-
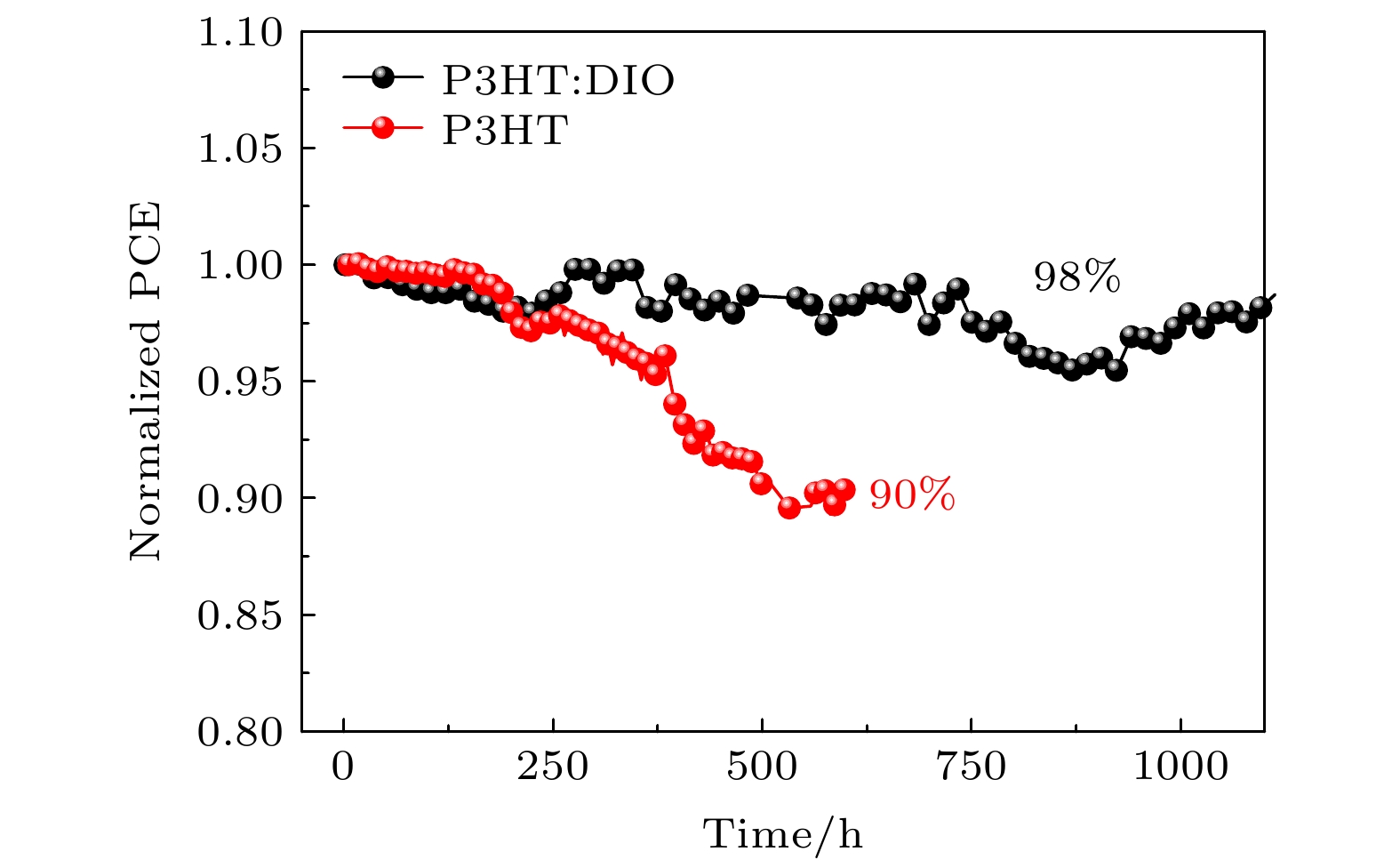
无空穴传输层的碳基钙钛矿太阳能(PSCs)电池拥有成本低、制备步骤简单、稳定性高的优点, 应用前景广阔. 但是碳电极与活性层的直接接触, 导致器件的光电转换效率普遍低于其他金属电极的钙钛矿太阳能电池. 本文使用聚(3-己基噻吩)(P3HT)作为器件的空穴传输层, 相比传统的有机空穴传输层材料Spiro-OMeTAD, 具有低成本和易于制造的优点, 并通过在P3HT中掺杂1,8-二碘辛烷(DIO)的方法优化其光电性能, 提升了载流子的迁移率, 阻挡电子的运输, 降低界面复合, 改善了碳电极与器件的界面接触, 提高了短路电流Jsc和填充因子FF, 使器件的初始光电转换效率从14.06%提升至15.11%. 器件在氮气环境下测试稳定性, 连续光照1000 h后器件的转换效率仍保持在98%以上.HTL-free carbon-based perovskite solar (PSCs) batteries have the advantages of low cost, simple preparation steps, and high stability, and have broad application prospects. However, the direct contact between the carbon electrode and the active layer causes the photoelectric conversion efficiency of the device to be generally lower than that of other metal electrode perovskite solar cells. Therefore, it is necessary to add a hole-transport layer between the perovskite layer and the electrode to improve the charge transport efficiency and optimize the performance. Poly(3-hexylthiophene) has excellent photoelectric properties and is regarded as one of the suitable hole transport materials for perovskite solar cells. In this paper, P3HT is used as the hole transport layer of the device. Compared with the traditional organic hole-transport layer Spiro-OMeTAD, the P3HT has the advantages of low cost and easy manufacture. However, in the current devices with using P3HT as the hole transport layer, due to the characteristics of the surface morphology and molecular ordering of the P3HT film, the carrier mobility in the film itself is low, resulting in unsatisfactory device performance. Studies have shown that the surface morphology and molecular arrangement of the P3HT film can be changed by doping, and the migration rate of charge-carriers inside the film can be accelerated, thereby improving the photovoltaic performance of the solar cell. In this paper, a printing process is used to print carbon paste on the hole transport layer as the electrode of the device, and spin coating is used to prepare the transport layer. And through the method of doping 1,8-diiodooctane (DIO) in P3HT to optimize the device performance, the photoelectric conversion efficiency of the carbon-based perovskite solar cell is improved, the mobility of holes is improved, and the transportation of electrons is blocked. The reduced interface recombination, the improved interface contact between the carbon electrode and the device, the increased short-circuit current Jsc and the fill factor FF lead the photoelectric conversion efficiency of the device to increase from 14.06% to 15.11%. We test the light stability of the device under the 1000-h continuous illumination in a nitrogen atmosphere, and the conversion efficiency of the device remains above 98%, indicating that the addition of DIO into P3HT improves not only the photoelectric conversion efficiency of the device, but also the stability.
-
Keywords:
- perovskite /
- solar cell /
- 3-hexylthiophene /
- 1, 8-diiodooctane
[1] Chapin D M, Fuller C S, Pearson G L 1954 J. Appl. Phys. 25 676
 Google Scholar
Google Scholar
[2] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[3] Kim H S, Lee C R, Im J H, Lee K B, Moehl T, Marchioro A, Moon S J, Humphry-Baker R, Yum J H, Moser J E, Graetzel M, Park N G 2012 Sci. Rep. 2 591
 Google Scholar
Google Scholar
[4] Im J H, Lee C R, Lee J W, Park S W, Park N G 2011 Nanoscale 3 4088
 Google Scholar
Google Scholar
[5] Liu M, Johnston M B, Snaith H J 2013 Nature 501 395
 Google Scholar
Google Scholar
[6] Abate A, Leijtens T, Pathak S, Teuscher J, Avolio R, Errico M E, Kirkpatrik J, Ball J M, Docampo P, McPherson I, Snaith H J 2013 PCCP 15 2572
 Google Scholar
Google Scholar
[7] Furube A, Katoh R, Hara K, Sato T, Murata S, Arakawa H, Tachiya M 2005 J. Phys. Chem. B 109 16406
 Google Scholar
Google Scholar
[8] Abbas H A, Kottokkaran R, Ganapathy B, Samiee M, Zhang L, Kitahara A, Noack M, Dalal V L 2015 APL Mater. 3 506
[9] Wang M J, Li S B, Zhang P, Wang Y F, Li H Q, Chen Z 2015 Chem. Phys. Lett. 639 283
 Google Scholar
Google Scholar
[10] Yan W, Li Y, Ye S, Li Y, Rao H, Liu Z, Wang S, Bian Z, Huang C 2016 Nano Res. 9 1600
 Google Scholar
Google Scholar
[11] Hauch A, Georg A 2002 Electrochim. Acta 46 3457
[12] Ikegami M, Miyoshi K, Miyasaka T, Teshima K, Wei T C, Wan C C, Wang Y Y 2007 Appl. Phys. Lett. 90 153122
 Google Scholar
Google Scholar
[13] Che M, Zhu L, Zhao Y L, Yao D S, Gu X Q, Song J, Qiang Y H 2016 Mater. Sci. Semicond. Process. 56 29
 Google Scholar
Google Scholar
[14] Mei A, Li X, Liu L, Ku Z, Liu T, Rong Y, Xu M, Hu M, Chen J, Yang Y, Graetzel M, Han H 2014 Science 345 295
 Google Scholar
Google Scholar
[15] Liu J, Li N, Dong Q, Li J, Qin C, Wang L 2019 Sci. China Mater. 62 173
 Google Scholar
Google Scholar
[16] Zhang H, Song K, Zhu L, Meng Q 2020 Carbon 168 372
 Google Scholar
Google Scholar
[17] Meng F, Zhou Y, Gao L, Li Y, Liu A, Li Y, Zhang C, Fan M, Wei G, Ma T 2021 Mater. Today Energy 19 100590
 Google Scholar
Google Scholar
[18] Chen R, Feng Y, Zhang C, Wang M, Jing L, Ma C, Bian J, Shi Y 2020 J. Mater. Chem. C 8 9262
 Google Scholar
Google Scholar
[19] Etgar L, Gao P, Xue Z, Peng Q, Chandiran A K, Liu B, Nazeeruddin M K, Grätzel M 2012 J. Am. Chem. Soc. 134 17396
 Google Scholar
Google Scholar
[20] Ku Z, Rong Y, Xu M, Liu T, Han H 2013 Sci. Rep. 3 3132
 Google Scholar
Google Scholar
[21] Liu Z Y, Sun B, Shi T L, Tang Z R, Liao G L 2016 J. Mater. Chem. A 4 10700
 Google Scholar
Google Scholar
[22] Ghoreishi F S, Ahmadi V, Poursalehi R, SamadPour M, Johansson M B, Boschloo G, Johansson E M J 2020 J. Power Sources 473 228492
 Google Scholar
Google Scholar
[23] Odabasi C, Yildirim R 2020 Sol. Energy Mater. Sol. Cells 205 110284
 Google Scholar
Google Scholar
[24] Long C Y, Wang N, Huang K Q, Li H Y, Liu B, Yang J L 2020 Chin. Phys. B 29 048801 8
[25] Zhang F, Yang X, Cheng M, Wang W, Sun L 2016 Nano Energy 20 108
 Google Scholar
Google Scholar
[26] Wu Z, Liu Z, Hu Z, Hawash Z, Qiu L, Jiang Y, Ono L K, Qi Y 2019 Adv. Mater. 31 1804284
 Google Scholar
Google Scholar
[27] Jung E H, Jeon N J, Park E Y, Moon C S, Shin T J, Yang T Y, Noh J H, Seo J 2019 Nature 567 511
 Google Scholar
Google Scholar
[28] Almohammedi A, Khan M T, Benghanem M, Aboud S W, Shkir M, AlFaify S 2020 Mater. Sci. Semicond. Process. 120 105272
 Google Scholar
Google Scholar
[29] Park M S, Meresa A A, Kwon C M, Kim F S 2019 Polymers 11 1682 11
[30] Kiymaz D, Kiymaz A, Zafer C 2021 Nanotechnology 32 105401
 Google Scholar
Google Scholar
[31] Su H, Xiao J Y, Li Q H, Peng C, Zhang X X, Mao C, Yao Q, Lu Y J, Ku Z L, Zhong J, Li W, Peng Y, Huang F Z, Cheng Y B 2020 Mater. Sci. Semicond. Process. 107 104809
 Google Scholar
Google Scholar
[32] Wang G M, Swensen J, Moses D, Heeger A J 2003 J. Appl. Phys. 93 6137
 Google Scholar
Google Scholar
-
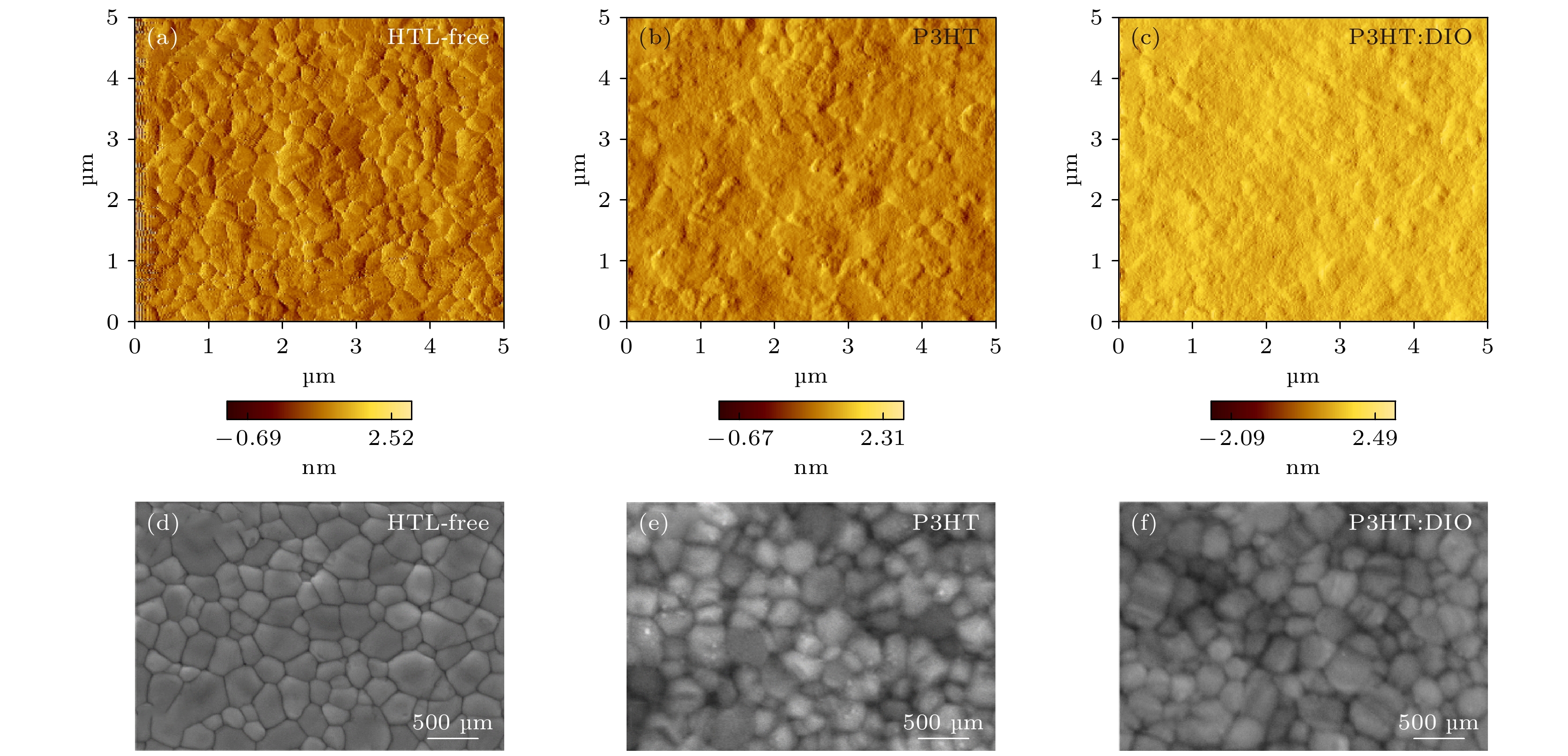
图 2 钙钛矿薄膜表面形貌 (a), (b), (c) 原子力显微镜(AFM)表征钙钛矿薄膜(HTL-free) (a), 旋涂了空穴传输层P3HT的钙钛矿薄膜(P3HT) (b), 旋涂了添加DIO的空穴传输层P3HT的钙钛矿薄膜(P3HT: DIO) (c)的表面形貌图; (e), (d), (f) 扫面电子显微镜表征钙钛矿薄膜(e), 旋涂了空穴传输层P3HT的钙钛矿薄膜(d), 旋涂了添加DIO的空穴传输层P3HT的钙钛矿薄膜(f)的表面形貌图
Fig. 2. Surface morphology of the perovskite films: (a), (b), (c) Atomic force microscopy (AFM) characterization of perovskite film (HTL-free) (a), perovskite film with P3HT (b), perovskite film with P3HT: DIO (c); (d), (e), (f) scanning electron microscopy characterization of perovskite film (HTL-free) (d), perovskite film with P3HT (e), perovskite film with P3HT: DIO (f).
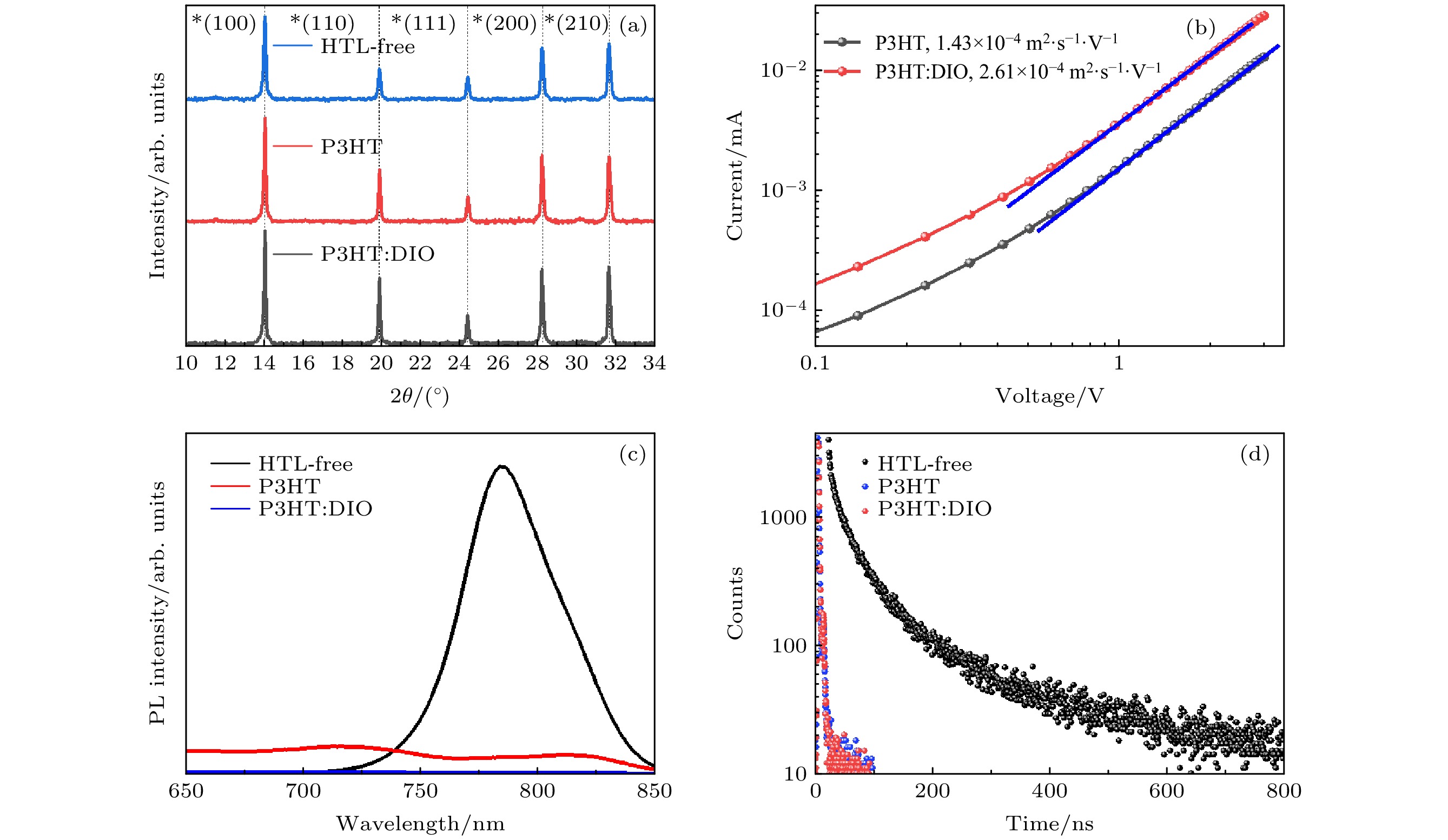
图 3 钙钛矿薄膜、旋涂空穴传输层P3HT的钙钛矿薄膜与旋涂空穴传输层中添加了DIO的P3HT薄膜的光电性能测试 (a) X射线衍射图谱; (b) 通过ITO/PEDT/P3HT结构器件测得的P3HT材料的载流子迁移率曲线; (c) 稳态光致发光曲线; (d) 时间分辨光致发光衰减曲线
Fig. 3. Photoelectric performance test of perovskite film, perovskite film with P3HT and perovskite with P3HT: DIO: (a) X-ray diffraction pattern; (b) carrier mobility fitting of the hole-transport layer; (c) steady-state photoluminescence; (d) time-resolved photoluminescence.
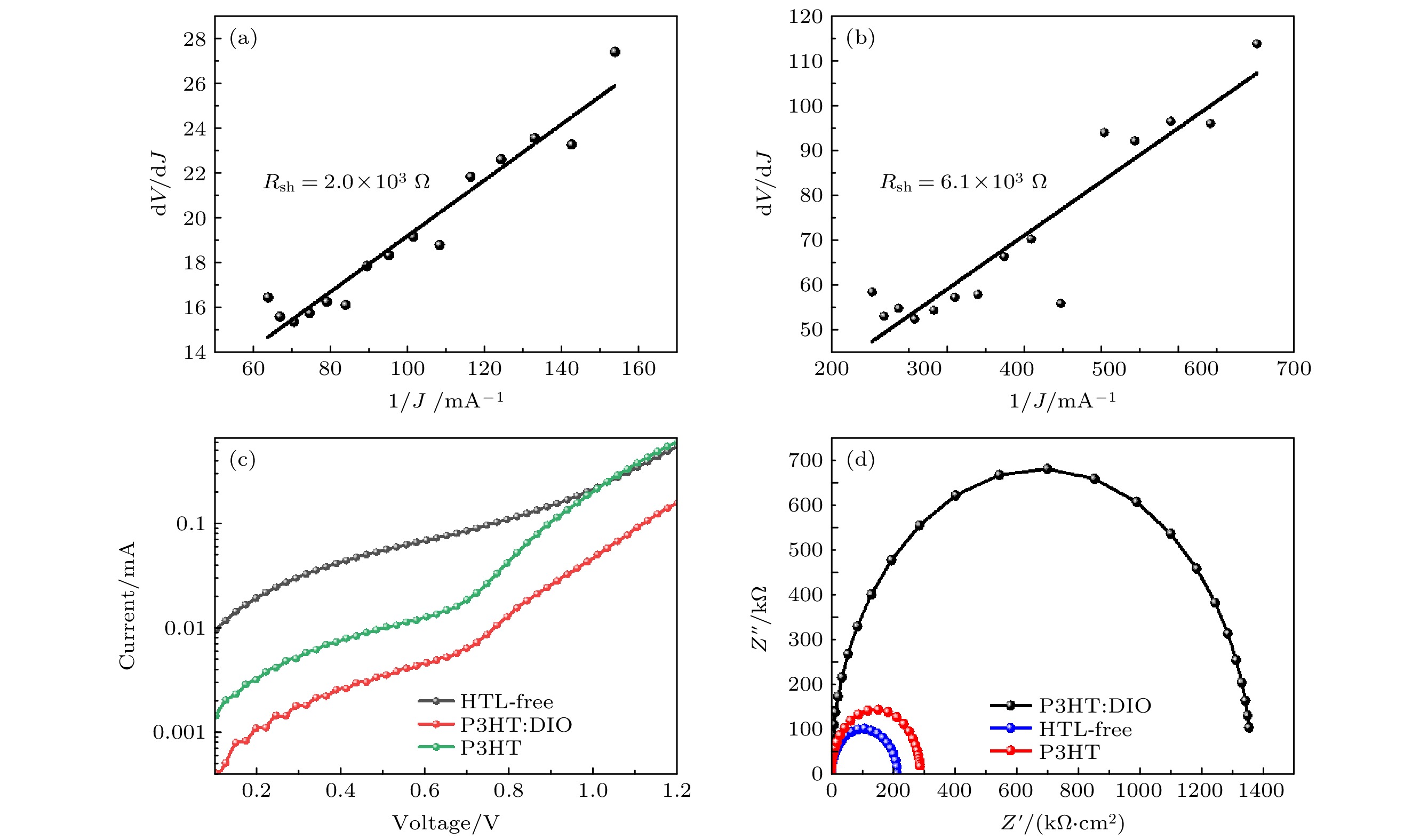
图 5 (a), (b) 旋涂P3HT和P3HT: DIO器件的暗电流测试曲线; (c) 无空穴传输层器件、旋涂P3HT和P3HT: DIO器件的暗电流测试曲线; (d) 无空穴传输层器件、旋涂P3HT和P3HT: DIO器件结构的电化学阻抗谱
Fig. 5. (a), (b) The dark current test curves of spin-coated P3HT and P3HT: DIO devices; (c) dark current test curves for holeless transport layer devices, spin-coated P3HT and P3HT: DIO devices.; (d) electrochemical impedance spectra of hole-free transport layer devices, spin-coated P3HT and P3HT:DIO device structures.
表 1 基于不同空穴传输材料的钙钛矿太阳能电池性能参数
Table 1. Photovoltaic parameters of perovskite solar cell based on different HTLs.
HTM JSC/(mA·cm–2) VOC/V FF/% PCE/% P3HT:DIO 22.39 0.90 74.86 15.11 P3HT 22.15 0.89 71.32 14.06 无 21.07 0.89 71.94 13.48 -
[1] Chapin D M, Fuller C S, Pearson G L 1954 J. Appl. Phys. 25 676
 Google Scholar
Google Scholar
[2] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[3] Kim H S, Lee C R, Im J H, Lee K B, Moehl T, Marchioro A, Moon S J, Humphry-Baker R, Yum J H, Moser J E, Graetzel M, Park N G 2012 Sci. Rep. 2 591
 Google Scholar
Google Scholar
[4] Im J H, Lee C R, Lee J W, Park S W, Park N G 2011 Nanoscale 3 4088
 Google Scholar
Google Scholar
[5] Liu M, Johnston M B, Snaith H J 2013 Nature 501 395
 Google Scholar
Google Scholar
[6] Abate A, Leijtens T, Pathak S, Teuscher J, Avolio R, Errico M E, Kirkpatrik J, Ball J M, Docampo P, McPherson I, Snaith H J 2013 PCCP 15 2572
 Google Scholar
Google Scholar
[7] Furube A, Katoh R, Hara K, Sato T, Murata S, Arakawa H, Tachiya M 2005 J. Phys. Chem. B 109 16406
 Google Scholar
Google Scholar
[8] Abbas H A, Kottokkaran R, Ganapathy B, Samiee M, Zhang L, Kitahara A, Noack M, Dalal V L 2015 APL Mater. 3 506
[9] Wang M J, Li S B, Zhang P, Wang Y F, Li H Q, Chen Z 2015 Chem. Phys. Lett. 639 283
 Google Scholar
Google Scholar
[10] Yan W, Li Y, Ye S, Li Y, Rao H, Liu Z, Wang S, Bian Z, Huang C 2016 Nano Res. 9 1600
 Google Scholar
Google Scholar
[11] Hauch A, Georg A 2002 Electrochim. Acta 46 3457
[12] Ikegami M, Miyoshi K, Miyasaka T, Teshima K, Wei T C, Wan C C, Wang Y Y 2007 Appl. Phys. Lett. 90 153122
 Google Scholar
Google Scholar
[13] Che M, Zhu L, Zhao Y L, Yao D S, Gu X Q, Song J, Qiang Y H 2016 Mater. Sci. Semicond. Process. 56 29
 Google Scholar
Google Scholar
[14] Mei A, Li X, Liu L, Ku Z, Liu T, Rong Y, Xu M, Hu M, Chen J, Yang Y, Graetzel M, Han H 2014 Science 345 295
 Google Scholar
Google Scholar
[15] Liu J, Li N, Dong Q, Li J, Qin C, Wang L 2019 Sci. China Mater. 62 173
 Google Scholar
Google Scholar
[16] Zhang H, Song K, Zhu L, Meng Q 2020 Carbon 168 372
 Google Scholar
Google Scholar
[17] Meng F, Zhou Y, Gao L, Li Y, Liu A, Li Y, Zhang C, Fan M, Wei G, Ma T 2021 Mater. Today Energy 19 100590
 Google Scholar
Google Scholar
[18] Chen R, Feng Y, Zhang C, Wang M, Jing L, Ma C, Bian J, Shi Y 2020 J. Mater. Chem. C 8 9262
 Google Scholar
Google Scholar
[19] Etgar L, Gao P, Xue Z, Peng Q, Chandiran A K, Liu B, Nazeeruddin M K, Grätzel M 2012 J. Am. Chem. Soc. 134 17396
 Google Scholar
Google Scholar
[20] Ku Z, Rong Y, Xu M, Liu T, Han H 2013 Sci. Rep. 3 3132
 Google Scholar
Google Scholar
[21] Liu Z Y, Sun B, Shi T L, Tang Z R, Liao G L 2016 J. Mater. Chem. A 4 10700
 Google Scholar
Google Scholar
[22] Ghoreishi F S, Ahmadi V, Poursalehi R, SamadPour M, Johansson M B, Boschloo G, Johansson E M J 2020 J. Power Sources 473 228492
 Google Scholar
Google Scholar
[23] Odabasi C, Yildirim R 2020 Sol. Energy Mater. Sol. Cells 205 110284
 Google Scholar
Google Scholar
[24] Long C Y, Wang N, Huang K Q, Li H Y, Liu B, Yang J L 2020 Chin. Phys. B 29 048801 8
[25] Zhang F, Yang X, Cheng M, Wang W, Sun L 2016 Nano Energy 20 108
 Google Scholar
Google Scholar
[26] Wu Z, Liu Z, Hu Z, Hawash Z, Qiu L, Jiang Y, Ono L K, Qi Y 2019 Adv. Mater. 31 1804284
 Google Scholar
Google Scholar
[27] Jung E H, Jeon N J, Park E Y, Moon C S, Shin T J, Yang T Y, Noh J H, Seo J 2019 Nature 567 511
 Google Scholar
Google Scholar
[28] Almohammedi A, Khan M T, Benghanem M, Aboud S W, Shkir M, AlFaify S 2020 Mater. Sci. Semicond. Process. 120 105272
 Google Scholar
Google Scholar
[29] Park M S, Meresa A A, Kwon C M, Kim F S 2019 Polymers 11 1682 11
[30] Kiymaz D, Kiymaz A, Zafer C 2021 Nanotechnology 32 105401
 Google Scholar
Google Scholar
[31] Su H, Xiao J Y, Li Q H, Peng C, Zhang X X, Mao C, Yao Q, Lu Y J, Ku Z L, Zhong J, Li W, Peng Y, Huang F Z, Cheng Y B 2020 Mater. Sci. Semicond. Process. 107 104809
 Google Scholar
Google Scholar
[32] Wang G M, Swensen J, Moses D, Heeger A J 2003 J. Appl. Phys. 93 6137
 Google Scholar
Google Scholar
计量
- 文章访问数: 8493
- PDF下载量: 137
- 被引次数: 0














 下载:
下载: