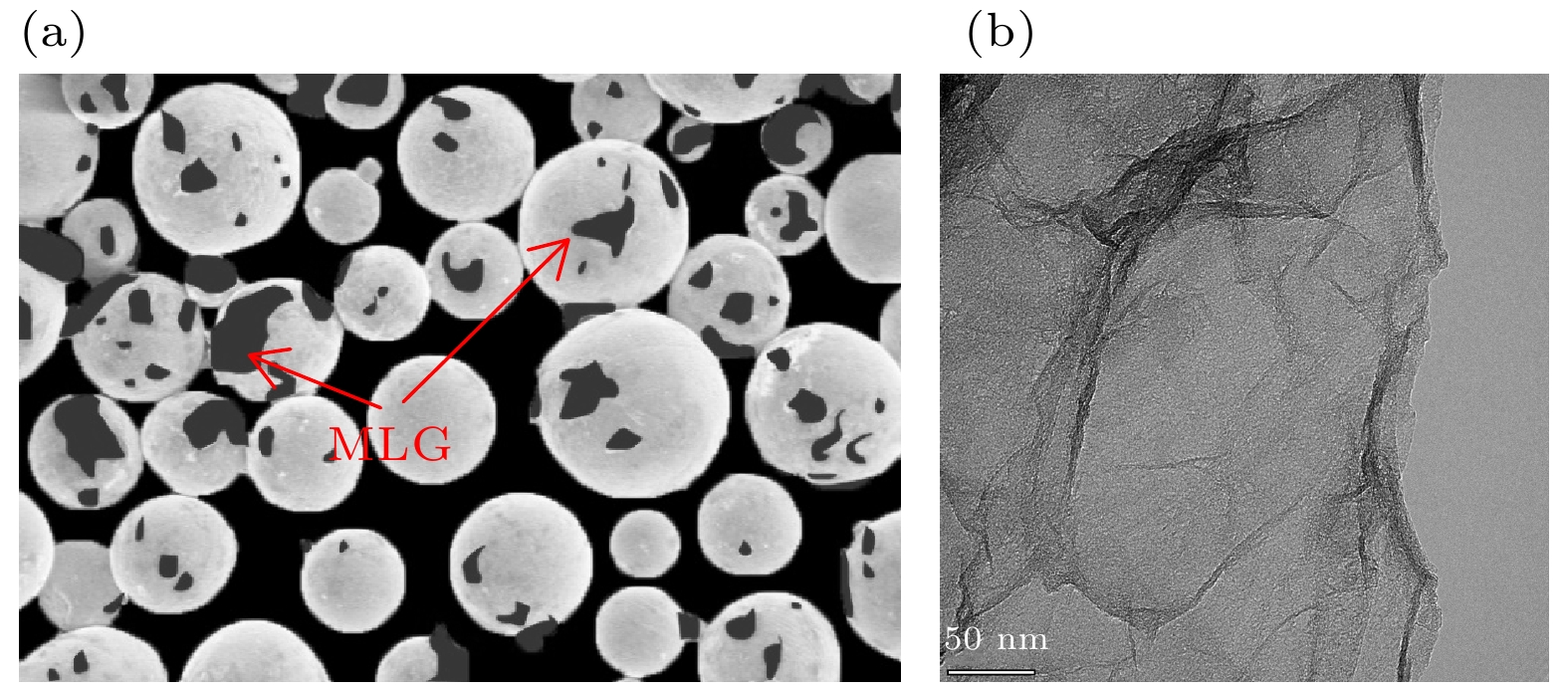
向TiAl合金中添加C元素反应形成的Ti2AlC相与Ti3AlC相分别具有改善TiAl合金塑性和强度的作用. 一般地, 液相烧结过程中会发生L + TiC→Ti2AlC(Ti3AlC)的包晶反应, 但在固相烧结过程中Ti2AlC与Ti3AlC的形成可能具有不同机制. 本文以多层石墨烯为碳源, 通过1100—1350 ℃的固相烧结获得C与TiAl合金的界面反应组织, 借助微观组织表征与分析, 发现Ti2AlC与Ti3AlC生成过程中没有TiC参与. 进一步计算发现TiC/TiAl, Ti2AlC/TiAl与Ti3AlC/TiAl的界面能分别约为1.2, 0.87和0.39 J·m2, 据此得出Ti2AlC与Ti3AlC可以不经TiC中间相直接形成. 此外, 研究还发现在1150—1250 ℃几乎只生成Ti2AlC相, 但1250—1350 ℃界面产物组成为Ti3AlC + 少量Ti2AlC相, 原因在于烧结温度对基体α相含量存在影响, 进而影响Ti2AlC与Ti3AlC的形成倾向. 在1150—1250 ℃, TiAl合金基体由γ + 少量α相组成, Ti2AlC具有较高形成倾向; 1250—1350 ℃基体几乎完全转化为α相, α相含量增大对Ti3AlC的形成具有促进作用. 研究结果表明, 通过控制TiAl合金与多层石墨烯的固相烧结温度, 可以调控Ti2AlC与Ti3AlC的相对含量, 进而有望改善TiAl合金的塑性与强度.Ti2AlC and Ti3AlC formed by the reaction between C and TiAl alloy can improve the plasticity and strength of TiAl alloy respectively. Generally, the peritectic reaction of L + TiC→Ti2AlC (Ti3AlC) occurs in the process of liquid-phase sintering, but different formation mechanisms of Ti2AlC and Ti3AlC may exist in the solid-state sintering. In this work, multilayer graphene is employed to fabricate the reaction interface with TiAl alloy under 1100–1350 ℃, which is the common solid-state sintering temperature of TiAl alloy. According to the microstructure characterization and analysis, Ti2AlC and Ti3AlC are verified to contain no TiC. The interface energy values of TiC/TiAl, Ti2AlC/TiAl and Ti3AlC/TiAl are calculated to be about 1.2, 0.87 and 0.39 J·m2, respectively, indicating that Ti2AlC and Ti3AlC can be formed directly without TiC mesophase. Besides, only Ti2AlC is observed to be formed at 1150–1250 ℃, while the interface products at 1250–1350 ℃ change into Ti3AlC with a small amount of Ti2AlC. The mechanism that the sintering temperature affects the formation tendency of Ti2AlC and Ti3AlC can be ascribed to the content of α phase. The TiAl alloy matrix is composed of γ and a few α phases at 1150–1250℃, but almost completely transforms into α phase at 1250–1350 ℃, and the increase in the α content can promote the formation of Ti3AlC. The above results demonstrate the possibility of regulating the relative content of Ti2AlC and Ti3AlC through controlling the sintering temperature, which provides a promising method to improve the plasticity and strength of TiAl alloy.
-
Keywords:
- titanium aluminide /
- solid sintering /
- interface reaction /
- interfacial energy
[1] Appel F, Clemens H, Fischer F D 2016 Prog. Mater. Sci 81 55
 Google Scholar
Google Scholar
[2] Ouyang P X, Mi G B, Cao J X, Huang X, He L J, Li P J 2018 Mater. Today Comm 16 364
 Google Scholar
Google Scholar
[3] Yamaguchi M, Inui H, Ito K 2000 Acta Mater 48 307
 Google Scholar
Google Scholar
[4] Gao B, Peng H, Liang Y, Lin J, Chen B 2021 Mater. Sci. Eng. A 881 141059
[5] Kan W, Chen B, Peng H, Liang Y, Lin J 2020 Mater. Lett. 259 126856
[6] Lapin J, Pelachova T, Bajana O 2019 J. Alloys Compd 797 754
 Google Scholar
Google Scholar
[7] Fang H, Chen R, Liu Y, Tan Y Guo J 2019 Intermetallics 115 106630
 Google Scholar
Google Scholar
[8] Gouma P I, Mills M J, Kim Y W 1998 Phil. Mag Lett 78 59
 Google Scholar
Google Scholar
[9] Wu H, Fan G H, Cui X P, Geng L, Yuan F, Pang J C, Wei L S, Huang M 2013 Mater. Sci. Eng. A 585 439
 Google Scholar
Google Scholar
[10] Wu M Y, Mi G B, Li P J, Huang X, Cao C X 2020 J. Aeron. Mater 40 45
[11] Nieto A, Bisht A, Lahiri D, Zhang C, Agarwal A 2016 Int. Mater. Rev 62 241
[12] Wu M Y, Mi G B, Li P J, Huang X, Cao C X 2022 Mater. Lett 310 131515
 Google Scholar
Google Scholar
[13] Guo B S, Ni S, Yi J H, Shen R J, Tang Z H, Du Y, Song M 2017 Mater. Sci. Eng. A 698 282
 Google Scholar
Google Scholar
[14] Song Y, Chen Y, Liu W W, Li W L, Wang Y G, Zhao D, Liu X B 2016 Mater. Des 109 256
 Google Scholar
Google Scholar
[15] Chen Y L, Yan M, Sun Y M, Mei B C, Zuo J Q 2009 Ceram. Int 35 1807
 Google Scholar
Google Scholar
[16] Song X J, Cui H Z, Hou N, Wei N, Han Y, Tian J, Song Q 2016 Ceram. Int 42 13586
 Google Scholar
Google Scholar
[17] MSIT, Cornish L, Cacciamani G, Cupid D M, Keyzer J D https://materials.springer.com/msi/docs/sm_msi_r_10_014870_02/ [2021-5-6]
[18] Kim Y W 1992 Acta Metall. Mater 40 1121
 Google Scholar
Google Scholar
[19] 王苹, 梅炳初, 洪小林, 朱教群, 周卫兵, 严明, 2007 武汉理工大学学报 29 5
Wang P, Mei B C, Hong X L, Zhu J Q, Zhou W B, Yan M 2007 J. Wuhan Univ. Technol. 29 5 (in Chinese)
[20] Pietzka M A, Schuster J C 1994 J. Phase Equilib 15 392
 Google Scholar
Google Scholar
[21] Chen Y, Chu M Y, Wang L J, Bao X H, Lin Y, Shen J Y 2011 Phys. Status Solidi A 208 1879
 Google Scholar
Google Scholar
[22] 胡庚祥 蔡珣 戎咏华 2013 材料科学基础 (上海: 上海交通大学出版社) 第130页
Hu G X, Cai X, Rong Y H 2013 Fundamentals of Materials Science (Shanghai: Shanghai Jiao Tong University Press) p130 (in Chinese)
[23] Merwe J, Woltersdorf J, Jesser W A 1986 Mater. Sci. Eng 81 1
 Google Scholar
Google Scholar
[24] 刘文胜, 黄伯云, 周科朝, 唐建成 2000 材料导报 14 19
Liu W S, Huang B Y, Zhou K C, Tang J C 2000 Mater. Rep. 14 19 (in Chinese)
[25] Zhang W J, Reddy B V, Deevi S C 2001 Scr. Mater 45 645
 Google Scholar
Google Scholar
[26] Kumpfert J 2001 Adv. Eng. Mater 3 851
 Google Scholar
Google Scholar
[27] Todorova T Z, Gaier M, Zwanziger J W, Plucknett K P 2019 J. Alloy. Compd 789 712
 Google Scholar
Google Scholar
[28] Arusei G K, Chepkoech M, Amolo G O, Wambua N 2020 arXiv: 2011.07102 v1 [cond-mat. mtrl]
[29] Zhang X W, Wang X H, Li F Z, Zhou Y C 2010 J. Am. Ceram. Soc 92 2698
[30] Schuster J C, Nowotny H, Vaccaro C 1980 J. Solid State Chem 32 213
 Google Scholar
Google Scholar
[31] Болецкая B М1979 Metal Sci. Heat Treatm. Metals 12 37(in Russian)
[32] Vanloo F, Bastin G F 1989 Metall. Meter. Trans. A 20 403
-
图 8 C与TiAl合金的反应路径 (a) 液相烧结经由TiC发生包晶反应; (b) 1150—1250 ℃固相烧结界面反应形成Ti2AlC; (c) 1250—1350 ℃固相烧结界面反应形成Ti3AlC
Fig. 8. Reaction paths of C and TiAl alloy: (a) peritectic reaction via TiC during liquid-phase sintering; (b) Ti2AlC formed at 1150—1250 ℃ by solid-state sintering interface reaction; (c) Ti3AlC formed at 1250—1350 ℃ by solid-state sintering interface reaction.
表 1 Ti-Al-C主要化合物的摩尔生成自由能
Table 1. Molar free energy of formation of compounds formed by Ti-Al-C.
化合物 摩尔生成自由能 ${\varDelta _{\text{f} } }G_{\text{m} }$/(kJ·mol–1) TiAl3 0.06522T – 289.37 TiAl 0.02224T – 91.078 Ti3Al 0.01678T – 109.75 TiC 0.01522T – 189.07 Ti2AlC 0.03045T – 387.13 Ti3AlC 0.1208T – 583.45 表 2 C与TiAl的摩尔反应自由能
${\varDelta _{\text{r}}}G_{\text{m}}$ (kJ·mol–1)Table 2. Molar free energy
${\varDelta _{\text{r}}}G_{\text{m}}$ of reaction between C and TiAl (kJ·mol–1).摩尔反应自由能 温度/℃ 1150 1250 $ \Delta {G_1} $ –3.72 –3.69 $ \Delta {G_2} $ –5.52 –5.50 $ \Delta {G_3} $ –6.88 –6.68 表 3 Ti-Al-C化合物晶体结构数据
Table 3. Crystal structure data of Ti-Al-C compounds.
序号 晶体 空间群 晶格常数 a TiAl ${ { {{Pm} }\bar 3 m} }$ a = b = 4.0051 Å, c = 4.0707 Å b Ti3Al ${{P63/mmc} }$ a = b = 5.764 Å, c = 4.664 Å c TiC ${ { {{Fm} }\bar 3 m} }$ a = b = c = 4.328 Å d Ti3AlC ${ { {{Pm} }\bar 3 m} }$ a = b = c = 4.156 Å e Ti2AlC ${{P63/mmc} }$ a = b = 3.063 Å, c = 13.668 Å 表 4 TiAl, Ti3Al, TiC, Ti2AlC, Ti3AlC的基本物理参数[24-30]
Table 4. Basic physical parameters of TiAl, Ti3Al, TiC, Ti2AlC and Ti3AlC[24-30].
相 TiAl Ti3Al TiC Ti2AlC Ti3AlC 弹性模量
G/GPa70 56 182 115 83 泊松比 ν 0.23 0.28 0.228 0.164 0.25 线膨胀系数
α/10–612—14.5 12—14.5 7.74 9.62 10.1 表 5 TiAl/TiC, TiAl/Ti2AlC, α/Ti2AlC, TiAl/Ti3AlC和α/Ti3AlC的界面能
Table 5. Interfacial energy of TiAl/TiC, TiAl/Ti2AlC, α/Ti2AlC, TiAl/Ti3AlC and α/Ti3AlC.
温度/
℃$ {\sigma _{{\text{γ /TiC}}}} $/
(J·m2)$ {\sigma _{{\text{γ /H}}}} $/
(J·m2)$ {\sigma _{{\text{α /H}}}} $/
(J·m2)$ {\sigma _{{\text{γ /P}}}} $/
(J·m2)$ {\sigma _{^{{\text{α /P}}}}} $/
(J·m2)1150 1.243 0.879 0.831 0.396 0.308 1200 1.230 0.872 0.827 0.390 0.303 1250 1.223 0.868 0.821 0.385 0.288 1300 1.220 0.866 0.819 0.384 0.288 1350 1.217 0.864 0.819 0.383 0.287 表 6 MLG与TiAl合金反应生成单C原子层的TiC, Ti2AlC, Ti3AlC的总能量变化
Table 6. Total energy change of the interface reaction between MLG and TiAl with the formation of TiC, Ti2AlC, Ti3AlC per C atom layer.
-
[1] Appel F, Clemens H, Fischer F D 2016 Prog. Mater. Sci 81 55
 Google Scholar
Google Scholar
[2] Ouyang P X, Mi G B, Cao J X, Huang X, He L J, Li P J 2018 Mater. Today Comm 16 364
 Google Scholar
Google Scholar
[3] Yamaguchi M, Inui H, Ito K 2000 Acta Mater 48 307
 Google Scholar
Google Scholar
[4] Gao B, Peng H, Liang Y, Lin J, Chen B 2021 Mater. Sci. Eng. A 881 141059
[5] Kan W, Chen B, Peng H, Liang Y, Lin J 2020 Mater. Lett. 259 126856
[6] Lapin J, Pelachova T, Bajana O 2019 J. Alloys Compd 797 754
 Google Scholar
Google Scholar
[7] Fang H, Chen R, Liu Y, Tan Y Guo J 2019 Intermetallics 115 106630
 Google Scholar
Google Scholar
[8] Gouma P I, Mills M J, Kim Y W 1998 Phil. Mag Lett 78 59
 Google Scholar
Google Scholar
[9] Wu H, Fan G H, Cui X P, Geng L, Yuan F, Pang J C, Wei L S, Huang M 2013 Mater. Sci. Eng. A 585 439
 Google Scholar
Google Scholar
[10] Wu M Y, Mi G B, Li P J, Huang X, Cao C X 2020 J. Aeron. Mater 40 45
[11] Nieto A, Bisht A, Lahiri D, Zhang C, Agarwal A 2016 Int. Mater. Rev 62 241
[12] Wu M Y, Mi G B, Li P J, Huang X, Cao C X 2022 Mater. Lett 310 131515
 Google Scholar
Google Scholar
[13] Guo B S, Ni S, Yi J H, Shen R J, Tang Z H, Du Y, Song M 2017 Mater. Sci. Eng. A 698 282
 Google Scholar
Google Scholar
[14] Song Y, Chen Y, Liu W W, Li W L, Wang Y G, Zhao D, Liu X B 2016 Mater. Des 109 256
 Google Scholar
Google Scholar
[15] Chen Y L, Yan M, Sun Y M, Mei B C, Zuo J Q 2009 Ceram. Int 35 1807
 Google Scholar
Google Scholar
[16] Song X J, Cui H Z, Hou N, Wei N, Han Y, Tian J, Song Q 2016 Ceram. Int 42 13586
 Google Scholar
Google Scholar
[17] MSIT, Cornish L, Cacciamani G, Cupid D M, Keyzer J D https://materials.springer.com/msi/docs/sm_msi_r_10_014870_02/ [2021-5-6]
[18] Kim Y W 1992 Acta Metall. Mater 40 1121
 Google Scholar
Google Scholar
[19] 王苹, 梅炳初, 洪小林, 朱教群, 周卫兵, 严明, 2007 武汉理工大学学报 29 5
Wang P, Mei B C, Hong X L, Zhu J Q, Zhou W B, Yan M 2007 J. Wuhan Univ. Technol. 29 5 (in Chinese)
[20] Pietzka M A, Schuster J C 1994 J. Phase Equilib 15 392
 Google Scholar
Google Scholar
[21] Chen Y, Chu M Y, Wang L J, Bao X H, Lin Y, Shen J Y 2011 Phys. Status Solidi A 208 1879
 Google Scholar
Google Scholar
[22] 胡庚祥 蔡珣 戎咏华 2013 材料科学基础 (上海: 上海交通大学出版社) 第130页
Hu G X, Cai X, Rong Y H 2013 Fundamentals of Materials Science (Shanghai: Shanghai Jiao Tong University Press) p130 (in Chinese)
[23] Merwe J, Woltersdorf J, Jesser W A 1986 Mater. Sci. Eng 81 1
 Google Scholar
Google Scholar
[24] 刘文胜, 黄伯云, 周科朝, 唐建成 2000 材料导报 14 19
Liu W S, Huang B Y, Zhou K C, Tang J C 2000 Mater. Rep. 14 19 (in Chinese)
[25] Zhang W J, Reddy B V, Deevi S C 2001 Scr. Mater 45 645
 Google Scholar
Google Scholar
[26] Kumpfert J 2001 Adv. Eng. Mater 3 851
 Google Scholar
Google Scholar
[27] Todorova T Z, Gaier M, Zwanziger J W, Plucknett K P 2019 J. Alloy. Compd 789 712
 Google Scholar
Google Scholar
[28] Arusei G K, Chepkoech M, Amolo G O, Wambua N 2020 arXiv: 2011.07102 v1 [cond-mat. mtrl]
[29] Zhang X W, Wang X H, Li F Z, Zhou Y C 2010 J. Am. Ceram. Soc 92 2698
[30] Schuster J C, Nowotny H, Vaccaro C 1980 J. Solid State Chem 32 213
 Google Scholar
Google Scholar
[31] Болецкая B М1979 Metal Sci. Heat Treatm. Metals 12 37(in Russian)
[32] Vanloo F, Bastin G F 1989 Metall. Meter. Trans. A 20 403
计量
- 文章访问数: 5776
- PDF下载量: 76
- 被引次数: 0













 下载:
下载: