-
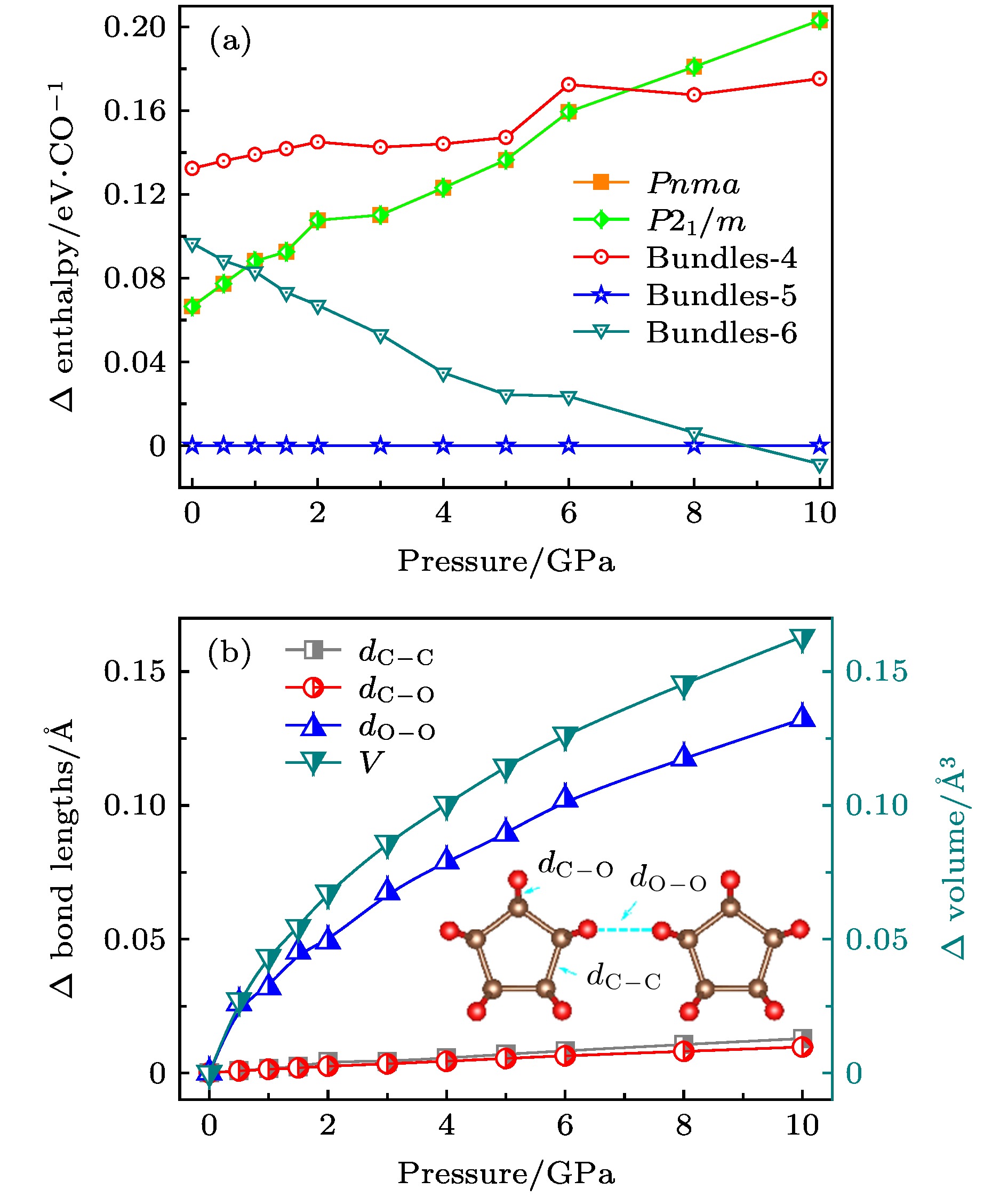
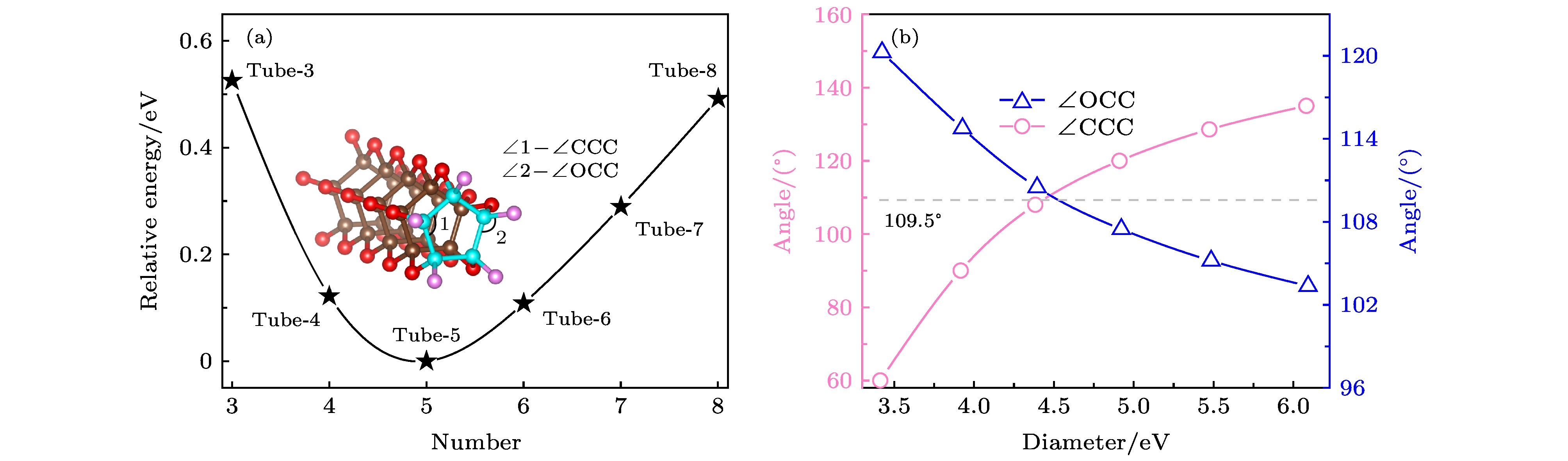
对低压下一氧化碳(CO)晶体结构的研究已有半个多世纪, 研究其晶体内部结构的方法有很多, 实验人员通过红外设备进行红外分析, 或者通过更先进的激光设备进行拉曼分析, 以求得到精准的CO晶体的内部结构. 通过基于不同原理的计算分析手段, 科学家可以预言或者验证碳原子和氧原子如何排序来形成CO固体结构. 基于密度泛函理论, 本文设计了一种类似于碳纳米管束状结构的CO晶体结构. 通过分析计算, 该CO纳米管束状晶体是宽带隙半导体, 与目前已经研究报道的最稳定的CO分子晶体和链状晶体相比, 具有能量的更稳定性. 截然不同的电子结构性质以及能量上的高度稳定性, 使得该结构不仅丰富了低压下CO晶体结构的多样性, 还为探究低压下CO晶体的内部结构提供了新的思路与方向.The crystal structure of carbon monoxide has been studied for more than half a century. The internal structures of low-pressure carbon monoxide crystals have been investigated by means of infrared analysis and Raman analysis, and the internal structure of carbon monoxide has also been studied through computational analysis. Previous studies showed that carbon monoxide can produce different phase transitions at different pressures, and thus forming new polymers with new physical properties such as electrical, optical and mechanical properties. In this paper, from first-principles calculations, we propose six nanotube structures made of carbon monoxide, named Tube-3–Tube-8. The nanotubes are packed into the nanotube bundles, and carbon monoxide nanotube bundle structures that are similar to carbon nanotube bundles are constructed by first-principles calculation. We study the structural, energy and electronic properties of the nanotubes and nanotube bundles. In order to evaluate the relative stability of the predicted nanotubes, we calculate the cohesive energy and phonon spectrum, and we also carry out the molecular dynamics analysis. The results show that there are three nanotubes (Tube-4–Tube-6) that are relatively stable, of which Tube-5 nanotube is the most stable phase. We attribute the stability of Tube-5 to sp3-hybridized C atoms being nearest to the hybridized atoms of diamond. Then we investigate nanotube bundles from the three stable nanotubes, and accordingly name them Bundles-4–Bundles-6. We calculate the enthalpy function under pressure and compare it with the enthalpy function of several known carbon monoxide molecular crystal and chain crystal, which are the most stable structures according to the current studies. More pleasingly, we find that these nanotube bundles are more stable than these carbon monoxide molecular crystal and chain crystal at low pressure. In addition, by calculating the energy bands of Tube-4–Tube-6, we can deduce that these nanotube bundles (Bundles-4– Bundles-6) are all wide band gap semiconductors, which are entirely different from molecular and chain crystals that are metals. We expect that the discovery of nanotube bundle structures will increase the diversity of carbon monoxide crystal under low pressure, and provide a new understanding of exploring the internal structure of carbon monoxide crystal.
-
Keywords:
- carbon monoxide crystal /
- nanotube bundle structure /
- low-pressure phase /
- first-principles
[1] Ashcroft N W 2004 Phys. Rev. Lett. 92 187002
 Google Scholar
Google Scholar
[2] Yoo C S, Cynn H, Gygi F, Galli G 1999 Phys. Rev. Lett. 83 5527
 Google Scholar
Google Scholar
[3] Eremets M I, Gavriliu K A G, Trojan I A, Dziven Ko D A, Boehler R 2004 Nat. Mater. 3 558
 Google Scholar
Google Scholar
[4] Yoo C S 2013 Phys. Chem. Chem. Phys. 15 7949
 Google Scholar
Google Scholar
[5] Santoro M, Gorelli F A, Bini R, Salamat A, Garbarino G, Levelut C, Cambon O, Haines J 2014 Nat. Commun. 5 3761
 Google Scholar
Google Scholar
[6] Zhou R L, Qu B Y, Dai J, Cheng Z 2014 Phys. Rev. X 4 011030
 Google Scholar
Google Scholar
[7] Evans W J, Lipp M J, Yoo C S, Cynn H 2006 Chem. Mater. 18 10
[8] Schettino V, Roberto B 2003 Phys. Chem. Chem. Phys. 5 1951
 Google Scholar
Google Scholar
[9] Raza Z, Pickard C J, Pinilla C, Saitta A M 2013 Phys. Rev. Lett. 111 235501
 Google Scholar
Google Scholar
[10] Naghavi S S, Crespo Y, Martoná K R, Tosatti1 E 2015 Phys. Rev. B 91 224108
 Google Scholar
Google Scholar
[11] Pic Kard C J, Needs R J 2009 Phys. Rev. Lett. 102 125702
 Google Scholar
Google Scholar
[12] Sun J, Klug D D, Martoná K R, Montoya J A, Lee M S, Scandolo S, Tosatti E 2009 Proc. Natl. Acad. Sci. U.S.A. 106 6077
 Google Scholar
Google Scholar
[13] Boulard E, Pan D, Galli G, Liu Z, Mao W L 2015 Nat. Commun. 6 6311
 Google Scholar
Google Scholar
[14] Lipp M, Evans W J, Garcia-Baonza V, Lorenzana H E 1998 Low Temp. Phys. 111 247
 Google Scholar
Google Scholar
[15] Bernard S, Chiarott G L, Scandolo S, Tosatti E 1998 Phys. Rev. Lett. 81 2092
 Google Scholar
Google Scholar
[16] Sun J, Klug D D, Pic Kard C J, Needs R J 2011 Phys. Rev. Lett. 106 145502
 Google Scholar
Google Scholar
[17] Lipp M J, Evans W J, Baer B J, Yoo C S 2005 Nat. Mater. 4 211
 Google Scholar
Google Scholar
[18] Cromer D T, Schiferl D, Lesar R, Mills R T 1983 Acta Crystallogr C 39 1146
 Google Scholar
Google Scholar
[19] Ma, Y M, Oganov A R, Li Z W, Xie Y, Kota Kos Ki J 2009 Phys. Rev. Lett. 102 065501
 Google Scholar
Google Scholar
[20] Santoro M, Gorelli F A 2006 Chem. Soc. Rev. 35 918
 Google Scholar
Google Scholar
[21] Plašienka D, Martoňák R 2014 Phys. Rev. B 89 134105
 Google Scholar
Google Scholar
[22] Lu C, Miao M, Ma Y 2013 Am. Chem. Soc. 135 14167
 Google Scholar
Google Scholar
[23] Datchi F Mallic K B, Salamat A, Rousse G, Ninet S, Garbarino G, Bouvier P, Mezouar M 2014 Phys. Rev. B 89 144101
 Google Scholar
Google Scholar
[24] Datchi F, Mallic K B, Salamat A, Ninet S 2012 Phys. Rev. Lett. 108 125701
 Google Scholar
Google Scholar
[25] Polian A, Loubeyre P, Boccara N 1989 Simple Molecular System at Very High Density (New York: Plenum Publishing Corporation) pp221−236
[26] Mills R L, Schiferl D, Katz A L, Olinger B W 1984 J. Phys. Colloq. 45 186
 Google Scholar
Google Scholar
[27] Yang N L, Snow A, Haubenstoc K H, Bramwell F B 1978 J. Polymer. Sci. Polymer. Chem. Ed. 16 1909
 Google Scholar
Google Scholar
[28] Ordejón P, Artacho E, Soler J M 1996 Phys. Rev. B 53 10441
 Google Scholar
Google Scholar
[29] Hohenberg P, Kohn W 1964 Phys. Rev. B 136 864
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[31] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[32] Perdew J P, Bur Ke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[34] Heyd J, Scuseria G E, Ernzerhof M J 2006 Chem. Phys. 124 219906
[35] Heyd J, Scuseria G E, Ernzerhof M 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
-
表 1 CO纳米管的键长dC—C和dC—O, 每个CO单元的总能量Etol和形成能Ecoh, 以及纳米管每个原胞中碳原子转移给氧原子的电荷数CCHG—OCHG
Table 1. Structural parameters of Tube-3−Tube-7, where dC—C is bond length between carbon atoms, dC—O is bond length between carbon atom and oxygen atom; total energy (Etol) and cohesive energy (Ecoh); electron transfer from carbon atom to oxygen atom (CCHG—OCHG)
dC—C/Å dC—O/Å Etol/eV·CO–1 Ecoh/eV·CO–1 CCHG—OCHG/e Tube-3 1.53 1.40 –14.61 0.16 0.99 Tube-4 1.58 1.40 –15.01 –0.24 0.99 Tube-5 1.58 1.41 –15.13 –0.36 0.98 Tube-6 1.61 1.41 –15.03 –0.25 0.95 Tube-7 1.64 1.40 –14.84 –0.07 0.96 Tube-8 1.67 1.40 –14.64 0.13 0.93 表 2 CO纳米管束不同密堆积方式的总能量 (单位: eV/CO)
Table 2. Total energy of different dense packing modes of nanometer tube bundles (in eV/CO)
Bundles-4 Bundles-5 Bundles-6 Square –15.149 –15.268 –15.159 Hexagon –15.146 –15.276 –15.161 -
[1] Ashcroft N W 2004 Phys. Rev. Lett. 92 187002
 Google Scholar
Google Scholar
[2] Yoo C S, Cynn H, Gygi F, Galli G 1999 Phys. Rev. Lett. 83 5527
 Google Scholar
Google Scholar
[3] Eremets M I, Gavriliu K A G, Trojan I A, Dziven Ko D A, Boehler R 2004 Nat. Mater. 3 558
 Google Scholar
Google Scholar
[4] Yoo C S 2013 Phys. Chem. Chem. Phys. 15 7949
 Google Scholar
Google Scholar
[5] Santoro M, Gorelli F A, Bini R, Salamat A, Garbarino G, Levelut C, Cambon O, Haines J 2014 Nat. Commun. 5 3761
 Google Scholar
Google Scholar
[6] Zhou R L, Qu B Y, Dai J, Cheng Z 2014 Phys. Rev. X 4 011030
 Google Scholar
Google Scholar
[7] Evans W J, Lipp M J, Yoo C S, Cynn H 2006 Chem. Mater. 18 10
[8] Schettino V, Roberto B 2003 Phys. Chem. Chem. Phys. 5 1951
 Google Scholar
Google Scholar
[9] Raza Z, Pickard C J, Pinilla C, Saitta A M 2013 Phys. Rev. Lett. 111 235501
 Google Scholar
Google Scholar
[10] Naghavi S S, Crespo Y, Martoná K R, Tosatti1 E 2015 Phys. Rev. B 91 224108
 Google Scholar
Google Scholar
[11] Pic Kard C J, Needs R J 2009 Phys. Rev. Lett. 102 125702
 Google Scholar
Google Scholar
[12] Sun J, Klug D D, Martoná K R, Montoya J A, Lee M S, Scandolo S, Tosatti E 2009 Proc. Natl. Acad. Sci. U.S.A. 106 6077
 Google Scholar
Google Scholar
[13] Boulard E, Pan D, Galli G, Liu Z, Mao W L 2015 Nat. Commun. 6 6311
 Google Scholar
Google Scholar
[14] Lipp M, Evans W J, Garcia-Baonza V, Lorenzana H E 1998 Low Temp. Phys. 111 247
 Google Scholar
Google Scholar
[15] Bernard S, Chiarott G L, Scandolo S, Tosatti E 1998 Phys. Rev. Lett. 81 2092
 Google Scholar
Google Scholar
[16] Sun J, Klug D D, Pic Kard C J, Needs R J 2011 Phys. Rev. Lett. 106 145502
 Google Scholar
Google Scholar
[17] Lipp M J, Evans W J, Baer B J, Yoo C S 2005 Nat. Mater. 4 211
 Google Scholar
Google Scholar
[18] Cromer D T, Schiferl D, Lesar R, Mills R T 1983 Acta Crystallogr C 39 1146
 Google Scholar
Google Scholar
[19] Ma, Y M, Oganov A R, Li Z W, Xie Y, Kota Kos Ki J 2009 Phys. Rev. Lett. 102 065501
 Google Scholar
Google Scholar
[20] Santoro M, Gorelli F A 2006 Chem. Soc. Rev. 35 918
 Google Scholar
Google Scholar
[21] Plašienka D, Martoňák R 2014 Phys. Rev. B 89 134105
 Google Scholar
Google Scholar
[22] Lu C, Miao M, Ma Y 2013 Am. Chem. Soc. 135 14167
 Google Scholar
Google Scholar
[23] Datchi F Mallic K B, Salamat A, Rousse G, Ninet S, Garbarino G, Bouvier P, Mezouar M 2014 Phys. Rev. B 89 144101
 Google Scholar
Google Scholar
[24] Datchi F, Mallic K B, Salamat A, Ninet S 2012 Phys. Rev. Lett. 108 125701
 Google Scholar
Google Scholar
[25] Polian A, Loubeyre P, Boccara N 1989 Simple Molecular System at Very High Density (New York: Plenum Publishing Corporation) pp221−236
[26] Mills R L, Schiferl D, Katz A L, Olinger B W 1984 J. Phys. Colloq. 45 186
 Google Scholar
Google Scholar
[27] Yang N L, Snow A, Haubenstoc K H, Bramwell F B 1978 J. Polymer. Sci. Polymer. Chem. Ed. 16 1909
 Google Scholar
Google Scholar
[28] Ordejón P, Artacho E, Soler J M 1996 Phys. Rev. B 53 10441
 Google Scholar
Google Scholar
[29] Hohenberg P, Kohn W 1964 Phys. Rev. B 136 864
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[31] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[32] Perdew J P, Bur Ke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[33] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[34] Heyd J, Scuseria G E, Ernzerhof M J 2006 Chem. Phys. 124 219906
[35] Heyd J, Scuseria G E, Ernzerhof M 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
计量
- 文章访问数: 9828
- PDF下载量: 79
- 被引次数: 0













 下载:
下载: