-
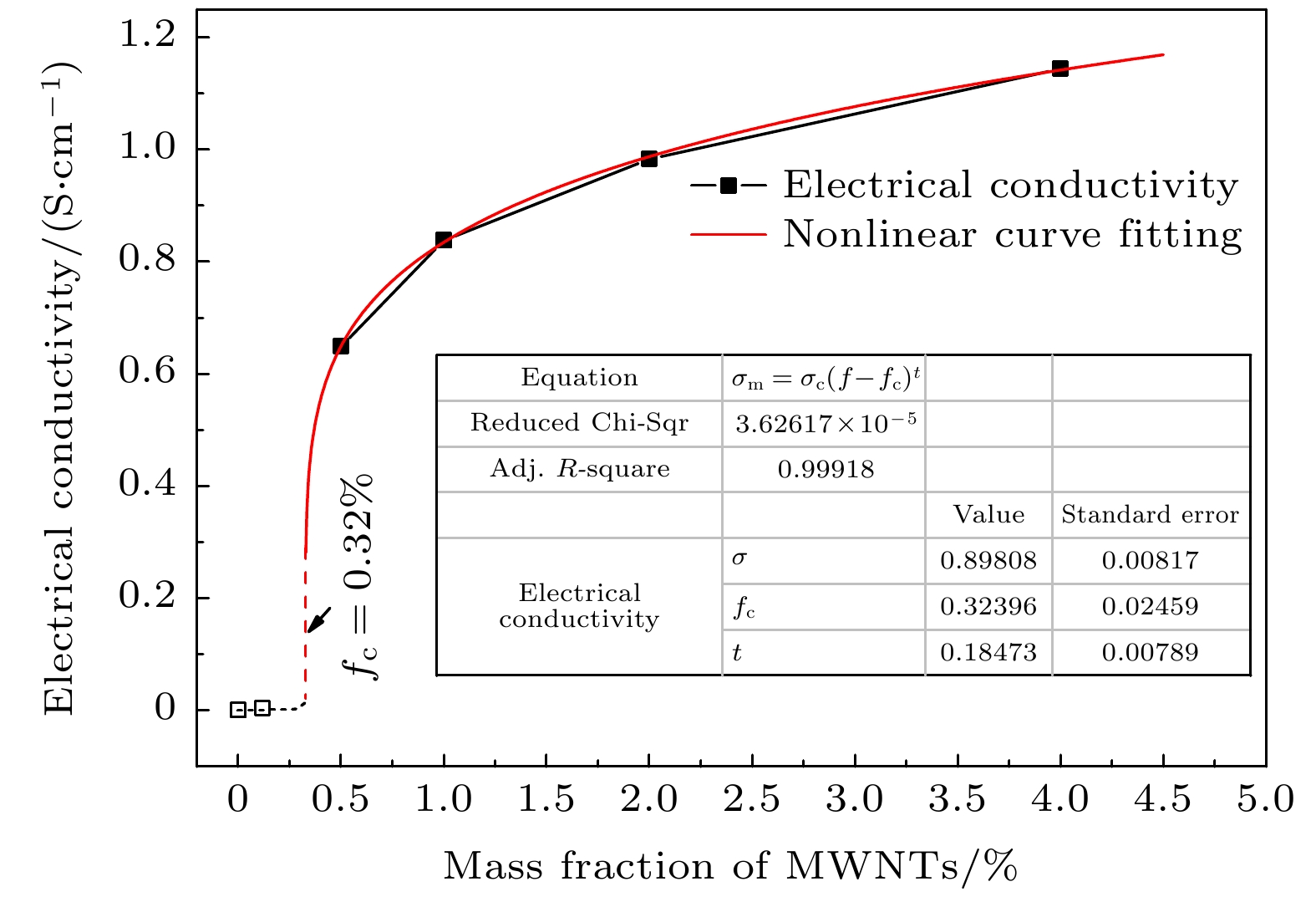
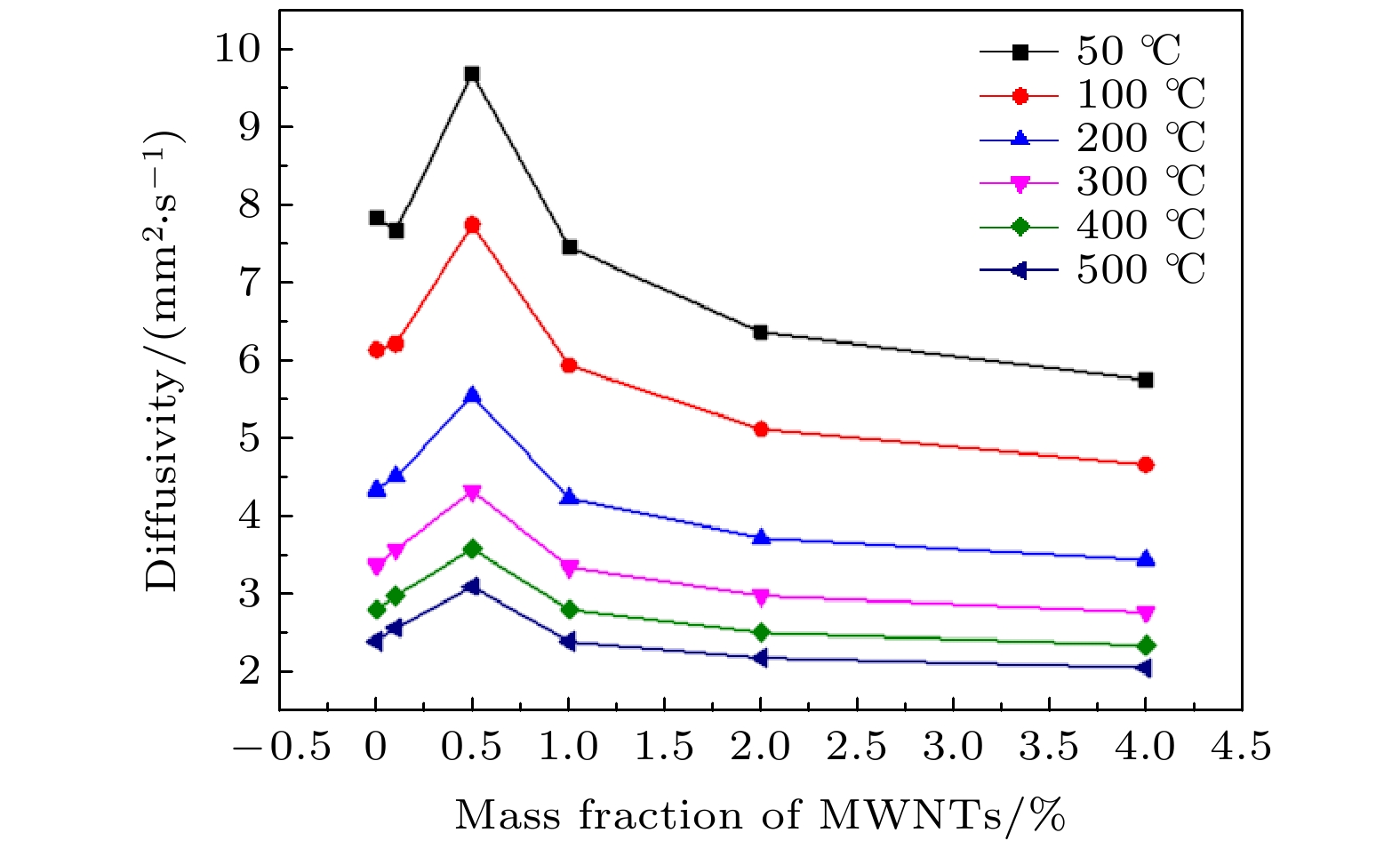
采用溶胶-喷雾制备了多壁碳纳米管增强氧化铝基球形复合粉体, 采用放电等离子真空快速烧结成型. SEM分析测试结果表明, 多壁碳纳米管在氧化铝基体中呈网络分布, 且主要位于晶界处, 少量呈穿晶分布. 复合材料性能分析测试结果表明, 当多壁碳纳米管的质量分数为0.5%时, 复合材料的维氏硬度相对纯的氧化铝提高了32.6%; 热扩散系数在不同测试温度下相对纯氧化铝的平均提高幅度为27.2%. 此外, 当多壁碳纳米管质量分数达到0.5%时复合材料呈导体, 根据渗流导电理论拟合得到实验制备复合材料的渗流阈值为0.32 wt.%, 说明多壁碳纳米管在氧化铝基体中分散良好.The spherical composite powders of multi-walled carbon nanotubes reinforced alumina are prepared by sol- spray. The results show that the multi-walled carbon nanotubes are well dispersed in the composites. The analyses of the composite properties show that most of the multi-walled carbon nanotubes are distributed in a network at the grain boundaries, and a small number of them are distributed in the grains. When the mass fraction of multi-walled carbon nanotubes accounts for 0.5%, the Vickers hardness of the composite increases by 32.6% relative to pure alumina; the thermal diffusion coefficient increased averagely by 27.2% with respect to pure alumina at different temperatures. The composites are conductive at 0.5% of multi-walled carbon nanotubes, and the percolation threshold of the composites prepared by this method is 0.32wt.% based on the fitting of the percolation conductivity theory, indicating that the multi-walled carbon nanotubes are well dispersed in the alumina matrix.
-
Keywords:
- carbon nanotubes /
- sol-spray /
- alumina /
- composites
[1] Iijima, Sumio 1991 Nature 354 56
 Google Scholar
Google Scholar
[2] Ke C, Jia C C, Li W S 2013 Appl. Phys. A 110 269
 Google Scholar
Google Scholar
[3] Chan K F, Zaid M, Mamat M S, Liza S, Yaakob Y 2021 Crystals 11 457
 Google Scholar
Google Scholar
[4] Park S S, Moorthy M S, Ha C S 2014 Korean J. Chem. Eng. 31 1707
 Google Scholar
Google Scholar
[5] Hassan R U, Shahzad F, Abbas N, Hussain, S 2019 J. Mater. Sci-Mater EI. 30 6304
[6] 康艳茹, 何禧佳, 殷正娥, 李亚利 2018 复合材料学报 35 150
Kang Y R, He X J, Yin Z E, Li Y L 2018 Acta Mater. Compos. Sin. 35 150
[7] Ngo I L, Jeon S, Chan B 2016 Int J Heat Mass Tran. 98 219
 Google Scholar
Google Scholar
[8] Li C, Liang T, Lu W, Tang C, Hu X, Cao M 2004 Compos. Sci. Technol. 64 2089
 Google Scholar
Google Scholar
[9] Lee T H, Cho S H, Lee T G 2018 J. Am. Ceram. Soc. 101 3156
 Google Scholar
Google Scholar
[10] Chung, D D L. 2001 Carbon 39 279
 Google Scholar
Google Scholar
[11] Singh M A, Sarma D K, Hanzel O, Sedláček J, Šajgalík P 2017 J. Eur. Ceram. Soc. 37 3107
 Google Scholar
Google Scholar
[12] Nan C W, Shi Z, Lin Y 2003 Chem. Phys. Lett. 375 666
 Google Scholar
Google Scholar
[13] Lanfant B, Leconte Y, Debski N, Bonnefont G, Bernard F 2018 Ceram. Int. 45 2566
[14] Kaiser A B, G Düsberg, Roth S 1998 Phys. Rev. B 57 1418
 Google Scholar
Google Scholar
[15] Momohjimoh I, Saheb N, Hussein M A, Laoui T, Aqeeli N. 2020 Ceram. Int. 46 16008
 Google Scholar
Google Scholar
[16] Lanfant B, Leconte Y, Debski N, Pinault M, Mayne M, Herlin N 2014 Tech. Connect. World Washington, June 15−18 2014 p131.
[17] Liu C, Ding J 2020 Procedia Manufacturing 48 763
 Google Scholar
Google Scholar
[18] Zhan G D, Mukherjee A K 2010 Int. J. Appl. Ceram. Tec. 1 161
[19] Saheb N, Hayat U 2017 Ceram. Int. 43 5715
 Google Scholar
Google Scholar
[20] Lee K, Chan B M, Park S B, Hong S H 2011 J. Am. Ceram. Soc. 94 3774
 Google Scholar
Google Scholar
[21] Kumari L, Zhang T, Du G H, Li W Z, Wang Q W, Datye A Wu K H 2009 Ceram. Int. 35 1775
 Google Scholar
Google Scholar
[22] Estili M, Sakka Y 2014 Sci. Technol. Adv. Mat. 15 064902
 Google Scholar
Google Scholar
[23] Zhang S C, Fahrenholtz W G, Hilmas G E, Yadlowsky E J 2010 J. Eur. Ceram. Soc. 30 1373
 Google Scholar
Google Scholar
[24] Barinov S M, Fateeva L V, Yurashev S V, Ballóková E, Rudnayová E 2002 Powder Metall. Prog. 22 61
[25] Lin J, Fan G, Li Z, Kai X, Di Z, Chen Z, Humphries S, Heness G, Yeung W Y Z 2011 Carbon 49 1965
 Google Scholar
Google Scholar
[26] Damavandi B Y, Xia Y, Ahmad I, Zhu Y 2017 Veruscript Functional Nanomaterials 41 1
[27] Groffman P M, Baron J S, Blett T, Gold A, Goodman I, Gunderson L H, Levinson B M, Palmer M A, Paerl H W, Peterson G D 2006 Ecosystems 9 1
 Google Scholar
Google Scholar
[28] S M 1994 Applications of Percolation Theory(Los Angeles: CRC Press) pp53−57.
[29] Bergman D J 1980 Phys. Rev. Lett. 44 1285
 Google Scholar
Google Scholar
[30] Liu Y, Lin, Y, Shi Z, Nan, C W, Shen Z J 2005 J. Am. Ceram. Soc. 88 1337
 Google Scholar
Google Scholar
[31] Meir Y 2012 Physica A 302 391
-
图 1 溶胶-喷雾制备的球形粉末SEM图 (a) 300 ℃喷雾后的水合氢氧化铝; (b) 900 ℃热处理后的Al2O3; (c) (d)300 ℃喷雾干燥后的1% MWNT/Al2O3粉末; (e) (f) 为(c)氩气中900 ℃热处理后的1% MWNT/Al2O3粉末
Fig. 1. SEM of spherical powder prepared by sol-spray: (a) Hydrated aluminum hydroxide after spray drying at 300 ℃; (b) Al2O3 after heat treatment at 900 ℃; (c) and (d) 1% MWNT/Al2O3 composites powder after spray drying at 300 ℃; (e) and (f) 1% MWNT/Al2O3 composites powder after heat treatment at 900 ℃ in argon.
表 1 MWNT/Al2O3复合材料性能测试结果
Table 1. Properties of MWNT/Al2O3 composite.
编号 样品名称及烧结参数 相对密度/% 维氏硬度/HV 电导率/(S·cm–1) 1# 0.0%WMNTs(3 min × 1450 ℃ × 40 MPa) 100 1436.7 10–13 2# 0.1%WMNTs(3 min × 1450 ℃ × 40 MPa) 99.1 1523.7 — 3# 0.5%WMNTs(3 min × 1450 ℃ × 40 MPa) 98.7 1901.4 0.649 4# 1.0%WMNTs(3 min × 1450 ℃ × 40 MPa) 98.5 1733.6 0.838 5# 2.0%WMNTs(3 min × 1450 ℃ × 40 MPa) 98.0 1698.6 0.983 6# 4.0%WMNTs(3 min × 1450 ℃ × 40 MPa) 97.3 1233.2 1.144 改变烧结参数对性能影响 7# 1.0%WMNTs(6 min × 1450 ℃ × 40 MPa) 98.5 1703.1 0.789 8# 1.0%WMNTs(9 min × 1450 ℃ × 40 MPa) 98.7 1687.5 0.917 9# 1.0%WMNTs(3 min × 1450 ℃ × 50 MPa) 98.8 1747.8 0.923 10# 1.0%WMNTs(3 min × 1500 ℃ × 40 MPa) 98.6 1673.1 0.768 表 2 5% MWNT/Al2O3复合材料和纯Al2O3热扩散系数
Table 2. Thermal diffusivity of 0.5% MWNT/Al2O3 composite and pure Al2O3.
测试
温度/℃纯Al2O3热
扩散系数
/(mm2·s–1)0.5% MWNT
/Al2O3热扩散
系数/(mm2·s–1)热扩散系数
提高幅度/%50 7.85 9.71 23.7 100 6.15 7.77 26.2 200 4.35 5.56 27.8 300 3.38 4.34 28.4 400 2.81 3.59 28.0 500 2.40 3.10 29.3 平均值 27.2 -
[1] Iijima, Sumio 1991 Nature 354 56
 Google Scholar
Google Scholar
[2] Ke C, Jia C C, Li W S 2013 Appl. Phys. A 110 269
 Google Scholar
Google Scholar
[3] Chan K F, Zaid M, Mamat M S, Liza S, Yaakob Y 2021 Crystals 11 457
 Google Scholar
Google Scholar
[4] Park S S, Moorthy M S, Ha C S 2014 Korean J. Chem. Eng. 31 1707
 Google Scholar
Google Scholar
[5] Hassan R U, Shahzad F, Abbas N, Hussain, S 2019 J. Mater. Sci-Mater EI. 30 6304
[6] 康艳茹, 何禧佳, 殷正娥, 李亚利 2018 复合材料学报 35 150
Kang Y R, He X J, Yin Z E, Li Y L 2018 Acta Mater. Compos. Sin. 35 150
[7] Ngo I L, Jeon S, Chan B 2016 Int J Heat Mass Tran. 98 219
 Google Scholar
Google Scholar
[8] Li C, Liang T, Lu W, Tang C, Hu X, Cao M 2004 Compos. Sci. Technol. 64 2089
 Google Scholar
Google Scholar
[9] Lee T H, Cho S H, Lee T G 2018 J. Am. Ceram. Soc. 101 3156
 Google Scholar
Google Scholar
[10] Chung, D D L. 2001 Carbon 39 279
 Google Scholar
Google Scholar
[11] Singh M A, Sarma D K, Hanzel O, Sedláček J, Šajgalík P 2017 J. Eur. Ceram. Soc. 37 3107
 Google Scholar
Google Scholar
[12] Nan C W, Shi Z, Lin Y 2003 Chem. Phys. Lett. 375 666
 Google Scholar
Google Scholar
[13] Lanfant B, Leconte Y, Debski N, Bonnefont G, Bernard F 2018 Ceram. Int. 45 2566
[14] Kaiser A B, G Düsberg, Roth S 1998 Phys. Rev. B 57 1418
 Google Scholar
Google Scholar
[15] Momohjimoh I, Saheb N, Hussein M A, Laoui T, Aqeeli N. 2020 Ceram. Int. 46 16008
 Google Scholar
Google Scholar
[16] Lanfant B, Leconte Y, Debski N, Pinault M, Mayne M, Herlin N 2014 Tech. Connect. World Washington, June 15−18 2014 p131.
[17] Liu C, Ding J 2020 Procedia Manufacturing 48 763
 Google Scholar
Google Scholar
[18] Zhan G D, Mukherjee A K 2010 Int. J. Appl. Ceram. Tec. 1 161
[19] Saheb N, Hayat U 2017 Ceram. Int. 43 5715
 Google Scholar
Google Scholar
[20] Lee K, Chan B M, Park S B, Hong S H 2011 J. Am. Ceram. Soc. 94 3774
 Google Scholar
Google Scholar
[21] Kumari L, Zhang T, Du G H, Li W Z, Wang Q W, Datye A Wu K H 2009 Ceram. Int. 35 1775
 Google Scholar
Google Scholar
[22] Estili M, Sakka Y 2014 Sci. Technol. Adv. Mat. 15 064902
 Google Scholar
Google Scholar
[23] Zhang S C, Fahrenholtz W G, Hilmas G E, Yadlowsky E J 2010 J. Eur. Ceram. Soc. 30 1373
 Google Scholar
Google Scholar
[24] Barinov S M, Fateeva L V, Yurashev S V, Ballóková E, Rudnayová E 2002 Powder Metall. Prog. 22 61
[25] Lin J, Fan G, Li Z, Kai X, Di Z, Chen Z, Humphries S, Heness G, Yeung W Y Z 2011 Carbon 49 1965
 Google Scholar
Google Scholar
[26] Damavandi B Y, Xia Y, Ahmad I, Zhu Y 2017 Veruscript Functional Nanomaterials 41 1
[27] Groffman P M, Baron J S, Blett T, Gold A, Goodman I, Gunderson L H, Levinson B M, Palmer M A, Paerl H W, Peterson G D 2006 Ecosystems 9 1
 Google Scholar
Google Scholar
[28] S M 1994 Applications of Percolation Theory(Los Angeles: CRC Press) pp53−57.
[29] Bergman D J 1980 Phys. Rev. Lett. 44 1285
 Google Scholar
Google Scholar
[30] Liu Y, Lin, Y, Shi Z, Nan, C W, Shen Z J 2005 J. Am. Ceram. Soc. 88 1337
 Google Scholar
Google Scholar
[31] Meir Y 2012 Physica A 302 391
计量
- 文章访问数: 8912
- PDF下载量: 88
- 被引次数: 0














 下载:
下载: