-
在超快激光激发下分子聚集体内有多个激子产生. 针对具有链间耦合的双分子链系统, 应用密度矩阵理论, 在偶极-偶极近似和算符算术平均值近似下通过求解量子主方程计算了不同双链构型下的多激子动力学过程. 研究发现在光激发作用下的多激子离域在双链分子系统中, 在能量表象下形成激子态能带. 激子态能带的宽度和激子占据能级随双分子链构型的不同而不同. 对于链间和链内均为H型排列的分子链, 激子态能带变宽, 激子态优先占据在高阶能级上. 在不同能级下的激子具有不同的动力学演变特点. 若链间耦合较强, 激子态通过链间耦合传递, 与分子是否直接受到激发没有直接关系, 激发态在两个链内的转移周期与最近邻的链间耦合有直接关系.Details of exciton dynamics in dye aggregates and supra-molecular complexes are substantially important for the functionality of molecular based opto-electronic devices. There are intensive theoretical studies of the multi-exciton dynamics in quantum dot structures but much less in molecular systems. Multiple excitons can be produced in molecular chains as well as two-dimensional and three-dimensional aggregates under an excitation of ultrafast strong laser pulse. According to the dipole arrangements of molecular chains, the coupled molecular chains are designed as H-H, H-J and J-H types of dipole configurations. In the scheme of density matrix theory, the dynamic processes of multiple excitons of different configurations are investigated by solving the quantum master equation through using the approximate dipole-dipole and expectation values of interest. The equations of motion for expectation values of interest governing the respective density operator are used to describe the temporal evolution of the multi-exciton states. It is found that the exciton energy band can be formed in the energy representation, and the multiple excitons are delocalized in the aggregates. The excitons represent different temporal evolutions excited by different resonant excitations. Compared with single-chain systems, double-chain systems have different degrees of blue shift or red shift due to interchain coupling. In the H-H type of aggregate, the electron population is lower if the double-molecule chain is simultaneously excited by the resonance frequency of a single molecule; the electron population increases to a certain extent if the frequency of the field is higher than the vibration frequency of a single molecule. The band width and the energy levels of the multiple excitons vary for different configurations of coupled molecular chains, and the wave packets show their own characters in these energetic levels. In the H-H type of aggregate, exciton state has priority to occupy the high-order energy level. The width of the exciton band of H-J type is significantly narrower than that of H single or H-H double chain configuration, because the Coulomb interaction of the inter-stranded dipole moment makes the whole energy low. In the J-H aggregates, the exciton states are more stable from the energy point of view, and the exciton energy band is wide because of the large Coulomb interaction. The energy of exciton state can be transferred via the interchain coupling, no matter which chain is excited. The transfer period directly correlates with the nearest interchain coupling.
-
Keywords:
- coupled bimolecular chain /
- approximation of dipole-dipole /
- density matrix theory /
- multi-exciton dynamics
[1] Kattoor V, Awasthi K, Jokar E, Diau E W G, Ohta N 2018 J. Phys. Chem. C 122 26623
 Google Scholar
Google Scholar
[2] Stiehm T, Schneider R, Kern J, Niehues I, Michaelis de Vasconcellos S, Bratschitsch R 2019 Rev. Sci. Instrum. 90 083102
 Google Scholar
Google Scholar
[3] Barkaoui H, Abid H, Yangui A, Triki S, Boukheddaden K, Abid Y 2018 J. Phys. Chem. C 122 24253
 Google Scholar
Google Scholar
[4] Jin X H, Price M, Finnegan J, Boott C, Richter J, Rao A, Menke S, Friend R, Whittell G, Manners I 2018 Science 360 897
 Google Scholar
Google Scholar
[5] Wei J, Xu J, Bai X, Liu J 2015 Microsc. Microanal. 21 1685
 Google Scholar
Google Scholar
[6] Ghosh S, Giannini S, Lively K, Blumberger J 2020 Faraday Discuss. 221 501
[7] Drexler M J, Woscholski R, Lippert S, Stolz W, Rahimi-Iman A, Koch M 2014 Phys. Rev. B 90 195304
 Google Scholar
Google Scholar
[8] Averkiev N S, Korotchenkov A V, Kosobukin V A 2019 Semiconductors 53 1042
 Google Scholar
Google Scholar
[9] Rais D, Menšík M, Paruzel B, Kurunthu D, Pfleger J 2017 Phys. Chem. Chem. Phys. 19 10562
 Google Scholar
Google Scholar
[10] Warrier A R, Bingi J, Vijayan C 2016 Plasmonics 11 953
 Google Scholar
Google Scholar
[11] Sharma R, Khan P, Aneesh J, Sangunni K S, Csarnovics I, Kokenyesi S, Jain H, Adarsh K V 2016 APL Mater. 4 106105
 Google Scholar
Google Scholar
[12] Rossi D, Wang H, Dong Y, Qiao T, Qian X, Son D H 2018 ACS Nano 12 12436
 Google Scholar
Google Scholar
[13] Dey S, Zhao J 2016 J. Phys. Chem. Lett. 7 2921
 Google Scholar
Google Scholar
[14] Garagiola M, Osenda O 2020 Physica E 116 113755
 Google Scholar
Google Scholar
[15] Tahara H, Kanemitsu Y 2019 ChemNanoMat 5 977
 Google Scholar
Google Scholar
[16] Mehata M S, Ratnesh R K 2019 Dalton Trans. 48 7619
 Google Scholar
Google Scholar
[17] Bednarz M, Malyshev V A, Knoester J 2004 J. Chem. Phys. 120 3827
 Google Scholar
Google Scholar
[18] Yan Y A, Kühn O 2012 New J. Phys. 14 105004
 Google Scholar
Google Scholar
[19] Abramavicius D, Mukamel S 2011 J. Chem. Phys. 134 174504
 Google Scholar
Google Scholar
[20] 吴建军, 张晓华, 王煜, 王英特, 晋卫军 2005 第八届全国发光分析暨动力学分析学术研讨会论文集 太原, 中国, 2005年7月15—19日 第87页
Wu J J, Zhang X H, Wang Y, Wang Y T, Jin W J 2005 Proceedings of the 8th National Symposium on Luminescence analysis and Dynamic Analysis Taiyuan, China July 15–19, 2005 p87 (in Chinese)
[21] Qiu Y 2015 Synth. Met. 199 21
 Google Scholar
Google Scholar
[22] Sun Z, Stafström S 2013 J. Chem. Phys. 138 164905
 Google Scholar
Google Scholar
[23] Zhang Y L, Liu X J, Sun Z, An Z 2013 J. Chem. Phys. 138 174906
 Google Scholar
Google Scholar
[24] Meng Y, Di B, Liu X J, An Z, Wu C Q 2008 J. Chem. Phys. 128 184903
 Google Scholar
Google Scholar
[25] Johansson Å, Stafström S 2002 Phys. Rev. B 66 085208
 Google Scholar
Google Scholar
[26] Wang L, May V 2016 Phys. Rev. B 94 195413
 Google Scholar
Google Scholar
[27] Yamagata H, Spano F C 2012 J. Chem. Phys. 136 184901
 Google Scholar
Google Scholar
-
图 1 双分子链耦合的结构示意图 (a)
${\rm{H - H}}$ 型排列; (b)${\rm{H - J}}$ 型排列; (c)${\rm{J - H}}$ 型排列.$J_{mn} $ 为分子间的耦合作用矩阵元Fig. 1. Schematic diagram of the coupling of two molecular chains: (a)
${\rm{H - H}}$ type; (b)${\rm{H - J}}$ type; (c)${\rm{J - H}}$ type.$J_{mn} $ is the matrix element of intermolecular coupling action.图 2 双分子链同时受到激发时, 链内总占据数
$\mathop P\nolimits_{{\rm{tot}}} $ 随激发场频率$\hbar \mathop \omega \nolimits_0 $ 变化曲线.每条链内有10个全同分子,$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 2\;{\rm{ ps}}$ ,$\mathop E\nolimits_0 = 5 \times {10^6}\;{{{\rm{ V}}} / {\rm{m}}}$ . 其中黑色实线表示$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ , 红色虚线表示$\mathop \varDelta \nolimits_{{\rm{chain}}} = 2.5\;{\rm{ nm}}$ , 蓝色点划线表示$\mathop \varDelta \nolimits_{{\rm{chain}}} = {\rm{3}}{\rm{.5\;nm}}$ . 分子链位形为 (a)${\rm{H - H}}$ 型; (b)${\rm{H - J}}$ 型; (c)${\rm{J - H}}$ 型Fig. 2. Curves of the total population in the chain
$\mathop P\nolimits_{{\rm{tot}}} $ varying with the frequency of the excitation field$\hbar \mathop \omega \nolimits_0 $ with the two molecular chain simultaneously excited: (a)${\rm{H - H}}$ type; (b)${\rm{H - J}}$ type; (c)${\rm{J - H}}$ type. There are 10 identical molecules in each chain,$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 2\;{\rm{ ps}}$ ,$\mathop E\nolimits_0 = 5 \times {10^6}\;{{{\rm{ V}}} / {\rm{m}}}$ . The black solid line is$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ , the red dotted line is$\mathop \varDelta \nolimits_{{\rm{chain}}} = 2.5\;{\rm{ nm}}$ and the blue stipple line is$\mathop \varDelta \nolimits_{{\rm{chain}}} = {\rm{3}}{\rm{.5\; nm}}$ .图 3 H型单分子链受到激发时, 分子激发态的电荷占据演变.
$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 20\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = 5 \times {10^7}\;{{\rm{V}} / {\rm{m}}}$ Fig. 3. Charge population evolution of excited molecular with monomolecular chain of H-type excited.
$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 20\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = 5 \times {10^7}\;{{\rm{V}} / {\rm{m}}}$ .图 4 H-H型双分子链同时受到激发时, 分子激发态的电荷占据演变.
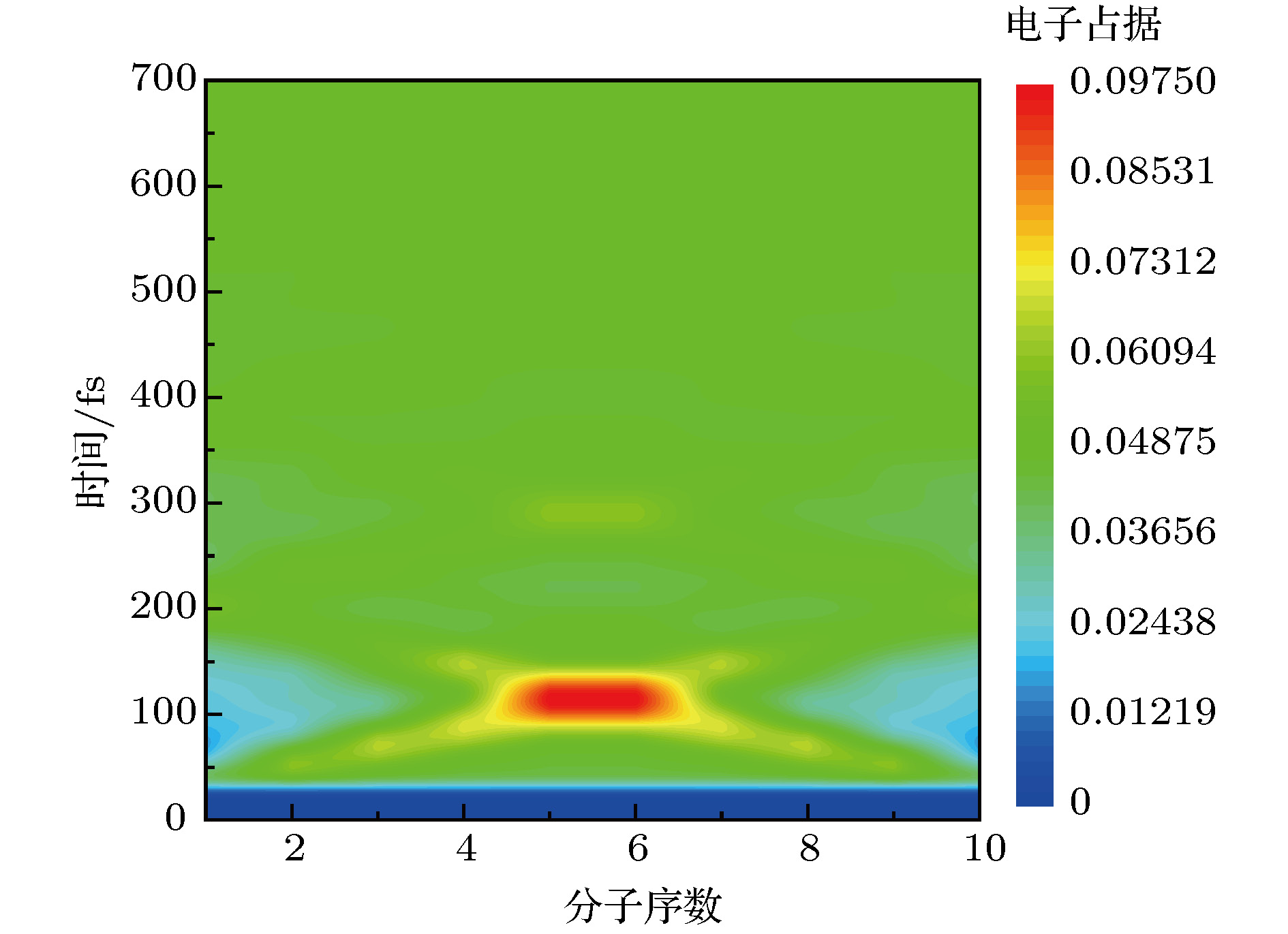
$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 20\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = 5 \times {10^7}\;{{\rm{V}} / {\rm{m}}}$ ,$\mathop \omega \nolimits_0 = 2.6\;{\rm{ eV}}$ Fig. 4. Charge population evolution of molecular excited states with two molecular chains of H-H type excited simultaneously.
$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 20\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = 5 \times {10^7}\;{{\rm{V}} / {\rm{m}}}$ ,$\mathop \omega \nolimits_0 = 2.6\;{\rm{ eV}}$ .图 5 H-H双分子链在不同共振能级下同时激发时, 分子激发态的电荷占据演变.
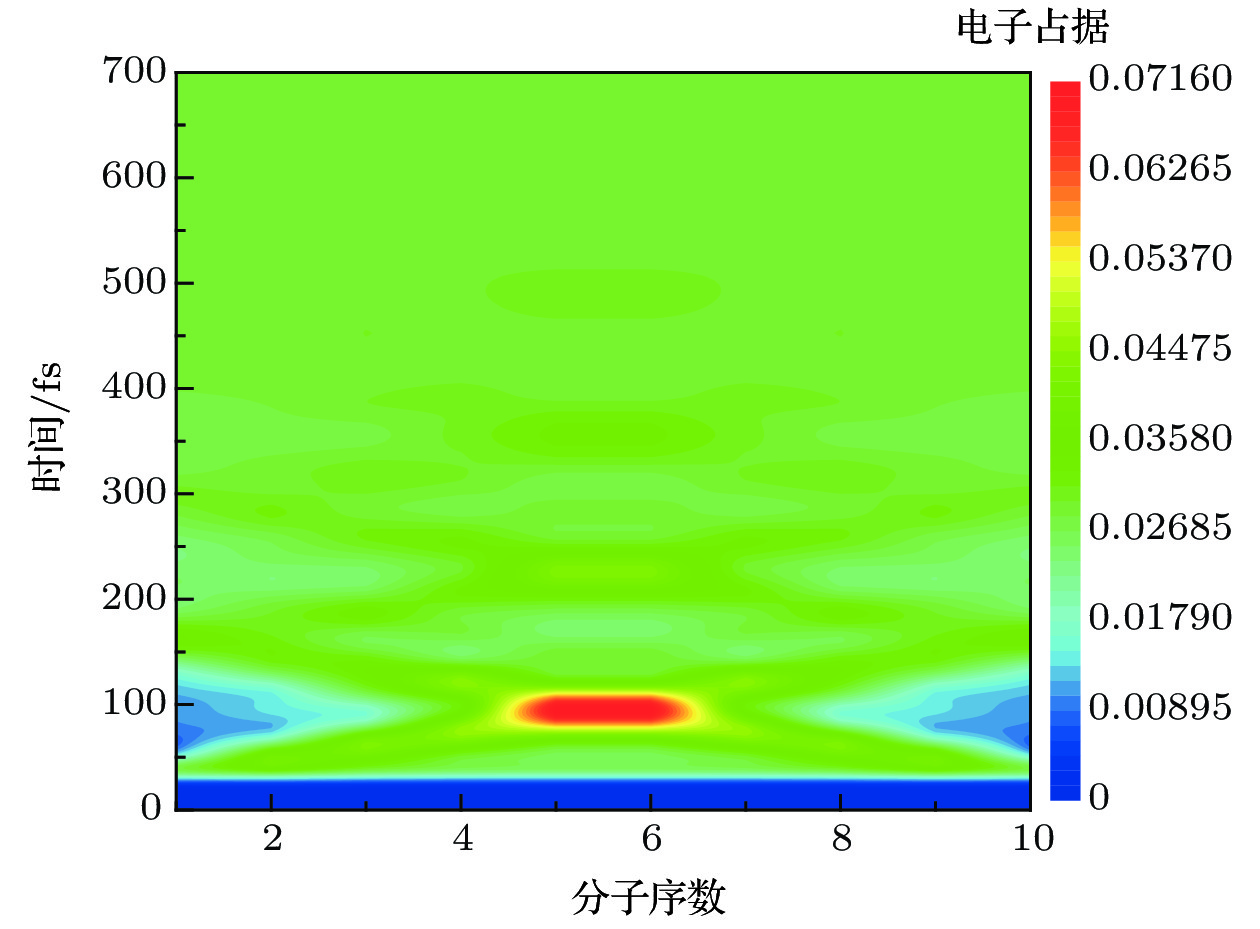
$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 1000\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = {10^6}\;{{\rm{V}} / {\rm{m}}}$ . (a)$\mathop \omega \nolimits_0 = 2.67\;{\rm{ eV}}$ ; (b)$\mathop \omega \nolimits_0 = 2.64\;{\rm{ eV}}$ ; (c)$\mathop \omega \nolimits_0 = 2.61\;{\rm{ eV}}$ ; (d)$\mathop \omega \nolimits_0 = 2.58\;{\rm{ eV}}$ Fig. 5. Charge population evolution of molecular excited states with two molecular chains of H-H type excited simultaneously at different resonance levels.
$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 1000\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = {10^6}\;{{\rm{V}} / {\rm{m}}}$ : (a)$\mathop \omega \nolimits_0 = 2.67\;{\rm{ eV}}$ ; (b)$\mathop \omega \nolimits_0 = 2.64\;{\rm{ eV}}$ ; (c)$\mathop \omega \nolimits_0 = 2.61\;{\rm{ eV}}$ ; (d)$\mathop \omega \nolimits_0 = 2.58\;{\rm{ eV}}$ 图 6
${\rm{H - H}}$ 型双分子链只有第一条链受到激发时, 分子激发态的电荷占据演变.$\mathop \varDelta \nolimits_{{\rm{mol}}} = 1.2\;{\rm{ nm}}$ ,$\mathop \tau \nolimits_{\rm{p}} = 20\;{\rm{ fs}}$ ,$\mathop E\nolimits_0 = 5 \times {10^7}\;{{\rm{V}}/ {\rm{m}}}$ . 其中, 第一行的图中分子链间距$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ ; 第二行的图中分子链间距$\mathop \varDelta \nolimits_{{\rm{chain}}} = 2.5\;{\rm{ nm}}$ ; 第三行中分子链间距$\mathop \varDelta \nolimits_{{\rm{chain}}} = 3.5\;{\rm{ nm}}$ ; (a) 列为被激发的分子链; (b) 列为未被激发的分子链; (c) 列为两条链各自的总占据数. 在(a) 列和(b) 列中的1—5分别对应分子链中第1—5个分子, 由于分子链关于分子中心对称, 图中只给出了5个分子的动力学演化过程Fig. 6. Charge population evolution of molecular excited states with only first chain excited in two molecular chains of H-H type. The spacing of the molecular chains in the first row is
$\mathop \varDelta \nolimits_{{\rm{chain}}} = 1.5\;{\rm{ nm}}$ ; The spacing of the molecular chains in the second row is$\mathop \varDelta \nolimits_{{\rm{chain}}} = 2.5\;{\rm{ nm}}$ ; The spacing of molecular chains in the third row is$\mathop \varDelta \nolimits_{{\rm{chain}}} = 3.5\;{\rm{ nm}}$ ; Column (a) is the chain of molecules that are excited; Column (b) is the unexcited molecular chain; Column (c) is the total number of the two chains. The 1—5 in Column (a) and (b) correspond to the 1—5 molecules in the molecular chain. Because the molecular chain is symmetric about the center of the molecular chain, the dynamic evolution process of only 5 molecules is shown in the figure.表 1 参数列表(具体说明见正文)
Table 1. Parameter list (see text for details).
$\mathop N\nolimits_{{\rm{mol}}} $ 20 $\mathop d\nolimits_{{\rm{mol}}} $ 8 D $\mathop E\nolimits_{{\rm{mol}}} $ $2.6\;{\rm{ eV}}$ $\mathop {\hbar k}\nolimits_{{\rm{mol}}} $ $3\;{\rm{ meV}}$ $\mathop \varDelta \nolimits_{ {\rm{mol} } }$ $1.2\;{\rm{ nm}}$ $\mathop \varDelta \nolimits_{ {\rm{chain} } }$ $1.5, \;2.5, \;3.5\;{\rm{ nm}}$ $\mathop E\nolimits_{\rm{0}} $ $\mathop {10}\nolimits^6 - \mathop {10}\nolimits^8\; {\rm{ }}{{\rm{V}} / {\rm{m}}}$ $\mathop \tau \nolimits_{\rm{p}} $ $20\;{\rm{ fs}} - 2\;{\rm{ ps}}$ -
[1] Kattoor V, Awasthi K, Jokar E, Diau E W G, Ohta N 2018 J. Phys. Chem. C 122 26623
 Google Scholar
Google Scholar
[2] Stiehm T, Schneider R, Kern J, Niehues I, Michaelis de Vasconcellos S, Bratschitsch R 2019 Rev. Sci. Instrum. 90 083102
 Google Scholar
Google Scholar
[3] Barkaoui H, Abid H, Yangui A, Triki S, Boukheddaden K, Abid Y 2018 J. Phys. Chem. C 122 24253
 Google Scholar
Google Scholar
[4] Jin X H, Price M, Finnegan J, Boott C, Richter J, Rao A, Menke S, Friend R, Whittell G, Manners I 2018 Science 360 897
 Google Scholar
Google Scholar
[5] Wei J, Xu J, Bai X, Liu J 2015 Microsc. Microanal. 21 1685
 Google Scholar
Google Scholar
[6] Ghosh S, Giannini S, Lively K, Blumberger J 2020 Faraday Discuss. 221 501
[7] Drexler M J, Woscholski R, Lippert S, Stolz W, Rahimi-Iman A, Koch M 2014 Phys. Rev. B 90 195304
 Google Scholar
Google Scholar
[8] Averkiev N S, Korotchenkov A V, Kosobukin V A 2019 Semiconductors 53 1042
 Google Scholar
Google Scholar
[9] Rais D, Menšík M, Paruzel B, Kurunthu D, Pfleger J 2017 Phys. Chem. Chem. Phys. 19 10562
 Google Scholar
Google Scholar
[10] Warrier A R, Bingi J, Vijayan C 2016 Plasmonics 11 953
 Google Scholar
Google Scholar
[11] Sharma R, Khan P, Aneesh J, Sangunni K S, Csarnovics I, Kokenyesi S, Jain H, Adarsh K V 2016 APL Mater. 4 106105
 Google Scholar
Google Scholar
[12] Rossi D, Wang H, Dong Y, Qiao T, Qian X, Son D H 2018 ACS Nano 12 12436
 Google Scholar
Google Scholar
[13] Dey S, Zhao J 2016 J. Phys. Chem. Lett. 7 2921
 Google Scholar
Google Scholar
[14] Garagiola M, Osenda O 2020 Physica E 116 113755
 Google Scholar
Google Scholar
[15] Tahara H, Kanemitsu Y 2019 ChemNanoMat 5 977
 Google Scholar
Google Scholar
[16] Mehata M S, Ratnesh R K 2019 Dalton Trans. 48 7619
 Google Scholar
Google Scholar
[17] Bednarz M, Malyshev V A, Knoester J 2004 J. Chem. Phys. 120 3827
 Google Scholar
Google Scholar
[18] Yan Y A, Kühn O 2012 New J. Phys. 14 105004
 Google Scholar
Google Scholar
[19] Abramavicius D, Mukamel S 2011 J. Chem. Phys. 134 174504
 Google Scholar
Google Scholar
[20] 吴建军, 张晓华, 王煜, 王英特, 晋卫军 2005 第八届全国发光分析暨动力学分析学术研讨会论文集 太原, 中国, 2005年7月15—19日 第87页
Wu J J, Zhang X H, Wang Y, Wang Y T, Jin W J 2005 Proceedings of the 8th National Symposium on Luminescence analysis and Dynamic Analysis Taiyuan, China July 15–19, 2005 p87 (in Chinese)
[21] Qiu Y 2015 Synth. Met. 199 21
 Google Scholar
Google Scholar
[22] Sun Z, Stafström S 2013 J. Chem. Phys. 138 164905
 Google Scholar
Google Scholar
[23] Zhang Y L, Liu X J, Sun Z, An Z 2013 J. Chem. Phys. 138 174906
 Google Scholar
Google Scholar
[24] Meng Y, Di B, Liu X J, An Z, Wu C Q 2008 J. Chem. Phys. 128 184903
 Google Scholar
Google Scholar
[25] Johansson Å, Stafström S 2002 Phys. Rev. B 66 085208
 Google Scholar
Google Scholar
[26] Wang L, May V 2016 Phys. Rev. B 94 195413
 Google Scholar
Google Scholar
[27] Yamagata H, Spano F C 2012 J. Chem. Phys. 136 184901
 Google Scholar
Google Scholar
计量
- 文章访问数: 7247
- PDF下载量: 71
- 被引次数: 0






















 下载:
下载: