-
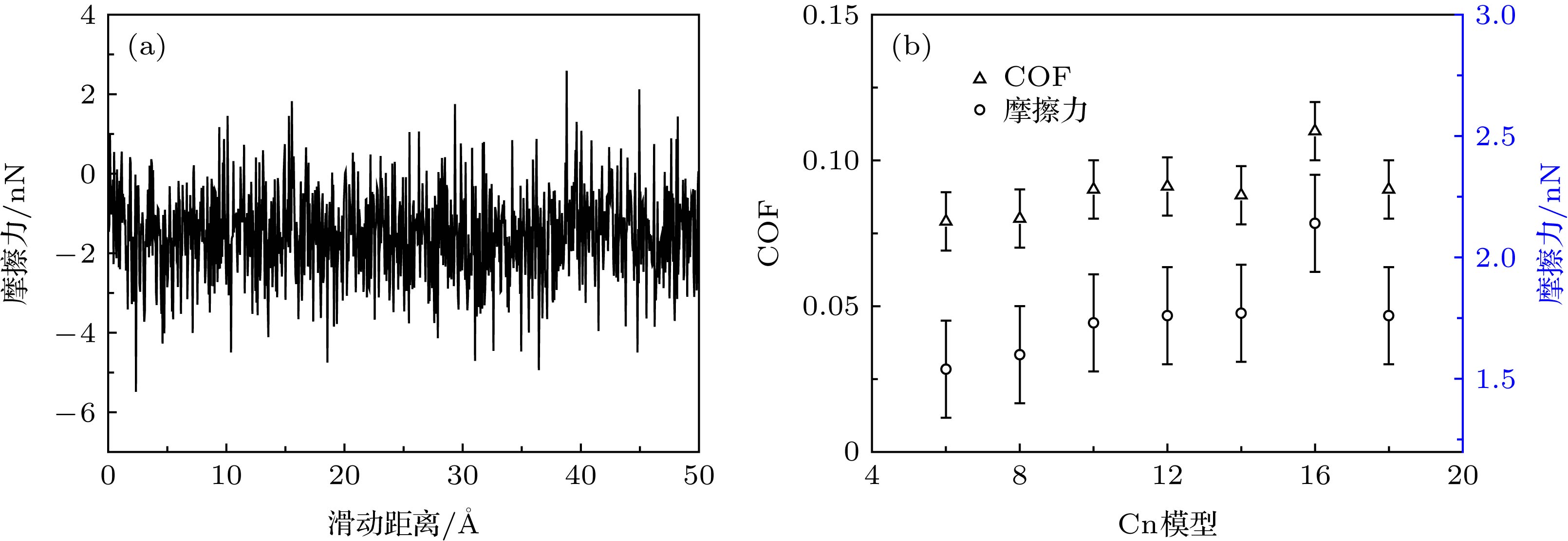
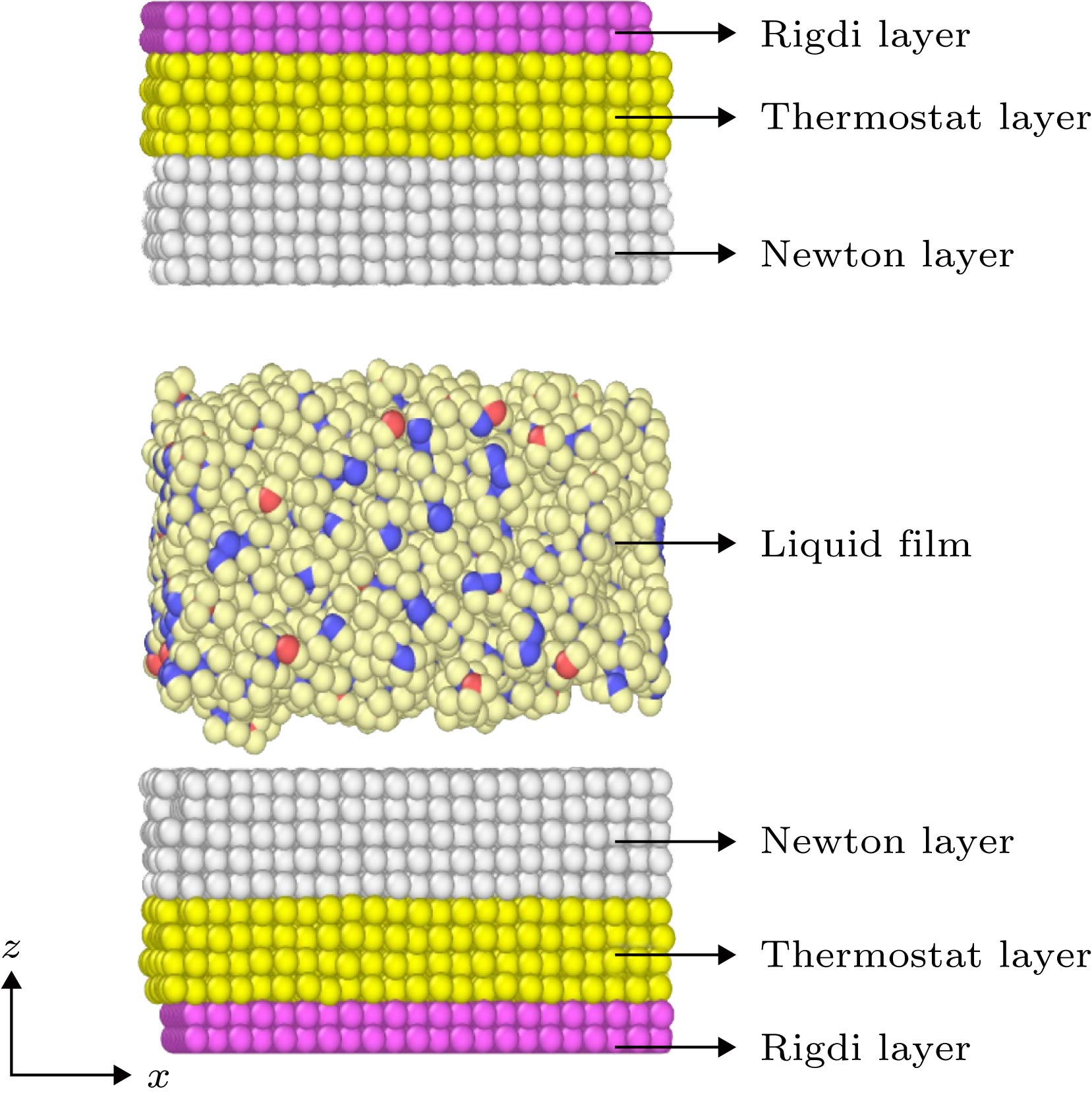
采用分子动力学方法, 模拟了两块金[111]基板及其间由不同链长的直链烷烃CnH2n + 2 (n = 6, 8, 10, 12, 14, 16, 18)组成的7种纯液体膜及6种混合分子液体膜的摩擦行为, 分析了分子链长对薄膜摩擦性质的影响以及滑动过程中的膜的结构变化机制. 结果表明: 在纯液体膜中, 十六烷液体膜的摩擦力最大; 碳原子数 n > 8时, 液体膜摩擦性质随着分子链长的增加而保持稳定. 在C6H14与CnH2n + 2的1∶1混合液体膜中, 己烷与十二烷混合液体膜的摩擦最大; 当长链分子CnH2n + 2的碳原子数n > 12时, 混合膜的摩擦性质较为稳定; 烷烃分子的碳原子数n > 10时, 加入短链分子会增强膜的摩擦. 滑动过程中在基板表面附近形成的多层高致密性分层是降低摩擦的主要原因, 单层或无分层结构导致较高摩擦. 液体膜与基板间相互作用对摩擦有贡献, 摩擦力主要来自膜内粘滞作用.How to overcome the friction between the micro components has become a key point of the successful operation of the micro/nano-electric mechanical systems. The understanding of the friction mechanism of the alkane liquid film confined between two substrates is important when the friction law on a macro/nano scale is not applicable. In this work, the molecular dynamics simulations are used to study the effect of the chain length on the friction properties of the liquid films that are confined between two golden substrates. There are seven pure alkane liquid films that are composed of one molecule CnH2n + 2(n = 6, 8, 10, 12, 14, 16, 18), and six mixed alkane liquid films that are composed of two molecules C6H14/CnH2n + 2(n = 8, 10, 12, 14, 16, 18) with a ratio of 1∶1. The results show that the friction force and the coefficient of friction of pure alkane liquid films both increase as the chain length increases when the carbon atom number is less than 12, whereas the friction property keeps stable when the carbon atom number of the alkane molecule is greater than 10 and the pure hexadecane liquid film has the largest friction force. In the mixed films, the addition of short chain alkane molecules can strengthen the friction, and the hexane/dodecane mixed film has the maximum friction force. The short chain molecule dilutes the C8H18 film and C10H22 film which cause the friction force to decrease. During the sliding progress, the formation of solid-like high density-packet layers is the main reason for the friction reduction. When no solid-like layer or just one solid-like layer is formed at the interface of golden base, the liquid alkane film is liquid-like and its viscosity becomes much larger than that in the normal state, which leads to high friction force. The short chain molecules reduce the density of the solid-like layers, which causes the film to transform from solid-like state to liquid state, thus resulting in the increase of friction. The friction property mainly depends on the layered structure, and the interaction between the golden surface and liquid film contributes to the friction. This study helps to understand the friction mechanism of ultra-thin liquid films.
-
Keywords:
- nanotribology /
- liquid film /
- chain length /
- structure
[1] Lewis J B, Vilt S G, Rivera J L, Jennings G K, Mccabe C 2012 Langmuir 28 14218
 Google Scholar
Google Scholar
[2] Yang G, Jin F, Yu L, Zhang P 2015 Tribo. Lett. 57 12
 Google Scholar
Google Scholar
[3] Cheng H, Hu Y 2012 Adv. Colloid Interface Sci. 171–172 53
 Google Scholar
Google Scholar
[4] 潘鹤, 滕淑华, 丁翠翠 2012 物理化学学报 28 917
 Google Scholar
Google Scholar
Pan H, Teng S H, Ding C C 2012 Acta Phys. Chim. Sin. 28 917
 Google Scholar
Google Scholar
[5] Mcdermott M T, Green J B D, Porter M D 1997 Langmuir 13 25040
[6] Booth B D, Vilt S G, Mccabe C, Jennings G K 2009 Langmuir 25 9995
 Google Scholar
Google Scholar
[7] 刘蕾, 宋仕永, 张平余 2012 物理化学学报 28 427
 Google Scholar
Google Scholar
Liu L, Song S Y, Zhang P Y 2012 Acta Phys. Chim. Sin. 28 427
 Google Scholar
Google Scholar
[8] 张兆慧, 韩奎, 曹娟, 王帆, 杨丽娟 2012 61 028701
 Google Scholar
Google Scholar
Zhang Z H, Han K, Cao J, Wang F, Yang L J 2012 Acta Phys. Sin. 61 028701
 Google Scholar
Google Scholar
[9] Gosvami N N, Egberts P, Bennewitz R 2011 J. Phys. Chem. A 115 6942
 Google Scholar
Google Scholar
[10] Granick S 1991 Science 253 1374
 Google Scholar
Google Scholar
[11] Cui S T, Cummings P T, Cochran H D 2001 Fluid Phase Equilibria 183–184 381
 Google Scholar
Google Scholar
[12] Vasko A A, Kutsenko V Y, Marchenko A A, Braun O M 2019 Tribo. Lett. 67 49
 Google Scholar
Google Scholar
[13] Ewen J P, Gattinoni C, Zhang J, Heyes D M, Spikes H A, Dini D 2017 Phys. Chem. Chem. Phys. 19 17883
 Google Scholar
Google Scholar
[14] Jorgensen W L, Tirado-Rives J 1988 J. Am. Chem. Soc. 110 1657
 Google Scholar
Google Scholar
[15] Maxwell D S, Tirado-Rives J, Jorgensen W L 1996 J. Am. Chem. Soc. 118 11225
 Google Scholar
Google Scholar
[16] Damm W, Frontera A, Tirado-Rives J, Jorgensen L W 1997 J. Comp. Chem. 18 1955
 Google Scholar
Google Scholar
[17] 张兆慧, 李海鹏, 韩奎 2013 62 158701
 Google Scholar
Google Scholar
Zhang Z H, Li H P, Han K 2013 Acta Phys. Sin. 62 158701
 Google Scholar
Google Scholar
[18] Jiang B W, Keffer J D, Edwards J B 2006 J. Fluorine Chem. 127 787
 Google Scholar
Google Scholar
[19] Daw M S, Baskes M I 1984 Phys. Rev. B 29 6443
 Google Scholar
Google Scholar
[20] Savio D, Fillot N, Vergne P, Zaccheddu M 2012 Tribo. Lett. 46 11
 Google Scholar
Google Scholar
[21] Plimpton S 1995 J. Comp. Phys. 117 1
 Google Scholar
Google Scholar
[22] Sabzevari S M, Mcgraw J D, Woodadams P 2016 RSC Adv. 6 91163
 Google Scholar
Google Scholar
[23] Mazyar O A, Jennings G K, Mccabe C 2009 Langmuir 25 5103
 Google Scholar
Google Scholar
-
表 1 纯液体膜中上基板与液体膜间相互作用 (kJ/mol)
Table 1. Interaction between upper substrate and liquid film (kJ/mol)
模型 C6 C8 C10 C12 C14 C16 C18 作用能 2285 2434 2438 2484 2496 2408 2488 表 2 混合液体膜中上基板与液体膜间相互作用(kJ/mol)
Table 2. Interaction between upper substrate and mixed liquid film (kJ/mol).
模型 C6C8 C6C10 C6C12 C6C14 C6C16 C6C18 作用能 2396 2396 2893 2438 2400 2505 -
[1] Lewis J B, Vilt S G, Rivera J L, Jennings G K, Mccabe C 2012 Langmuir 28 14218
 Google Scholar
Google Scholar
[2] Yang G, Jin F, Yu L, Zhang P 2015 Tribo. Lett. 57 12
 Google Scholar
Google Scholar
[3] Cheng H, Hu Y 2012 Adv. Colloid Interface Sci. 171–172 53
 Google Scholar
Google Scholar
[4] 潘鹤, 滕淑华, 丁翠翠 2012 物理化学学报 28 917
 Google Scholar
Google Scholar
Pan H, Teng S H, Ding C C 2012 Acta Phys. Chim. Sin. 28 917
 Google Scholar
Google Scholar
[5] Mcdermott M T, Green J B D, Porter M D 1997 Langmuir 13 25040
[6] Booth B D, Vilt S G, Mccabe C, Jennings G K 2009 Langmuir 25 9995
 Google Scholar
Google Scholar
[7] 刘蕾, 宋仕永, 张平余 2012 物理化学学报 28 427
 Google Scholar
Google Scholar
Liu L, Song S Y, Zhang P Y 2012 Acta Phys. Chim. Sin. 28 427
 Google Scholar
Google Scholar
[8] 张兆慧, 韩奎, 曹娟, 王帆, 杨丽娟 2012 61 028701
 Google Scholar
Google Scholar
Zhang Z H, Han K, Cao J, Wang F, Yang L J 2012 Acta Phys. Sin. 61 028701
 Google Scholar
Google Scholar
[9] Gosvami N N, Egberts P, Bennewitz R 2011 J. Phys. Chem. A 115 6942
 Google Scholar
Google Scholar
[10] Granick S 1991 Science 253 1374
 Google Scholar
Google Scholar
[11] Cui S T, Cummings P T, Cochran H D 2001 Fluid Phase Equilibria 183–184 381
 Google Scholar
Google Scholar
[12] Vasko A A, Kutsenko V Y, Marchenko A A, Braun O M 2019 Tribo. Lett. 67 49
 Google Scholar
Google Scholar
[13] Ewen J P, Gattinoni C, Zhang J, Heyes D M, Spikes H A, Dini D 2017 Phys. Chem. Chem. Phys. 19 17883
 Google Scholar
Google Scholar
[14] Jorgensen W L, Tirado-Rives J 1988 J. Am. Chem. Soc. 110 1657
 Google Scholar
Google Scholar
[15] Maxwell D S, Tirado-Rives J, Jorgensen W L 1996 J. Am. Chem. Soc. 118 11225
 Google Scholar
Google Scholar
[16] Damm W, Frontera A, Tirado-Rives J, Jorgensen L W 1997 J. Comp. Chem. 18 1955
 Google Scholar
Google Scholar
[17] 张兆慧, 李海鹏, 韩奎 2013 62 158701
 Google Scholar
Google Scholar
Zhang Z H, Li H P, Han K 2013 Acta Phys. Sin. 62 158701
 Google Scholar
Google Scholar
[18] Jiang B W, Keffer J D, Edwards J B 2006 J. Fluorine Chem. 127 787
 Google Scholar
Google Scholar
[19] Daw M S, Baskes M I 1984 Phys. Rev. B 29 6443
 Google Scholar
Google Scholar
[20] Savio D, Fillot N, Vergne P, Zaccheddu M 2012 Tribo. Lett. 46 11
 Google Scholar
Google Scholar
[21] Plimpton S 1995 J. Comp. Phys. 117 1
 Google Scholar
Google Scholar
[22] Sabzevari S M, Mcgraw J D, Woodadams P 2016 RSC Adv. 6 91163
 Google Scholar
Google Scholar
[23] Mazyar O A, Jennings G K, Mccabe C 2009 Langmuir 25 5103
 Google Scholar
Google Scholar
计量
- 文章访问数: 8759
- PDF下载量: 65
- 被引次数: 0













 下载:
下载: