-
MgO是具有强极性的离子化合物, 电场诱导MgO吸附H2是一种有效的储氢方法, 但外加的电场很强, 如何降低所需电场的强度是需要解决的关键问题. 本文在密度泛函理论水平上研究了电场中H2在(MgO)4团簇上的吸附性质. 结果表明(MgO)4能承受强电场并保持立方结构, 可用于电场储氢. 电荷分析表明(MgO)4在电场中被极化, 其偶极矩增大为场强0.005 a.u. 和0.010 a.u.时的1.67和3.33 Debye. H2能稳定吸附在单个Mg/O原子上. H2在Mg上为侧位吸附, 而在O上为端位吸附. 外加电场可提高其吸附强度. 仅需0.010 a. u.的外电场, 就可使H2在Mg/O上的吸附能由无电场时–0.118/–0.060 eV提高到–0.225/–0.150 eV. 所需电场强度小于较大尺寸的 (MgO)9团簇, 表明降低团簇尺寸是减少所需电场强度的一种可能方式. 利用QTAIM方法研究了H2与(MgO)4间的弱相互作用, 表明电场使团簇及氢分子极化, 从而增强了其间的静电作用. 当团簇尺寸降低时, 更多的原子位于表面, 且具有较低的配位数, 更容易被极化, 因此储氢所需的电场强度更低. 电场中(MgO)4中最多能吸附16个H2, 相应的质量密度为16.7 wt%, 表明(MgO)4是一种可能的电场储氢材料.MgO is a typical ionic compound with strong polarity. Hydrogen absorbed by MgO materials subjected to an external electric field is a potential method to store hydrogen. However, the method requires an extremely high intensity of electric field, which is difficult to achieve. Therefore, reducing field intensity has become a key problem in the field of hydrogen storage. In this paper, the hydrogen storage properties of an (MgO)4 cluster under an external electric field are investigated. The results show that under the external electric field, (MgO)4 keeps the frame of cube structure but with little distortion, which implies that (MgO)4 cluster can sustain the strong electric field. The (MgO)4 is also polarized by the external electric field and its dipole momentum increases to 1.67 and 3.33 Debye when the field intensity is 0.005 and 0.010 a.u., respectively. H2 can be adsorbed on a single Mg/O atom: H2 is adsorbed at lateral position of Mg atom, while at the top of O atom. The adsorption strength is substantially enhanced under an external electric field. Under only 0.010 a. u. of electric field, the adsorption energy of H2 on the Mg or O atoms increases from –0.118 eV to –0.225 eV or from –0.060 eV to –0.150 eV, respectively. The electric field required is significantly lower than that of a large (MgO)9 cluster. This result suggests that reducing the size of material is a possible method toreduce the electric field for hydrogen storage in polarizable materials. The weak interaction between H2 and (MgO)4 is analyzed by the quantum theory of atoms in molecules. The results indicate that under an electric field, (MgO)4 and H2 are effectively polarized, and the electrostatic interaction between them is subsequently enhanced. Meanwhile, the small cluster is easily polarized because most of the atoms are in the surface and have low coordination. Therefore, the electric field required can be substantially reduced. Moreover, (MgO)4 adsorbs 16 H2 molecules at most. The corresponding mass density of hydrogen storage reaches 16.7 wt%, indicating that (MgO)4 can be used as a hydrogen storage material under an electric field.
-
Keywords:
- electric field /
- (MgO)4 /
- hydrogen storage /
- electronic structure
[1] Lubitz W, Tumas W 2007 Chem. Rev. 107 3900
 Google Scholar
Google Scholar
[2] Yu X, Tang Z, Sun D, Ouyang L, Zhu M 2017 Prog. Mater. Sci. 88 1
 Google Scholar
Google Scholar
[3] Ren J, Musyoka N M, Langmi H W, Mathe M, Liao S 2017 Int. J. Hydrogen Energy 42 289
 Google Scholar
Google Scholar
[4] Jena P 2011 J. Phys. Chem. Lett. 2 206
 Google Scholar
Google Scholar
[5] Bhatia S K, Myers A L 2006 Langmuir 22 1688
 Google Scholar
Google Scholar
[6] Ao Z M, Hernandez-Nieves A D, Peeters F M, Li S 2012 Phys. Chem. Chem. Phys. 14 1463
 Google Scholar
Google Scholar
[7] Guo J H, Zhang H 2011 Struct. Chem. 22 1039
 Google Scholar
Google Scholar
[8] Zhou J, Wang Q, Sun Q, Jena P, Chen X S 2010 Proc. Natl. Acad. Sci. 107 2801
 Google Scholar
Google Scholar
[9] Sun X, Jiann Y H, Shang Z S 2010 J. Phys. Chem. C 114 7178
 Google Scholar
Google Scholar
[10] Ao Z M, Peeters F M 2010 J. Phys. Chem. C 114 14503
[11] Liu W, Zhao Y H, Nguyen J, Li Y, Jiang Q, Lavernia E J 2009 Carbon 47 3452
 Google Scholar
Google Scholar
[12] Wu G, Zhang J, Wu Y, Li Q, Chou K, Bao X 2009 J. Alloys Compd. 480 788
 Google Scholar
Google Scholar
[13] Dawoud J N, Sallabi A K, Fasfous I I, Jack D B 2009 e-J. Surf. Sci. Nanotechnol. 7 207
 Google Scholar
Google Scholar
[14] Larese J Z, Frazier L, Adams M A, Arnold T, Hinde R J, Ramirez-Cuesta A 2006 Phys. B: Cond. Matter 385 144
[15] Skofronick J G, Toennies J P, Traeger F, Weiss H 2003 Phys. Rev. B 67 035413
 Google Scholar
Google Scholar
[16] Sawabe K, Koga N, Morokuma K, Iwasawa Y 1994 J. Chem. Phys. 101 4819
 Google Scholar
Google Scholar
[17] Zhang G, Sheng Y 2018 Chin. Phys. B 27 093601
[18] Kwapien K, Sierka M, Dbler J, Sauer J 2010 Chem. Cat. Chem. 2 819
[19] 陈宏善, 陈华君 2011 60 073601
 Google Scholar
Google Scholar
Chen H S, Chen H J 2011 Acta Phys. Sin. 60 073601
 Google Scholar
Google Scholar
[20] Yin Y H, Chen H S 2016 Comput. Theor. Chem. 108 1
[21] Roberts C, Johnston R L 2001 Phys. Chem. Chem. Phys. 3 5024
 Google Scholar
Google Scholar
[22] de La Puente E, Aguado A, Ayuela A, López J M 1997 Phys. Rev. B 56 7607
 Google Scholar
Google Scholar
[23] Ziemann P J, Castleman Jr A W 1991 J. Chem. Phys. 94 718
 Google Scholar
Google Scholar
[24] Becke A D 1993 J. Chem. Phys. 98 5648
 Google Scholar
Google Scholar
[25] Miehlich B, Savin A, Stoll H, Preuss H 1989 Chem. Phys. Lett. 157 200
 Google Scholar
Google Scholar
[26] Ditchfield R, Hehre W J, Pople J A 1971 J. Chem. Phys. 54 724
 Google Scholar
Google Scholar
[27] Frisch M J, Trucks G, Schlegel H, et al. 2013 Gaussian09 Revision D.01 Wallingford CT: Gaussian, Inc.
[28] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[29] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[30] Castleman Jr A W, Khanna S N 2009 J. Phys. Chem. C 113 2664
-
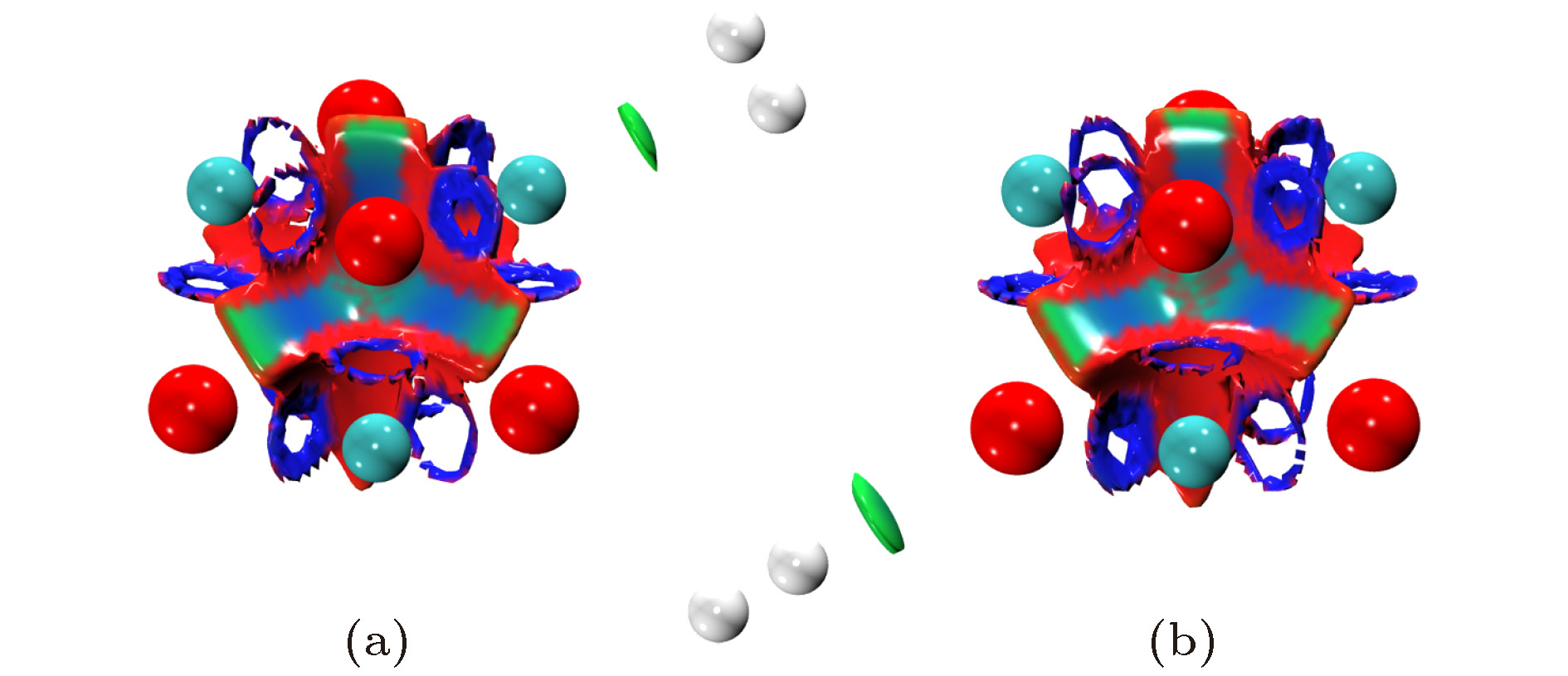
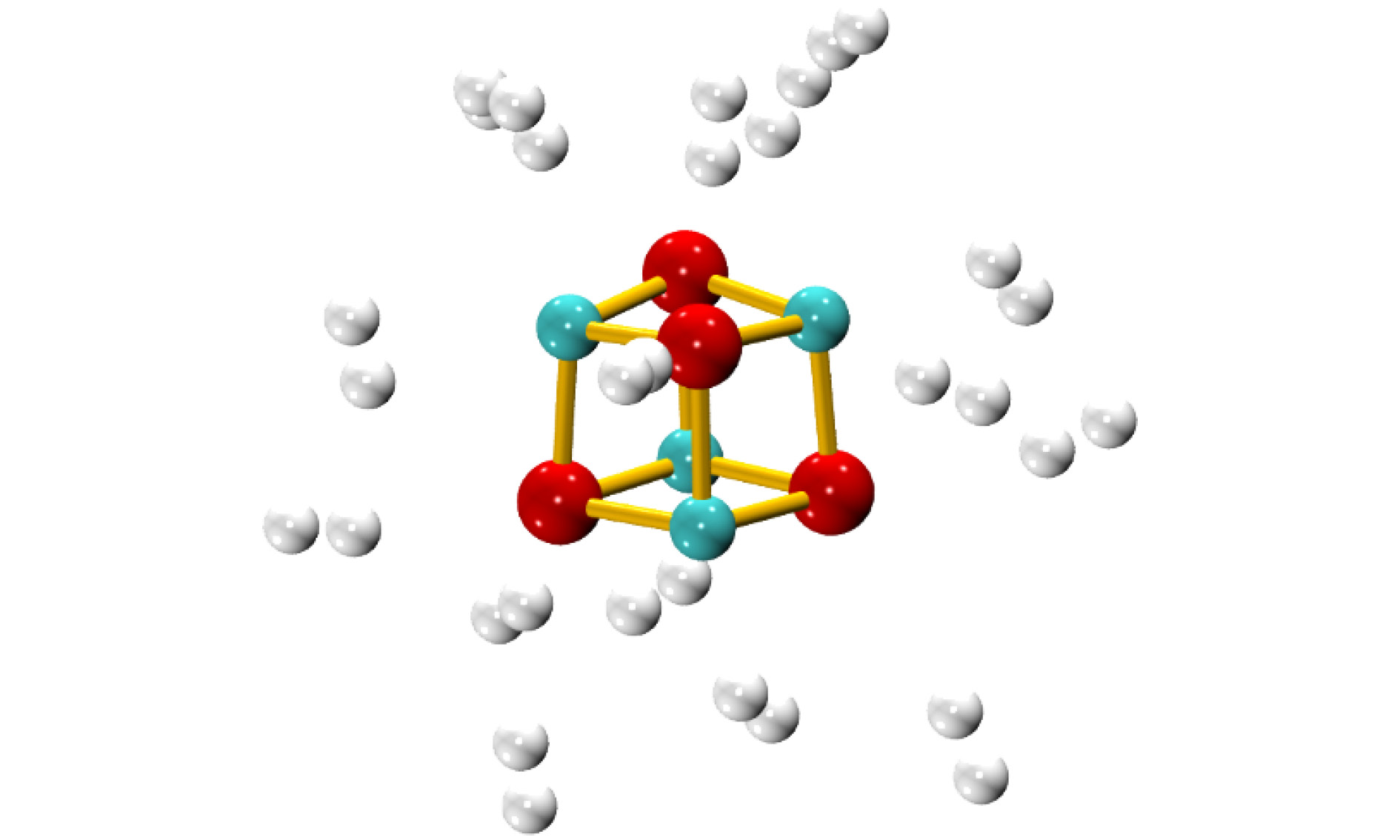
图 1 不同场强下(MgO)4的稳定结构(原子颜色与电荷得失相关, 由蓝色至红色表示失电子越多到得电子越多)
Fig. 1. The stable structures of (MgO)4 under the electric fields with different intensities(the color is correlated with the gaining or losing of electrons, from blue to red, it represents the variation of from the losing to obtaining electrons).
表 1 不同场强下(MgO)4中两类Mg/O原子的NPA 电荷(e)及Mg—O键长(Å) (QMgI/QMgII和QOI/QOII分别是Mg2, Mg4, Mg8/Mg6和O3, O5, O7/O1上的电荷; Mg—O键长RI = R12 = R14 = R18 = R63 = R65 = R67, 而RII = R23 = R25 = R43 = R47 = R85 = R87)
Table 1. The NPA charges for the two types of Mg/O atoms and the Mg—O distances (QMgI/QMgII and QOI/QOII are the charges of Mg2, Mg4, Mg8/Mg6 and O3, O5, O7/O1, respectively. The Mg—O distances RI = R12 = R14 = R18 = R63 = R65 = R67, while RII = R23 = R25 = R43= R47 = R85 = R87).
F/a.u. QMgI/QMgII QOI/QOII RI RII 0 1.493/1.493 –1.493/–1.493 1.96 1.96 0.005 1.476/1.527 –1.484/–1.503 1.94 1.97 0.010 1.460/1.554 –1.474/–1.512 1.93 1.99 表 2 H2在(MgO)4上的吸附能、到团簇距离RH-Mg/O, H—H键长RH—H及H和H2的NPA 电荷
Table 2. The adsorption energies Ea, H—H bond lengths RH—H, distances between H2 and cluster RH2-Mg/O and NPA charges of H atoms and H2 for (MgO)4H2
Site F/a. u. Ea/eV RH—H/Å RH—Mg/O/Å QH QH2 H2 on Mg 0 –0.118 0.751 2.217 0.023/0.023 0.046 0.005 –0.172 0.752 2.179 0.030/0.030 0.060 0.010 –0.225 0.753 2.093 0.044/0.044 0.088 H2 on O 0 –0.060 0.750 2.365 0.044/–0.068 –0.024 0.005 –0.101 0.755 2.248 0.069/–0.110 –0.041 0.010 –0.150 0.763 2.136 0.094/–0.157 –0.063 表 3 H2在(MgO)4上吸附结构的拓扑参数
Table 3. The topological parameters for the adsorption structures of H2 on (MgO)4
F/a. u. H2 on Mg H2 on O BCP ρ ${\nabla ^2}\rho $ H(r) ELF BCP ρ ${\nabla ^2}\rho $ H(r) ELF 0.000 Mg—H 0.013 0.059 0.002 0.029 O—H 0.011 0.038 0.002 0.045 H—H 0.266 –1.167 –0.292 1.000 H—H 0.265 –1.167 –0.292 1.000 Mg—O 0.056 0.403 0.005 0.057 Mg—O 0.056 0.403 0.005 0.057 0.005 Mg—H 0.014 0.063 0.002 0.031 O—H 0.015 0.049 0.002 0.060 H—H 0.260 –1.165 –0.292 1.000 H—H 0.261 –1.142 –0.287 1.000 Mg—O 0.054—0.059 0.382—0.426 0.004—0.005 0.056—0.059 Mg—O 0.055—0.059 0.390—0.427 0.004—0.005 0.057—0.059 0.010 Mg—H 0.016 0.069 0.002 0.034 O—H 0.019 0.063 0.002 0.080 H—H 0.255 –1.162 –0.291 0.999 H—H 0.255 –1.102 –0.278 0.999 Mg—O 0.051—0.061 0.341—0.533 0.004—0.005 0.055—0.060 Mg—O 0.025—0.061 0.358—0.444 0.004—0.005 0.054—0.060 -
[1] Lubitz W, Tumas W 2007 Chem. Rev. 107 3900
 Google Scholar
Google Scholar
[2] Yu X, Tang Z, Sun D, Ouyang L, Zhu M 2017 Prog. Mater. Sci. 88 1
 Google Scholar
Google Scholar
[3] Ren J, Musyoka N M, Langmi H W, Mathe M, Liao S 2017 Int. J. Hydrogen Energy 42 289
 Google Scholar
Google Scholar
[4] Jena P 2011 J. Phys. Chem. Lett. 2 206
 Google Scholar
Google Scholar
[5] Bhatia S K, Myers A L 2006 Langmuir 22 1688
 Google Scholar
Google Scholar
[6] Ao Z M, Hernandez-Nieves A D, Peeters F M, Li S 2012 Phys. Chem. Chem. Phys. 14 1463
 Google Scholar
Google Scholar
[7] Guo J H, Zhang H 2011 Struct. Chem. 22 1039
 Google Scholar
Google Scholar
[8] Zhou J, Wang Q, Sun Q, Jena P, Chen X S 2010 Proc. Natl. Acad. Sci. 107 2801
 Google Scholar
Google Scholar
[9] Sun X, Jiann Y H, Shang Z S 2010 J. Phys. Chem. C 114 7178
 Google Scholar
Google Scholar
[10] Ao Z M, Peeters F M 2010 J. Phys. Chem. C 114 14503
[11] Liu W, Zhao Y H, Nguyen J, Li Y, Jiang Q, Lavernia E J 2009 Carbon 47 3452
 Google Scholar
Google Scholar
[12] Wu G, Zhang J, Wu Y, Li Q, Chou K, Bao X 2009 J. Alloys Compd. 480 788
 Google Scholar
Google Scholar
[13] Dawoud J N, Sallabi A K, Fasfous I I, Jack D B 2009 e-J. Surf. Sci. Nanotechnol. 7 207
 Google Scholar
Google Scholar
[14] Larese J Z, Frazier L, Adams M A, Arnold T, Hinde R J, Ramirez-Cuesta A 2006 Phys. B: Cond. Matter 385 144
[15] Skofronick J G, Toennies J P, Traeger F, Weiss H 2003 Phys. Rev. B 67 035413
 Google Scholar
Google Scholar
[16] Sawabe K, Koga N, Morokuma K, Iwasawa Y 1994 J. Chem. Phys. 101 4819
 Google Scholar
Google Scholar
[17] Zhang G, Sheng Y 2018 Chin. Phys. B 27 093601
[18] Kwapien K, Sierka M, Dbler J, Sauer J 2010 Chem. Cat. Chem. 2 819
[19] 陈宏善, 陈华君 2011 60 073601
 Google Scholar
Google Scholar
Chen H S, Chen H J 2011 Acta Phys. Sin. 60 073601
 Google Scholar
Google Scholar
[20] Yin Y H, Chen H S 2016 Comput. Theor. Chem. 108 1
[21] Roberts C, Johnston R L 2001 Phys. Chem. Chem. Phys. 3 5024
 Google Scholar
Google Scholar
[22] de La Puente E, Aguado A, Ayuela A, López J M 1997 Phys. Rev. B 56 7607
 Google Scholar
Google Scholar
[23] Ziemann P J, Castleman Jr A W 1991 J. Chem. Phys. 94 718
 Google Scholar
Google Scholar
[24] Becke A D 1993 J. Chem. Phys. 98 5648
 Google Scholar
Google Scholar
[25] Miehlich B, Savin A, Stoll H, Preuss H 1989 Chem. Phys. Lett. 157 200
 Google Scholar
Google Scholar
[26] Ditchfield R, Hehre W J, Pople J A 1971 J. Chem. Phys. 54 724
 Google Scholar
Google Scholar
[27] Frisch M J, Trucks G, Schlegel H, et al. 2013 Gaussian09 Revision D.01 Wallingford CT: Gaussian, Inc.
[28] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[29] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[30] Castleman Jr A W, Khanna S N 2009 J. Phys. Chem. C 113 2664
计量
- 文章访问数: 10072
- PDF下载量: 80
- 被引次数: 0














 下载:
下载: