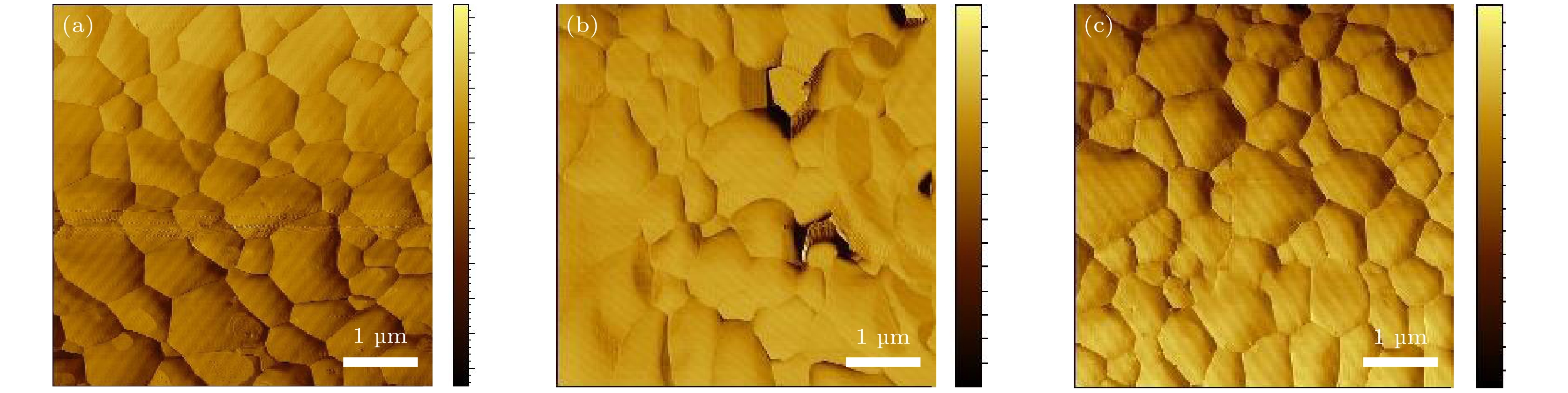
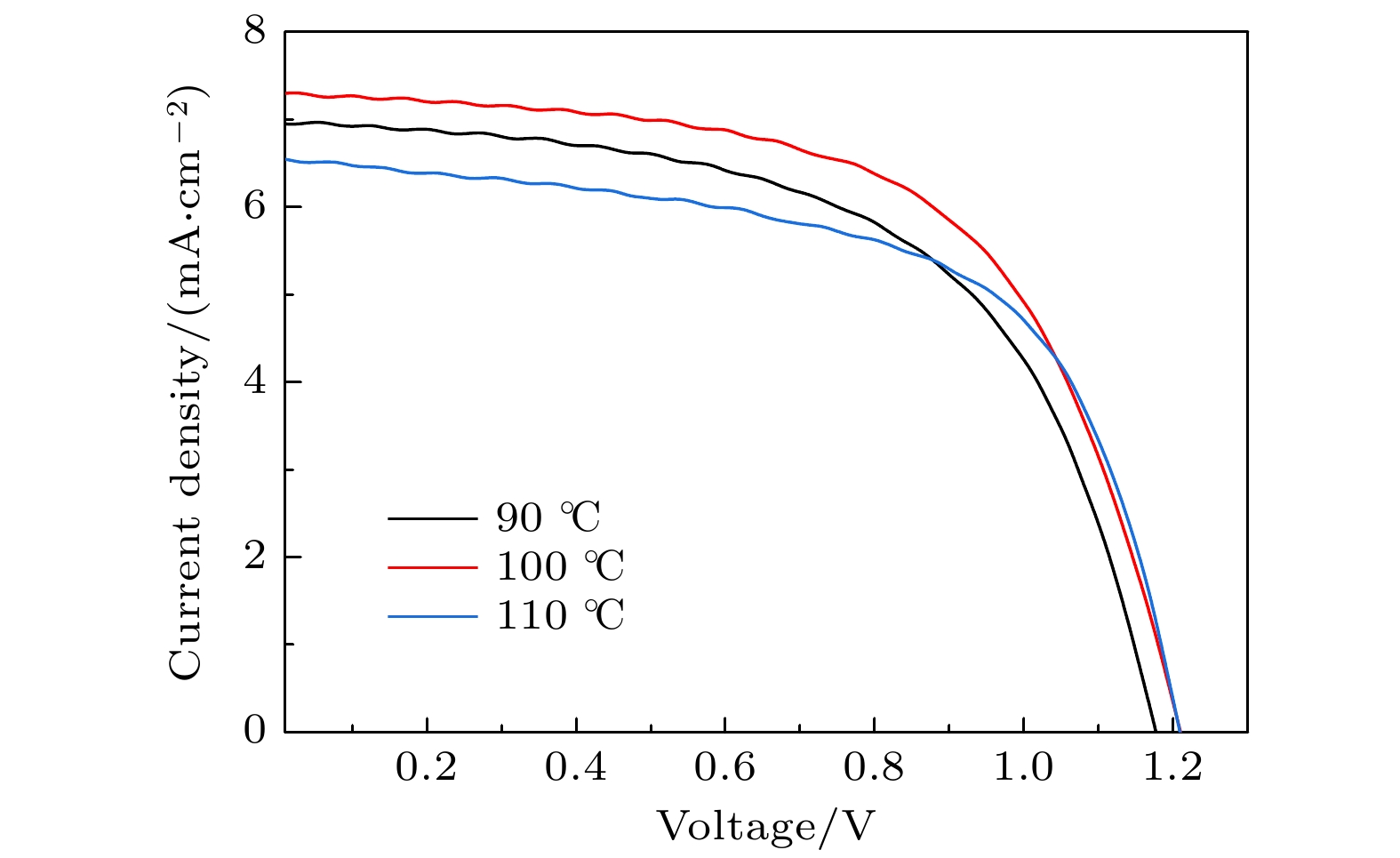
-
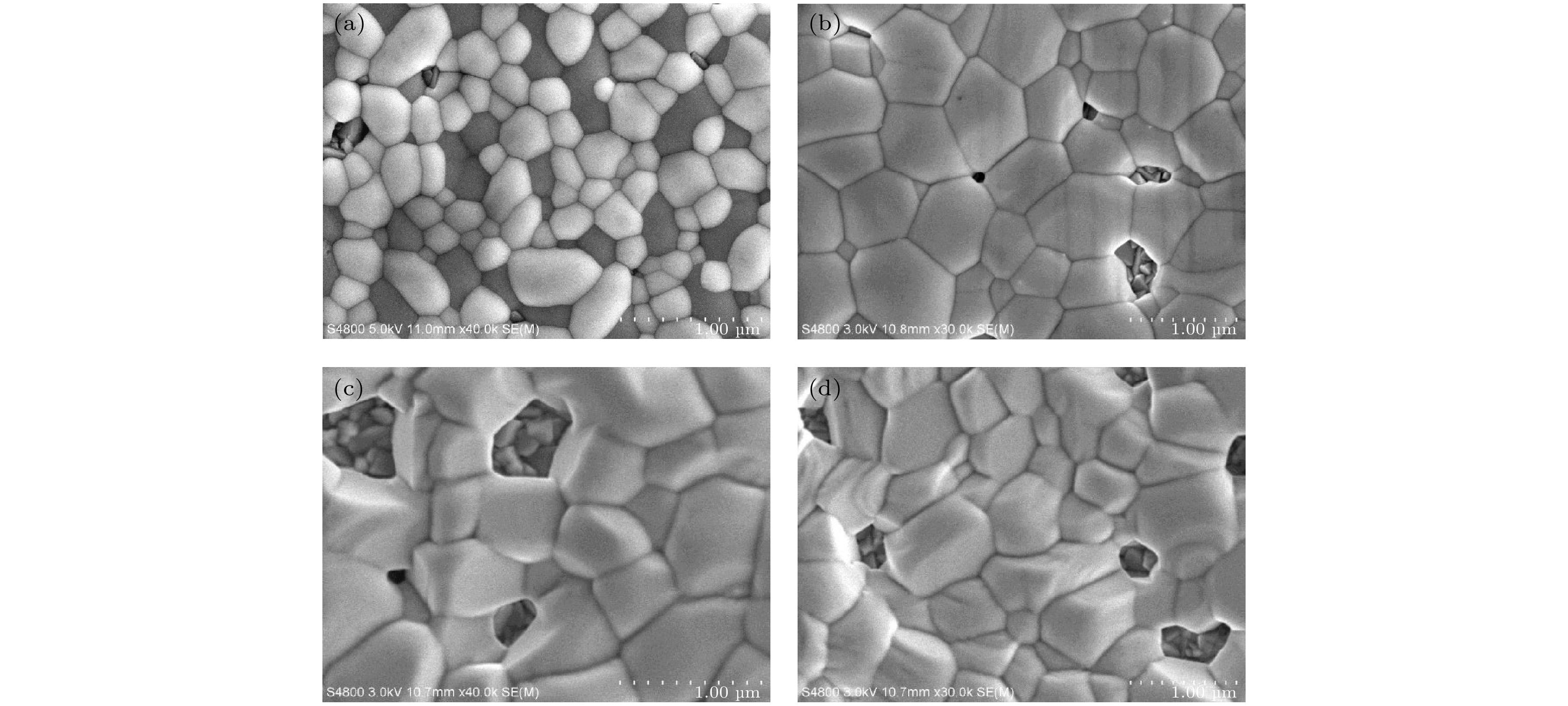
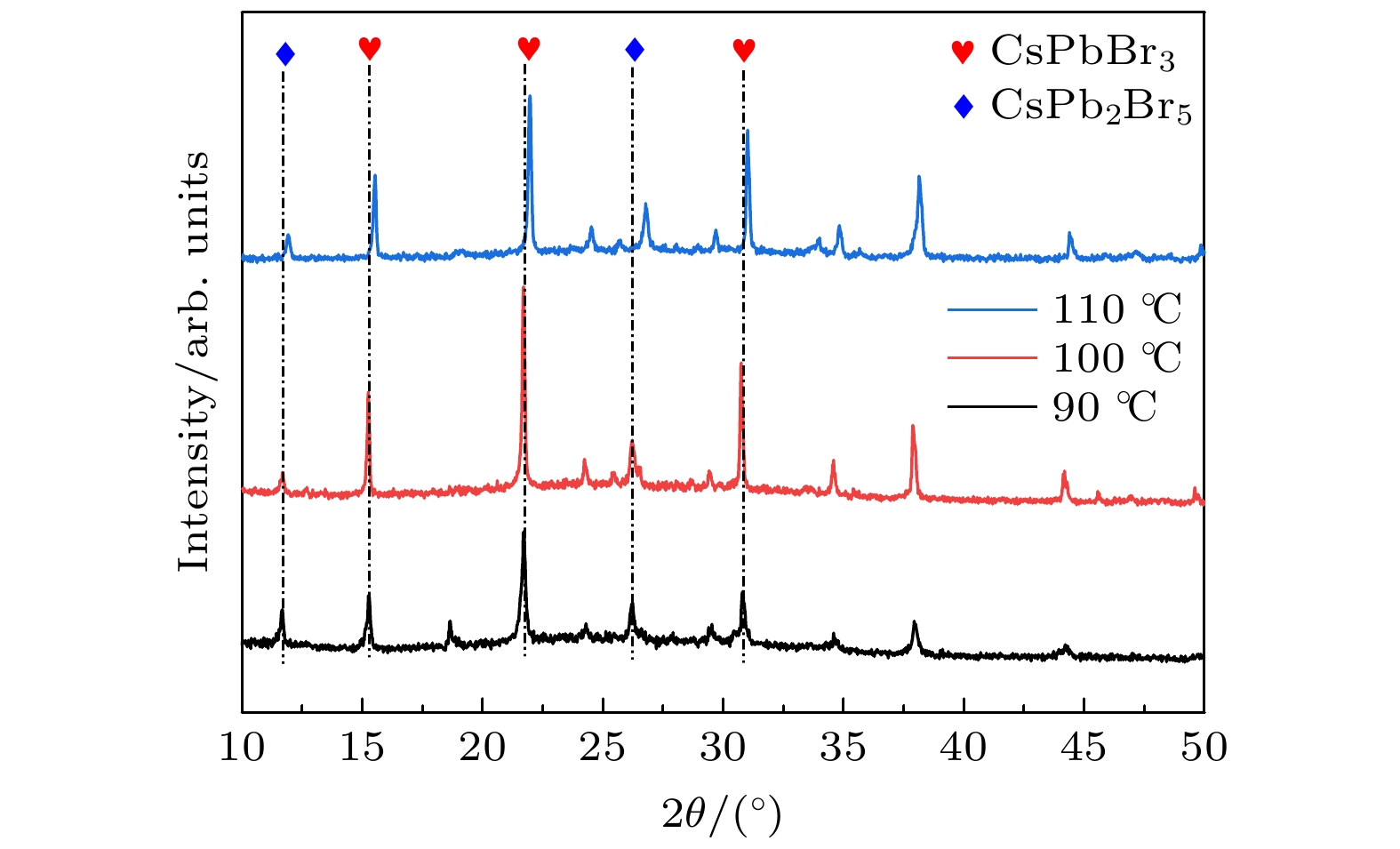
全无机CsPbBr3钙钛矿材料因其本征稳定性好、成本低廉从而在光伏领域展现出巨大的应用潜力, 但目前CsPbBr3太阳能电池的光电转换效率仍远低于其他体系的钙钛矿太阳能电池. 本文以无空穴传输层结构的碳基CsPbBr3全无机钙钛矿电池作为研究对象, 以多步旋涂法为基础, 通过在PbBr2(DMF)溶液中添加2-苯乙胺溴盐(PEABr)来调控CsPbBr3薄膜的结晶质量, 降低薄膜缺陷态密度, 钝化晶粒间界, 并对其中的关键工艺参数包括CsBr的用量(旋涂次数)、旋涂PbBr2薄膜时的衬底预热温度以及退火温度进行了优化. 最终在大气环境下获得了兼具稳定和高效的无空穴传输层结构的碳基CsPbBr3太阳能电池, 器件的光电转换效率达到8.25%, 并在无封装条件下保存1500 h仍可保持90%以上的效率, 对于进一步拓展CsPbBr3钙钛矿电池的优化设计思路具有重要意义.
In recent years, organic-inorganic hybrid perovskite solar cells have become a research hotspot in the photovoltaic field because of their excellent power conversion efficiency. However, this hybrid perovskite material's intrinsic instability and the harsh preparation environment limit its further commercial application. All-inorganic CsPbBr3 perovskite materials have attracted much attention because of their good stability, low cost and can be prepared in an atmospheric environment, showing great application potential. The controllable preparation and growth kinetics of CsPbBr3 materials need to be further studied, and the conversion efficiency of photovoltaic devices is still low. Considering the instability caused by traditional organic hole transport materials and their high preparation cost, this work focuses on the systematical studies of CsPbBr3 all-inorganic perovskite cells without a hole transport layer. Growth kinetics material of CsPbBr3 is controlled by adding 2-phenylethylamine bromide to precursor solution. The main research contents and results are described as follows. Based on multi-step spin-coating preparation of CsPbBr3 perovskite films, the perovskite cell preparation method is studied, and the critical process parameters including the spin-coating PbBr2, amount and number of spin-coating of CsBr, substrate preheating temperature, and the annealing temperature, are optimized. The optimization tests show that the optimal spin-coating of CsBr is obtained by being optimized five times and the spin-coating PbBr2 is conducted in the atmospheric environment. The optimal preheating temperature of the substrate is 80 ℃, and the optimal annealing temperature is 100 ℃. The perovskite films prepared under this condition are compact, each with a continuous high phase purity and good crystallization performance. The PbBr2 in DMF is first adopted and the 2-phenylethylamine bromide (PEABr) solution is added to regulate the CsPbBr3 crystalline quality of the film. The effects of PEABr on the perovskite crystallization process and device performance are systematically investigated. The results show that the introduction of PEABr can effectively optimize the CsPbBr3. The crystalline properties of the two-dimensional perovskite materials can improve the grain boundaries and improve their transport properties. The prepared perovskite solar cell with PEABr shows the highest power conversion efficiency of 8.25%, and it can maintain the efficiency of more than 90% when being stored for 1500 h under the condition of no encapsulation. Finally, stable, efficient and low-cost all-inorganic CsPbBr3 solar cells without a hole layer are obtained. -
Keywords:
- multi-step spin-coating method /
- CsPbBr3 /
- PEABr /
- process optimization /
- perovskite solar cells
[1] Liao C S, Yu Z L, He P B, Liu B, Zeng R, Wan Q, Cai M Q 2021 J. Colloid Interface Sci. 597 233
 Google Scholar
Google Scholar
[2] Li Q H, Ding Y F, He P B, Zeng R, Wan Q, Cai M Q 2021 J. Phys. Chem. Lett. 12 3809
 Google Scholar
Google Scholar
[3] Liao C S, Yu Z L, He P B, Zhao Y Q, Liu B, Cai M Q 2020 J. Power Sources 478 229078
 Google Scholar
Google Scholar
[4] Yu Z L, Zhao Y Q, Wan Q, Liu B, Yang J L, Cai M Q 2020 J. Phys. Chem. C 124 23052
 Google Scholar
Google Scholar
[5] Yu Z L, Zhao Y Q, He P B, Liu B, Yang J L, Cai M Q 2020 J. Phys. Condens. Matter 32 065002
 Google Scholar
Google Scholar
[6] Lee M M, Teuscher J, Miyasaka T, Murakami T N, Snaith H J 2012 Science 338 643
 Google Scholar
Google Scholar
[7] Jeong J, Kim M, Seo J, et al. 2021 Nature 592 381
 Google Scholar
Google Scholar
[8] Shi L, Hao H, Dong J, Zhong T, Zhang C, Hao J, Xing J, Liu H 2019 Nanomaterials (Basel) 9 915
 Google Scholar
Google Scholar
[9] Zhong T, Shi L, Hao H, Dong J, Tang K, Xu X, Hamukwaya S L, Liu H, Xing J 2021 ACS Sustainable Chem. Eng. 9 13010
 Google Scholar
Google Scholar
[10] Park B W, Seok S I 2019 Adv. Mater. 31 e1805337
 Google Scholar
Google Scholar
[11] Zhao Z, Gu F, Rao H, Ye S, Liu Z, Bian Z, Huang C 2019 Adv. Energy Mater. 9 1802671
 Google Scholar
Google Scholar
[12] Wang P, Zhang X, Zhou Y, Jiang Q, Ye Q, Chu Z, Li X, Yang X, Yin Z, You J 2018 Nat. Commun. 9 2225
 Google Scholar
Google Scholar
[13] Ding X, Cai M, Liu X, Ding Y, Liu X, Wu Y, Hayat T, Alsaedi A, Dai S 2019 ACS Appl. Mater. Interfaces 11 37720
 Google Scholar
Google Scholar
[14] Qiu Z, Li N, Huang Z, Chen Q, Zhou H 2020 Small Methods 4 1900877
 Google Scholar
Google Scholar
[15] Duan J, Wang Y, Yang X, Tang Q 2020 Angew. Chem. Int. Ed. 59 4391
 Google Scholar
Google Scholar
[16] Yuan H, Zhao Y, Duan J, Wang Y, Yang X, Tang Q 2018 J. Mater. Chem. A 6 24324
 Google Scholar
Google Scholar
[17] Wang H, Liu H, Li W, Zhu L, Chen H 2020 Nano Energy 77 105160
 Google Scholar
Google Scholar
[18] Guo Z N, Chen S, Wang Z Z, Yang Z Y, Liu F, Xu Y H, Wang J H, Yi Y, Zhang H, Liao L, Chu P K, Yu X F 2017 Adv. Mater. 29 1703811
 Google Scholar
Google Scholar
[19] Ku Z, Rong Y, Xu M, Liu T, Han H 2013 Sci. Rep. 3 3132
 Google Scholar
Google Scholar
[20] Zhao F, Guo Y, Wang X, Tao J, Li Z, Zheng D, Jiang J, Hu Z, Chu J 2020 J. Alloys Compd. 842 155984
 Google Scholar
Google Scholar
[21] Cao X, Zhang G, Cai Y, Jiang L, Chen Y, He X, Zeng Q, Jia Y, Xing G, Wei J 2020 Appl. Surf. Sci. 529 147119
 Google Scholar
Google Scholar
[22] Gao B, Meng J 2020 Solar Energy 211 1223
 Google Scholar
Google Scholar
[23] Xu C, Zhang Z, Hu Y, Sheng Y, Jiang P, Han H, Zhang J 2018 J. Energy Chem. 27 764
 Google Scholar
Google Scholar
[24] Cao X, Zhang G, Jiang L, Cai Y, Wang Y, He X, Zeng Q, Chen J, Jia Y, Wei J 2021 Green Chem. 23 2104
 Google Scholar
Google Scholar
[25] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. Int. Ed. 57 3787
 Google Scholar
Google Scholar
[26] Ding J, Duan J, Guo C, Tang Q 2018 J. Mater. Chem. A 6 21999
 Google Scholar
Google Scholar
[27] Li M H, Yeh H H, Chiang Y H, et al. 2018 Adv. Mater. 30 e1801401
 Google Scholar
Google Scholar
[28] Chen B, Rudd P N, Yang S, Yuan Y, Huang J 2019 Chem. Soc. Rev. 48 3842
 Google Scholar
Google Scholar
-
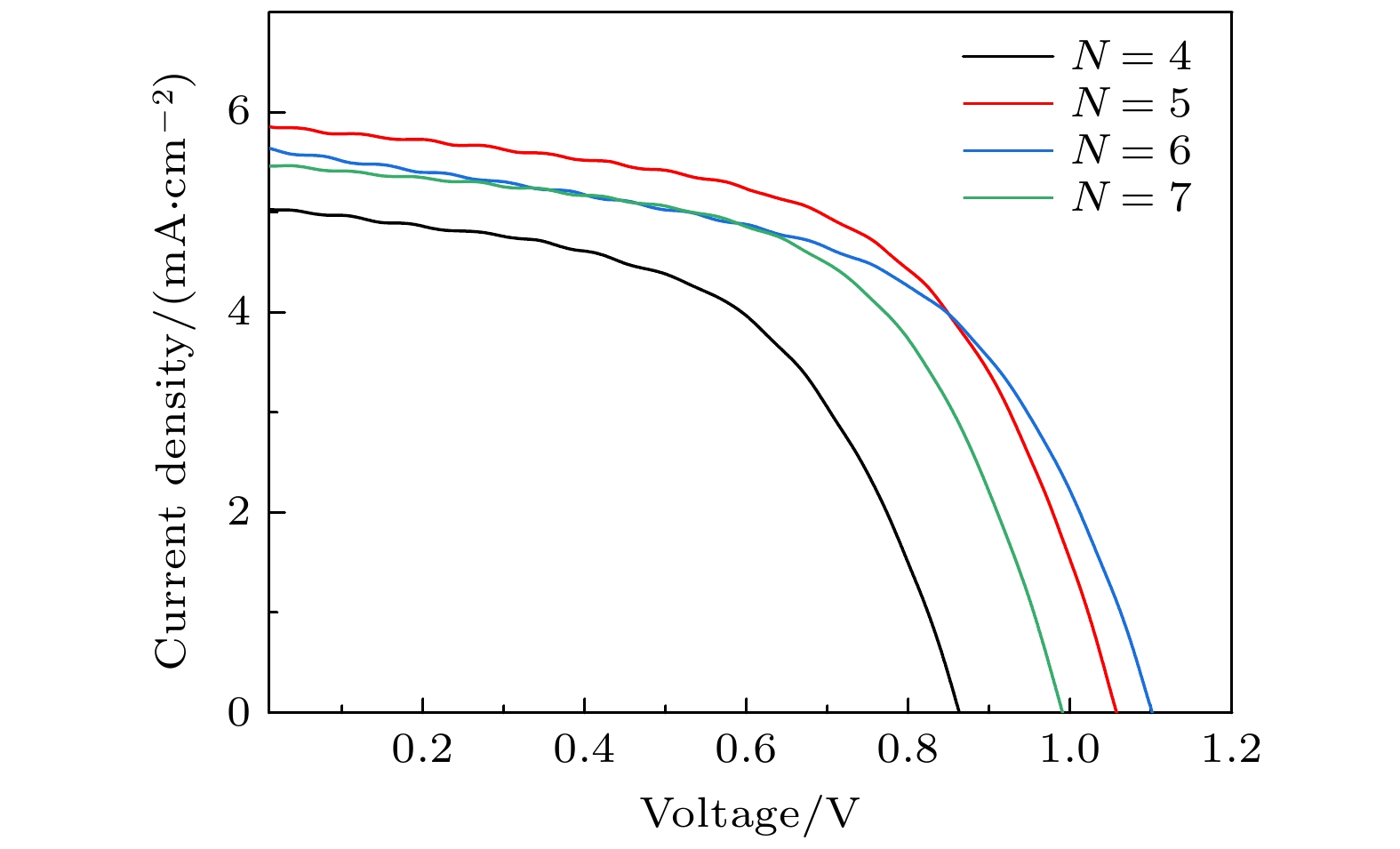
表 1 旋涂不同CsBr次数所制备太阳能电池的具体参数
Table 1. Parameters of solar cells prepared by spin-coating different times of CsBr.
旋涂次数 VOC
/VJSC
/(mA·cm–2)PCE
/%FF
/%4 0.85 5.0 2.37 55 5 1.06 5.8 3.58 58 6 1.10 5.6 3.41 55 7 0.99 5.4 3.16 59 表 2 在衬底不同预热温度下制备的钙钛矿电池的具体参数
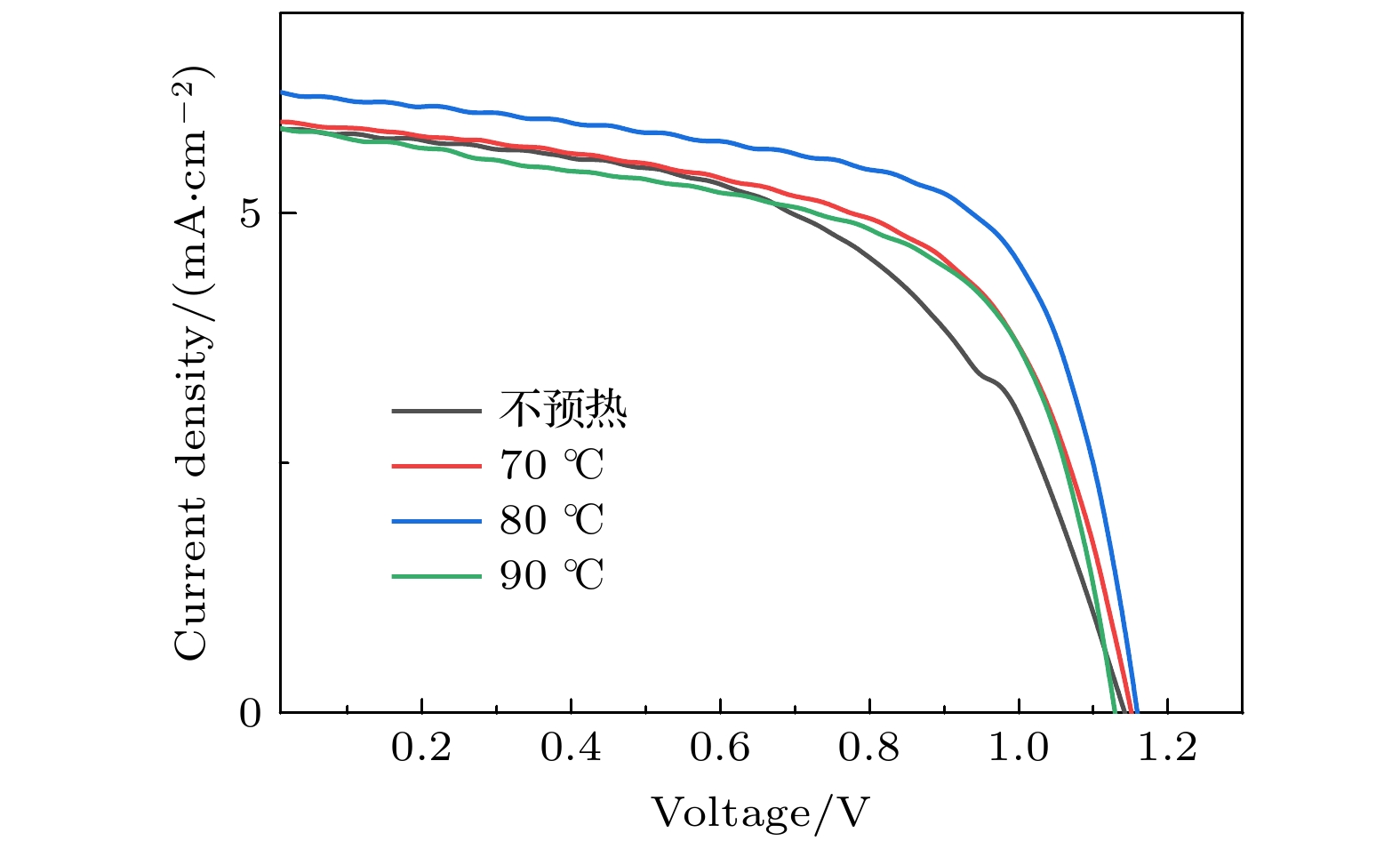
Table 2. Parameters of solar cells prepared at different preheating temperatures of the substrate.
衬底温度 VOC
/VJSC
/(mA·cm–2)PCE
/%FF
/%不预热 1.13 5.6 3.6 56 70 ℃ 1.15 5.9 4.1 60 80 ℃ 1.15 6.2 4.7 65 90 ℃ 1.13 5.8 4.0 61 表 3 PbBr2层不同退火温度所制备的太阳能电池的具体参数
Table 3. Parameters of solar cells prepared by PbBr2 layer at different annealing temperatures.
退火温度/℃ VOC
/VJSC
/(mA·cm–2)PCE
/%FF
/%90 1.17 6.9 4.74 58 100 1.20 7.3 5.25 60 110 1.19 6.5 4.82 62 表 4 PEABr不同添加量所制备的CsPbBr3电池的具体参数
Table 4. Parameters of solar cells prepared with different amounts of PEABr.
引入量
/(mg·mL–1)VOC
/VJSC
/(mA·cm–2)PCE
/%FF
/%w/o 1.20 7.30 5.25 60 5 1.28 8.40 7.16 66 10 1.31 8.51 8.25 73 15 1.30 8.16 7.37 69 -
[1] Liao C S, Yu Z L, He P B, Liu B, Zeng R, Wan Q, Cai M Q 2021 J. Colloid Interface Sci. 597 233
 Google Scholar
Google Scholar
[2] Li Q H, Ding Y F, He P B, Zeng R, Wan Q, Cai M Q 2021 J. Phys. Chem. Lett. 12 3809
 Google Scholar
Google Scholar
[3] Liao C S, Yu Z L, He P B, Zhao Y Q, Liu B, Cai M Q 2020 J. Power Sources 478 229078
 Google Scholar
Google Scholar
[4] Yu Z L, Zhao Y Q, Wan Q, Liu B, Yang J L, Cai M Q 2020 J. Phys. Chem. C 124 23052
 Google Scholar
Google Scholar
[5] Yu Z L, Zhao Y Q, He P B, Liu B, Yang J L, Cai M Q 2020 J. Phys. Condens. Matter 32 065002
 Google Scholar
Google Scholar
[6] Lee M M, Teuscher J, Miyasaka T, Murakami T N, Snaith H J 2012 Science 338 643
 Google Scholar
Google Scholar
[7] Jeong J, Kim M, Seo J, et al. 2021 Nature 592 381
 Google Scholar
Google Scholar
[8] Shi L, Hao H, Dong J, Zhong T, Zhang C, Hao J, Xing J, Liu H 2019 Nanomaterials (Basel) 9 915
 Google Scholar
Google Scholar
[9] Zhong T, Shi L, Hao H, Dong J, Tang K, Xu X, Hamukwaya S L, Liu H, Xing J 2021 ACS Sustainable Chem. Eng. 9 13010
 Google Scholar
Google Scholar
[10] Park B W, Seok S I 2019 Adv. Mater. 31 e1805337
 Google Scholar
Google Scholar
[11] Zhao Z, Gu F, Rao H, Ye S, Liu Z, Bian Z, Huang C 2019 Adv. Energy Mater. 9 1802671
 Google Scholar
Google Scholar
[12] Wang P, Zhang X, Zhou Y, Jiang Q, Ye Q, Chu Z, Li X, Yang X, Yin Z, You J 2018 Nat. Commun. 9 2225
 Google Scholar
Google Scholar
[13] Ding X, Cai M, Liu X, Ding Y, Liu X, Wu Y, Hayat T, Alsaedi A, Dai S 2019 ACS Appl. Mater. Interfaces 11 37720
 Google Scholar
Google Scholar
[14] Qiu Z, Li N, Huang Z, Chen Q, Zhou H 2020 Small Methods 4 1900877
 Google Scholar
Google Scholar
[15] Duan J, Wang Y, Yang X, Tang Q 2020 Angew. Chem. Int. Ed. 59 4391
 Google Scholar
Google Scholar
[16] Yuan H, Zhao Y, Duan J, Wang Y, Yang X, Tang Q 2018 J. Mater. Chem. A 6 24324
 Google Scholar
Google Scholar
[17] Wang H, Liu H, Li W, Zhu L, Chen H 2020 Nano Energy 77 105160
 Google Scholar
Google Scholar
[18] Guo Z N, Chen S, Wang Z Z, Yang Z Y, Liu F, Xu Y H, Wang J H, Yi Y, Zhang H, Liao L, Chu P K, Yu X F 2017 Adv. Mater. 29 1703811
 Google Scholar
Google Scholar
[19] Ku Z, Rong Y, Xu M, Liu T, Han H 2013 Sci. Rep. 3 3132
 Google Scholar
Google Scholar
[20] Zhao F, Guo Y, Wang X, Tao J, Li Z, Zheng D, Jiang J, Hu Z, Chu J 2020 J. Alloys Compd. 842 155984
 Google Scholar
Google Scholar
[21] Cao X, Zhang G, Cai Y, Jiang L, Chen Y, He X, Zeng Q, Jia Y, Xing G, Wei J 2020 Appl. Surf. Sci. 529 147119
 Google Scholar
Google Scholar
[22] Gao B, Meng J 2020 Solar Energy 211 1223
 Google Scholar
Google Scholar
[23] Xu C, Zhang Z, Hu Y, Sheng Y, Jiang P, Han H, Zhang J 2018 J. Energy Chem. 27 764
 Google Scholar
Google Scholar
[24] Cao X, Zhang G, Jiang L, Cai Y, Wang Y, He X, Zeng Q, Chen J, Jia Y, Wei J 2021 Green Chem. 23 2104
 Google Scholar
Google Scholar
[25] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. Int. Ed. 57 3787
 Google Scholar
Google Scholar
[26] Ding J, Duan J, Guo C, Tang Q 2018 J. Mater. Chem. A 6 21999
 Google Scholar
Google Scholar
[27] Li M H, Yeh H H, Chiang Y H, et al. 2018 Adv. Mater. 30 e1801401
 Google Scholar
Google Scholar
[28] Chen B, Rudd P N, Yang S, Yuan Y, Huang J 2019 Chem. Soc. Rev. 48 3842
 Google Scholar
Google Scholar
计量
- 文章访问数: 10299
- PDF下载量: 235
- 被引次数: 0














 下载:
下载: