-
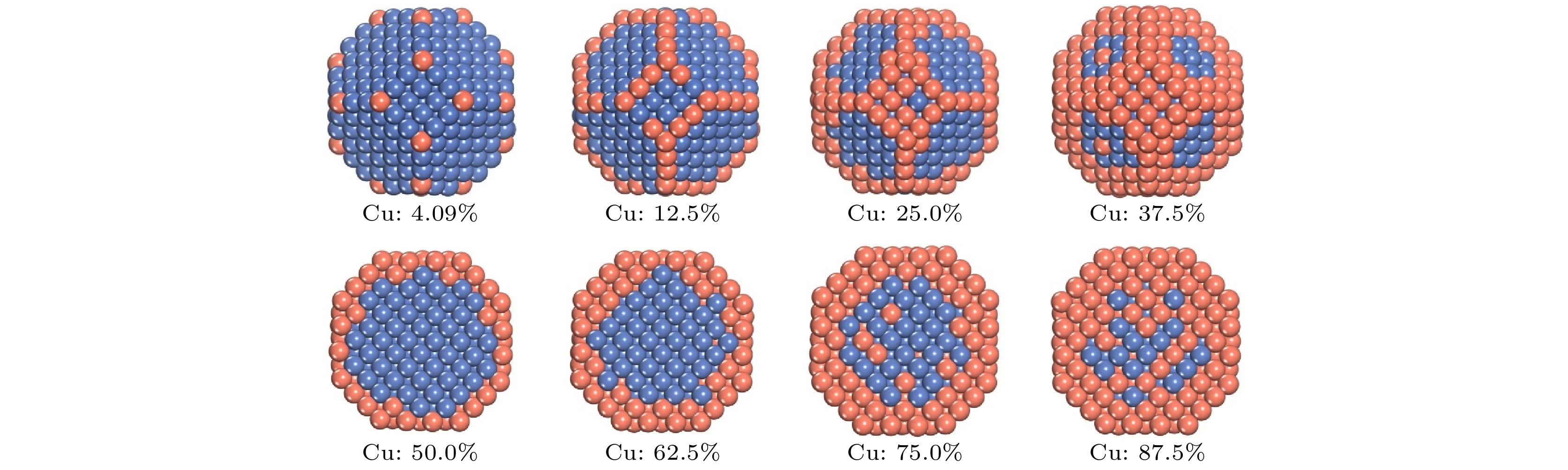
NiCu双金属核壳纳米粒子不仅由于其优异的稳定性、选择性以及磁学和催化性能而受到广泛关注, 而且可以通过改变其纳米粒子的形貌、表面元素分布和粒径大小而具有可调谐性能. 采用分子动力学与蒙特罗方法并结合嵌入原子势对NiCu双金属纳米粒子的表面偏析、结构特征以及Cu吸附原子在Ni基底沉积生长与表面扩散进行了研究, 结果表明Cu原子在Ni基底表面具有强的偏析倾向. 随着Cu原子浓度的增加, Cu原子优先占据纳米粒子的顶点、边、(100) 和 (111) 面, 最终形成完美的Ni核/Cu壳纳米粒子. 在生长温度T = 400 K时, 形成的Ni核/Cu壳结构最稳定. 进一步采用肘弹性波方法模拟计算在Ni基表面Cu吸附原子的扩散势垒, 结果表明, Cu吸附原子无论是交换还是扩散, 都需要克服较大的ES势垒, 从而难以在Ni基底表面进行面间扩散. 与Ni基底相反, 在Cu基底上沉积Ni原子, Ni吸附原子很容易从 (111) 面迁移至 (100) 面, 且在当前模拟温度下, Ni吸附原子无法在 (100) 面进行迁移, 导致生长构型朝正八面体的形状发展, 且其八个顶角几乎被Ni原子所占据. 本文经过深入研究, 从原子的角度出发, 对NiCu纳米催化剂的初步设计提供了一种新的思路和方法.
-
关键词:
- NiCu双金属 /
- 表面偏析 /
- 扩散 /
- 沉积生长 /
- Ni核/Cu壳纳米粒子
Bimetallic core-shell nanoparticles such as NiCu are of great interest not only due to their excellent stability, selectivity, and magnetic and catalytic properties, but also because they are tunable by changing the morphology, surface element distribution, and particle size of the nanoparticles. The surface segregation and structural features of NiCu bimetallic nanoparticles, the deposition growth and the surface diffusion of Cu adsorbed atoms on the Ni substrate surface are studied by using molecular dynamics and the Montero method combined with embedded atomic potential. The results show that the Cu atom has a strong tendency of surface segregation. With the increase of concentration of Cu atoms, Cu atoms preferentially occupy the vertex, edge, (100), and (111) facet of nanoparticles due to the difference in configuration energy between Cu atoms and surface Ni atoms with different coordination numbers after the exchange, and finally form perfect Ni-core/Cu-shell nanoparticles. When growth temperature T = 400 K, the Ni-core/Cu-shell structure formed is the most stable. By observing the NiCu core-shell structure’s growth sequence, it is found that a few Ni atoms are replaced by Cu atoms on the step edge of the Ni substrate. The diffusion energy barrier of Cu atoms adsorbed on a Ni substrate surface is calculated by using the nudged elastic band method. The results show that Cu atoms adsorbed need to overcome a large ES barrier for both exchange and diffusion, making it difficult to diffuse between the facets of Ni substrate surface in a temperature range of 200–800 K. The lowest energy barrier for the diffusion of Cu atoms between facets of Ni substrate surface is 0.43 eV, and the diffusion path is from (111) facet to (100) facet. In contrast to Ni substrate, Ni atoms deposited on Cu substrate can easily migrate from the (111) facet to the (100) facet with a diffusion energy barrier of only about 0.12 eV, and at the present simulated temperature, Ni adsorbed atoms are unable to migrate on the (100) facet, resulting in a growth configuration toward an octahedral shape with its eight apex angles almost occupied by Ni atoms. In this paper, a new idea and method are provided for the preliminary design of NiCu nano-catalysts from atoms.-
Keywords:
- NiCu bimetallic nanoparticles /
- surface segregation /
- diffusion /
- growth /
- core-shell structure
[1] Yang J Y, Hu W Y, Dai X Y 2018 Comput. Mater. Sci 154 371
 Google Scholar
Google Scholar
[2] Riccardo F, Julius J, Johnston R L 2008 Chem. Rev. 108 845
 Google Scholar
Google Scholar
[3] Nguyen N H, Hu A, Persic J, Wen J Z 2011 Chem. Phys. Lett. 503 112
 Google Scholar
Google Scholar
[4] Huang R, Wen Y H, Shao G F, Sun S G 2013 J. Phys. Chem. C 117 4278
 Google Scholar
Google Scholar
[5] Yang J Y, Hu W Y, Tang J F 2015 Thin Solid Films 593 137
 Google Scholar
Google Scholar
[6] Huang R, Wen Y H, Zhu Z Z, Sun S G 2012 J. Phys. Chem. C 116 8664
 Google Scholar
Google Scholar
[7] Mobedpour B, Rajabdoust S, Roumina R 2018 Comput. Mater. Sci 151 132
 Google Scholar
Google Scholar
[8] Henz B J, Hawa T, Zachariah M 2009 Mol. Simul. 35 804
 Google Scholar
Google Scholar
[9] Yang L Y, Gan X L, Xu C, Lang L, Jian Z Y, Xiao S F, Deng H Q, Li X F, Tian Z A, Hu W Y 2019 Comput. Mater. Sci. 156 47
 Google Scholar
Google Scholar
[10] Jian L G, Qiang W, Tian L H, Kai W, Cheng H J 2008 Chin. Phys. B 17 3343
 Google Scholar
Google Scholar
[11] Yang J Y, Hu W Y, Wu Y Q, Dai X Y 2012 Cryst. Growth Des. 12 2978
 Google Scholar
Google Scholar
[12] Yang J Y, Hu W Y, Wu Y R, Dai X Y 2012 Surf. Sci. 606 971
 Google Scholar
Google Scholar
[13] Tang J F, Yang J Y 2013 Thin Solid Films 536 318
 Google Scholar
Google Scholar
[14] Dai X Y, Hu W Y, Yang J Y, Chen C P 2015 Physica B 458 144
 Google Scholar
Google Scholar
[15] Yang J Y, Hu W Y, Tang J F, Dai X Y 2013 Comput. Mater. Sci. 74 160
 Google Scholar
Google Scholar
[16] Baletto F, Mottet C, Ferrando R 2002 Phys. Rev. B 66 155420
 Google Scholar
Google Scholar
[17] Baletto F, Mottet C, Ferrando R 2003 Phys. Rev. Lett. 90 135504
 Google Scholar
Google Scholar
[18] Baletto F, Mottet C, Ferrando R 2003 Eur. Phys. J. D 24 233
 Google Scholar
Google Scholar
[19] Baletto F, Mottet C, Rapallo A, Rossi G, Ferrando R 2004 Surf. Sci. 566-568 192
[20] Deng L, Hu W Y, Deng H Q, Xiao S F 2010 J. Phys. Chem. C 114 11026
 Google Scholar
Google Scholar
[21] Deng L, Hu W Y, Deng H Q, Xiao S F, Tang J F 2011 J. Phys. Chem. C 115 11355
[22] Deng L, Deng H Q, Xiao S F, Tang J F, Hu W Y 2013 Faraday Discuss. 162 293
 Google Scholar
Google Scholar
[23] Tang J, Deng L, Deng H, Xiao S, Zhang X, Hu W 2014 J. Phys. Chem. C 118 27850
 Google Scholar
Google Scholar
[24] Tang J, Deng L, Xiao S, Deng H, Zhang X, Hu W 2015 J. Phys. Chem. C 119 21515
 Google Scholar
Google Scholar
[25] Austin N, Butina B, Mpourmpakis G 2016 Prog. Nat. Sci. 26 487
 Google Scholar
Google Scholar
[26] Tripathi A, Hareesh C, Sinthika S, Andersson G, Thapa R 2020 Appl. Surf. Sci. 528 146964
 Google Scholar
Google Scholar
[27] Aslan M 2021 Mater. Today. Commun 26 101821
 Google Scholar
Google Scholar
[28] Buendia F, Anzaldo A T, Vital C, Beltran M R 2020 J. Chem. Phys. 152 024303
 Google Scholar
Google Scholar
[29] Yamauchi T, Tsukahara Y, Sakata T, Mori H, Yanagida T, Kawai T, Wada Y 2010 Nanoscale 2 515
 Google Scholar
Google Scholar
[30] Bonet F, Grugeon S, Dupont L, Herrera U R, Guéry C, Tarascon J M 2003 J. Solid State Chem. 172 111
 Google Scholar
Google Scholar
[31] Chatterjee J, Bettge M, Haik Y, Jen Chen C 2005 J. Magn. Magn. Mater. 293 303
 Google Scholar
Google Scholar
[32] Hristova E, Dong Y, Grigoryan V G, Springborg M 2008 J. Phys. Chem. A 112 7905
 Google Scholar
Google Scholar
[33] Xiao K, Qi X, Bao Z, Wang X, Zhong L, Fang K, Lin M, Sun Y 2013 Catal. Sci. Technol 3 1591
 Google Scholar
Google Scholar
[34] Quaino P, Belletti G, Shermukhamedov S A, Glukhov D V, Santos E, Schmickler W, Nazmutdinov R 2017 Phys. Chem. Chem. Phys. 19 26812
 Google Scholar
Google Scholar
[35] Guisbiers G, Khanal S, Ruiz-Zepeda F, Roque de la Puente J, Jose-Yacaman M 2014 Nanoscale 6 14630
 Google Scholar
Google Scholar
[36] Panizon E, Olmos-Asar J A, Peressi M, Ferrando R 2015 Phys. Chem. Chem. Phys. 17 28068
 Google Scholar
Google Scholar
[37] Mendes P C D, Justo S G, Mucelini J, Soares M D, Batista K E A, Quiles M G, Piotrowski M J, Da Silva J L F 2019 J. Phys. Chem. C 124 1158
[38] Hardeveld R, Hartog F 1969 Surf. Sci. 15 189
 Google Scholar
Google Scholar
[39] Wang G, Hove M A, Ross P, Baskes M I 2005 Prog. Surf. Sci. 79 28
[40] Dzhurakhalov A, Marc H 2007 Phys. Rev. B 76 045429
 Google Scholar
Google Scholar
[41] Martin T P 1996 Phys. Rep. 273 199
 Google Scholar
Google Scholar
[42] Onat B, Durukanoglu S 2013 J. Phys. Condes. Matter 26 035404
[43] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[44] Stukowski A 2010 Model. Simul. Mater. Sci. Eng 18 015012
 Google Scholar
Google Scholar
[45] Cleveland C L, Luedtke W D, Landman U 1999 Phys. Rev. B 60 5065
 Google Scholar
Google Scholar
[46] Zhi Z, Hu W Y, Xiao S F 2006 Phys. Rev. B 73 125443
 Google Scholar
Google Scholar
[47] Huang R, Wen Y H, Zhu Z Z, Sun S G 2012 J. Phys. Chem. C 116 11837
[48] Mottet C, Rossi G, Baletto F, Ferrando R 2005 Phys. Rev. Lett. 95 035501
 Google Scholar
Google Scholar
[49] Feng D, Feng Y, Yuan S, Zhang X, Wang G 2017 Appl. Therm. Eng 111 1457
 Google Scholar
Google Scholar
[50] Hamid I, Fang M, Duan H 2015 AIP Adv 5 047129
 Google Scholar
Google Scholar
[51] Ding F, Rosén A, Bolton K 2004 Phys. Rev. B 70 075416
 Google Scholar
Google Scholar
[52] Wang Q, Wang X, Liu J, Yang Y 2017 J. Nanopart. Res 19 25
 Google Scholar
Google Scholar
[53] Vitos L, Ruban A V, Skriver H L, Kollár J 1998 Surf. Sci. 411 186
 Google Scholar
Google Scholar
[54] Abbaspour M, Akbarzadeh H, Lotfi S 2018 J. Alloys. Compd. 764 323
 Google Scholar
Google Scholar
[55] Wang H, Hu T, Qin J Y, Zhang T 2012 J. Appl. Phys. 112 073520
 Google Scholar
Google Scholar
[56] Strohl J K, King T S 1989 J. Catal. 116 540
 Google Scholar
Google Scholar
[57] Tang J F, Yang J Y 2013 J. Nanopart. Res 15 2050
 Google Scholar
Google Scholar
[58] 高明, 邓永和, 文大东, 田泽安, 赵鹤平, 彭平 2020 69 046401
 Google Scholar
Google Scholar
Gao M, Deng Y H, Wen D D, Tian Z A, Zhao H P, Peng P 2020 Acta Phys Sin 69 046401
 Google Scholar
Google Scholar
[59] Zhang Y W, Deng Y H, Zeng Q F, Wen D D, Zhao H P, Gao M, Dai X Y, Wu A R 2020 Chin. Phys. B 29 116601
 Google Scholar
Google Scholar
[60] 张宇文, 邓永和, 文大东, 赵鹤平, 高明 2020 69 136601
 Google Scholar
Google Scholar
Zhang Y W, Dong Y H, Wen D D, Zhao H P, Gao M 2020 Acta Phys Sin 69 136601
 Google Scholar
Google Scholar
-
图 1 (a) TOC结构纳米粒子的总原子数与表面原子数比例随尺寸的关系; (b) TOC纳米粒子的表面催化位点比例随尺寸的关系
Fig. 1. (a) The total atom number and surface atom percentage of TOC structure nanoparticles as a function of nanoparticle size; (b) the relationship between the surface catalytic sites density of TOC structure nanoparticles and the size of nanoparticles.
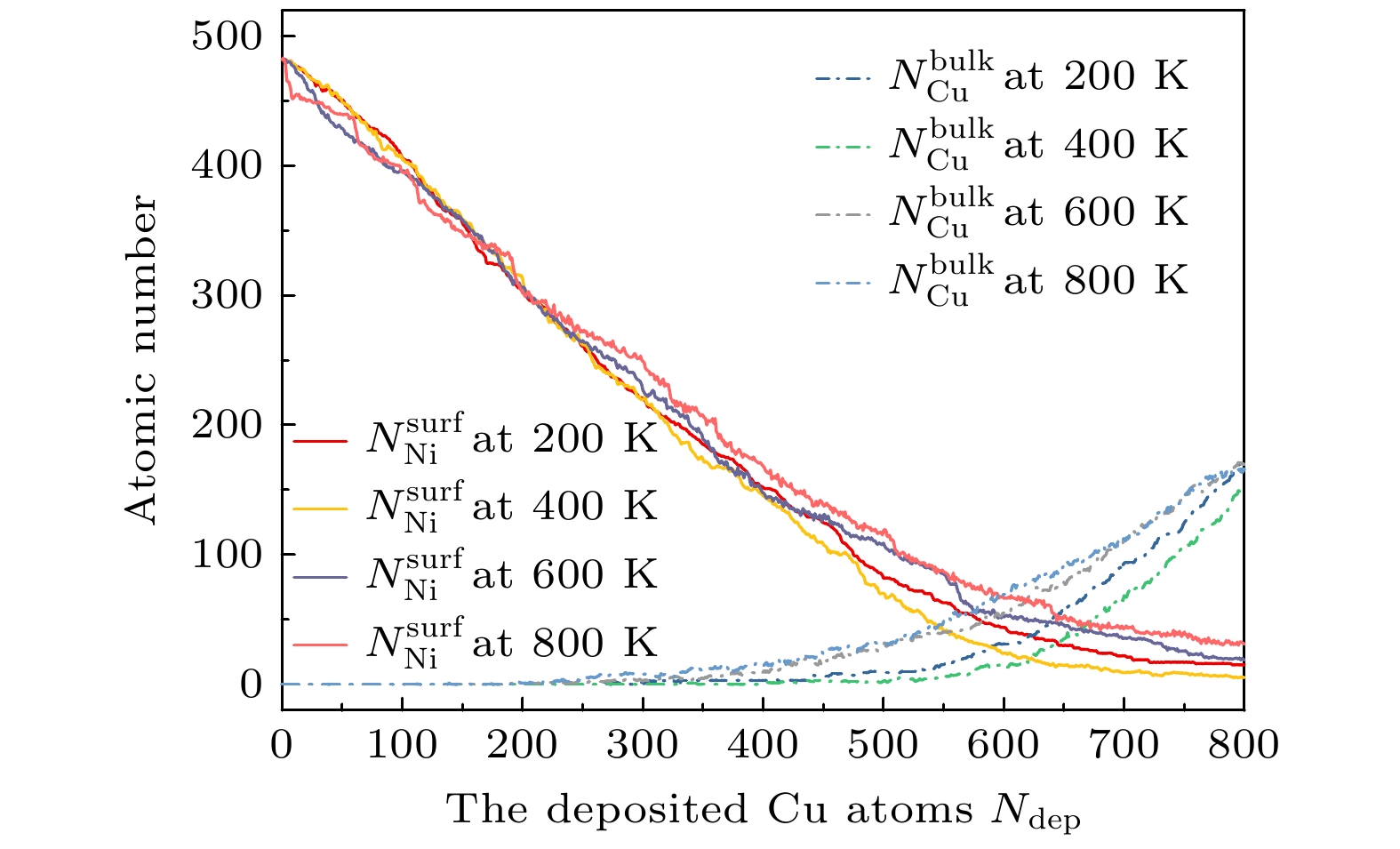
图 7 在温度T = 200, 400, 600和800 K下,
$N_{\rm{Ni}}^{{\rm{surf}}} $ 和$N_{\rm{Cu}}^{{\rm{bulk}}} $ 与沉积的Cu原子数的函数关系Fig. 7. The growth of Cu atoms on the TOC Ni substrate with 1289 atoms at T = 200, 400, 600, and 800 K, the
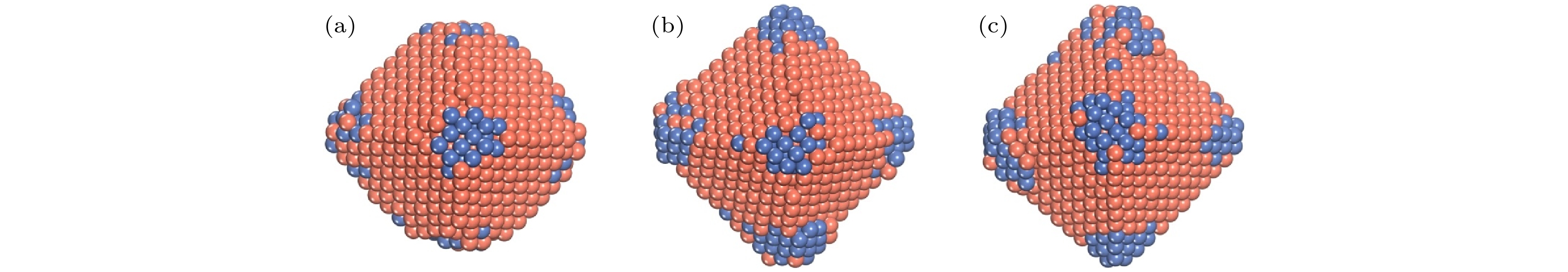
$N_{\rm{Ni}}^{{\rm{surf}}} $ and$N_{\rm{Cu}}^{{\rm{bulk}}} $ as a function of the deposited Cu atoms (Ndep).图 8 T = 400 K, Al原子在Ni TOC1289基底上的生长序列 (a) Ndep = 100; (b) Ndep = 200; (c) Ndep = 300; (d) Ndep = 400; (e) Ndep = 500; (f) Ndep = 600. 橘红色和海蓝色的球分别表示Cu原子和Ni原子
Fig. 8. Growth sequence of NiCu clusters at T = 400 K. The snapshots of the growth simulation for various Ndep of 100, 200, 300, 400, 500, and 600. The orange-red and sea-blue ball show the Cu and the Ni atom, respectively.
图 9 Cu (Ni) 吸附原子在Ni TOC1289 (Cu TOC1289) 基底表面的扩散势垒 (a) Ni-基底 (111)→(100); (b) Ni基底 (111)→(111); (c) Cu基底 (111)→(100); (d) Cu基底 (111)→(111). 橘红色和海蓝色的球分别表示Cu原子和Ni原子
Fig. 9. The diffusion energy barrier of Cu adatom on the surface of the Ni TOC1289: (a) Ni-base (111)→(100) facet; (b) Ni-base (111)→(111) facet; (c) Cu-base (111)→(100) facet; (d) Cu-base (111)→(111) facet. The orange-red and sea-blue balls represent Cu atoms and Ni atoms, respectively.
表 1 对于586原子的TOC纳米粒子, 内部Cu原子与表面Ni原子交换后构型能差值 (∆E, 单位: eV/atom), 数字6, 7, 8, 9和12分别表示顶点、边缘、(100) 面、(111) 面和体内的位置
Table 1. Configuration energy difference values (∆E, unit: eV/ atom) for a bulk Cu atom exchanging with surface Ni atoms for the TOC nanoparticles of 586 atoms. The numbers 6, 7, 8, 9, and 12 are represent the sites at the vertex, edge, (100) facet, (111) facet, and bulk, respectively
Configuration ∆E/(eV·atom–1) 12 → 6 –0.395 12 → 7 –0.303 12 → 8 –0.194 12 → 9 –0.149 -
[1] Yang J Y, Hu W Y, Dai X Y 2018 Comput. Mater. Sci 154 371
 Google Scholar
Google Scholar
[2] Riccardo F, Julius J, Johnston R L 2008 Chem. Rev. 108 845
 Google Scholar
Google Scholar
[3] Nguyen N H, Hu A, Persic J, Wen J Z 2011 Chem. Phys. Lett. 503 112
 Google Scholar
Google Scholar
[4] Huang R, Wen Y H, Shao G F, Sun S G 2013 J. Phys. Chem. C 117 4278
 Google Scholar
Google Scholar
[5] Yang J Y, Hu W Y, Tang J F 2015 Thin Solid Films 593 137
 Google Scholar
Google Scholar
[6] Huang R, Wen Y H, Zhu Z Z, Sun S G 2012 J. Phys. Chem. C 116 8664
 Google Scholar
Google Scholar
[7] Mobedpour B, Rajabdoust S, Roumina R 2018 Comput. Mater. Sci 151 132
 Google Scholar
Google Scholar
[8] Henz B J, Hawa T, Zachariah M 2009 Mol. Simul. 35 804
 Google Scholar
Google Scholar
[9] Yang L Y, Gan X L, Xu C, Lang L, Jian Z Y, Xiao S F, Deng H Q, Li X F, Tian Z A, Hu W Y 2019 Comput. Mater. Sci. 156 47
 Google Scholar
Google Scholar
[10] Jian L G, Qiang W, Tian L H, Kai W, Cheng H J 2008 Chin. Phys. B 17 3343
 Google Scholar
Google Scholar
[11] Yang J Y, Hu W Y, Wu Y Q, Dai X Y 2012 Cryst. Growth Des. 12 2978
 Google Scholar
Google Scholar
[12] Yang J Y, Hu W Y, Wu Y R, Dai X Y 2012 Surf. Sci. 606 971
 Google Scholar
Google Scholar
[13] Tang J F, Yang J Y 2013 Thin Solid Films 536 318
 Google Scholar
Google Scholar
[14] Dai X Y, Hu W Y, Yang J Y, Chen C P 2015 Physica B 458 144
 Google Scholar
Google Scholar
[15] Yang J Y, Hu W Y, Tang J F, Dai X Y 2013 Comput. Mater. Sci. 74 160
 Google Scholar
Google Scholar
[16] Baletto F, Mottet C, Ferrando R 2002 Phys. Rev. B 66 155420
 Google Scholar
Google Scholar
[17] Baletto F, Mottet C, Ferrando R 2003 Phys. Rev. Lett. 90 135504
 Google Scholar
Google Scholar
[18] Baletto F, Mottet C, Ferrando R 2003 Eur. Phys. J. D 24 233
 Google Scholar
Google Scholar
[19] Baletto F, Mottet C, Rapallo A, Rossi G, Ferrando R 2004 Surf. Sci. 566-568 192
[20] Deng L, Hu W Y, Deng H Q, Xiao S F 2010 J. Phys. Chem. C 114 11026
 Google Scholar
Google Scholar
[21] Deng L, Hu W Y, Deng H Q, Xiao S F, Tang J F 2011 J. Phys. Chem. C 115 11355
[22] Deng L, Deng H Q, Xiao S F, Tang J F, Hu W Y 2013 Faraday Discuss. 162 293
 Google Scholar
Google Scholar
[23] Tang J, Deng L, Deng H, Xiao S, Zhang X, Hu W 2014 J. Phys. Chem. C 118 27850
 Google Scholar
Google Scholar
[24] Tang J, Deng L, Xiao S, Deng H, Zhang X, Hu W 2015 J. Phys. Chem. C 119 21515
 Google Scholar
Google Scholar
[25] Austin N, Butina B, Mpourmpakis G 2016 Prog. Nat. Sci. 26 487
 Google Scholar
Google Scholar
[26] Tripathi A, Hareesh C, Sinthika S, Andersson G, Thapa R 2020 Appl. Surf. Sci. 528 146964
 Google Scholar
Google Scholar
[27] Aslan M 2021 Mater. Today. Commun 26 101821
 Google Scholar
Google Scholar
[28] Buendia F, Anzaldo A T, Vital C, Beltran M R 2020 J. Chem. Phys. 152 024303
 Google Scholar
Google Scholar
[29] Yamauchi T, Tsukahara Y, Sakata T, Mori H, Yanagida T, Kawai T, Wada Y 2010 Nanoscale 2 515
 Google Scholar
Google Scholar
[30] Bonet F, Grugeon S, Dupont L, Herrera U R, Guéry C, Tarascon J M 2003 J. Solid State Chem. 172 111
 Google Scholar
Google Scholar
[31] Chatterjee J, Bettge M, Haik Y, Jen Chen C 2005 J. Magn. Magn. Mater. 293 303
 Google Scholar
Google Scholar
[32] Hristova E, Dong Y, Grigoryan V G, Springborg M 2008 J. Phys. Chem. A 112 7905
 Google Scholar
Google Scholar
[33] Xiao K, Qi X, Bao Z, Wang X, Zhong L, Fang K, Lin M, Sun Y 2013 Catal. Sci. Technol 3 1591
 Google Scholar
Google Scholar
[34] Quaino P, Belletti G, Shermukhamedov S A, Glukhov D V, Santos E, Schmickler W, Nazmutdinov R 2017 Phys. Chem. Chem. Phys. 19 26812
 Google Scholar
Google Scholar
[35] Guisbiers G, Khanal S, Ruiz-Zepeda F, Roque de la Puente J, Jose-Yacaman M 2014 Nanoscale 6 14630
 Google Scholar
Google Scholar
[36] Panizon E, Olmos-Asar J A, Peressi M, Ferrando R 2015 Phys. Chem. Chem. Phys. 17 28068
 Google Scholar
Google Scholar
[37] Mendes P C D, Justo S G, Mucelini J, Soares M D, Batista K E A, Quiles M G, Piotrowski M J, Da Silva J L F 2019 J. Phys. Chem. C 124 1158
[38] Hardeveld R, Hartog F 1969 Surf. Sci. 15 189
 Google Scholar
Google Scholar
[39] Wang G, Hove M A, Ross P, Baskes M I 2005 Prog. Surf. Sci. 79 28
[40] Dzhurakhalov A, Marc H 2007 Phys. Rev. B 76 045429
 Google Scholar
Google Scholar
[41] Martin T P 1996 Phys. Rep. 273 199
 Google Scholar
Google Scholar
[42] Onat B, Durukanoglu S 2013 J. Phys. Condes. Matter 26 035404
[43] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[44] Stukowski A 2010 Model. Simul. Mater. Sci. Eng 18 015012
 Google Scholar
Google Scholar
[45] Cleveland C L, Luedtke W D, Landman U 1999 Phys. Rev. B 60 5065
 Google Scholar
Google Scholar
[46] Zhi Z, Hu W Y, Xiao S F 2006 Phys. Rev. B 73 125443
 Google Scholar
Google Scholar
[47] Huang R, Wen Y H, Zhu Z Z, Sun S G 2012 J. Phys. Chem. C 116 11837
[48] Mottet C, Rossi G, Baletto F, Ferrando R 2005 Phys. Rev. Lett. 95 035501
 Google Scholar
Google Scholar
[49] Feng D, Feng Y, Yuan S, Zhang X, Wang G 2017 Appl. Therm. Eng 111 1457
 Google Scholar
Google Scholar
[50] Hamid I, Fang M, Duan H 2015 AIP Adv 5 047129
 Google Scholar
Google Scholar
[51] Ding F, Rosén A, Bolton K 2004 Phys. Rev. B 70 075416
 Google Scholar
Google Scholar
[52] Wang Q, Wang X, Liu J, Yang Y 2017 J. Nanopart. Res 19 25
 Google Scholar
Google Scholar
[53] Vitos L, Ruban A V, Skriver H L, Kollár J 1998 Surf. Sci. 411 186
 Google Scholar
Google Scholar
[54] Abbaspour M, Akbarzadeh H, Lotfi S 2018 J. Alloys. Compd. 764 323
 Google Scholar
Google Scholar
[55] Wang H, Hu T, Qin J Y, Zhang T 2012 J. Appl. Phys. 112 073520
 Google Scholar
Google Scholar
[56] Strohl J K, King T S 1989 J. Catal. 116 540
 Google Scholar
Google Scholar
[57] Tang J F, Yang J Y 2013 J. Nanopart. Res 15 2050
 Google Scholar
Google Scholar
[58] 高明, 邓永和, 文大东, 田泽安, 赵鹤平, 彭平 2020 69 046401
 Google Scholar
Google Scholar
Gao M, Deng Y H, Wen D D, Tian Z A, Zhao H P, Peng P 2020 Acta Phys Sin 69 046401
 Google Scholar
Google Scholar
[59] Zhang Y W, Deng Y H, Zeng Q F, Wen D D, Zhao H P, Gao M, Dai X Y, Wu A R 2020 Chin. Phys. B 29 116601
 Google Scholar
Google Scholar
[60] 张宇文, 邓永和, 文大东, 赵鹤平, 高明 2020 69 136601
 Google Scholar
Google Scholar
Zhang Y W, Dong Y H, Wen D D, Zhao H P, Gao M 2020 Acta Phys Sin 69 136601
 Google Scholar
Google Scholar
计量
- 文章访问数: 11157
- PDF下载量: 159
- 被引次数: 0














 下载:
下载: