-
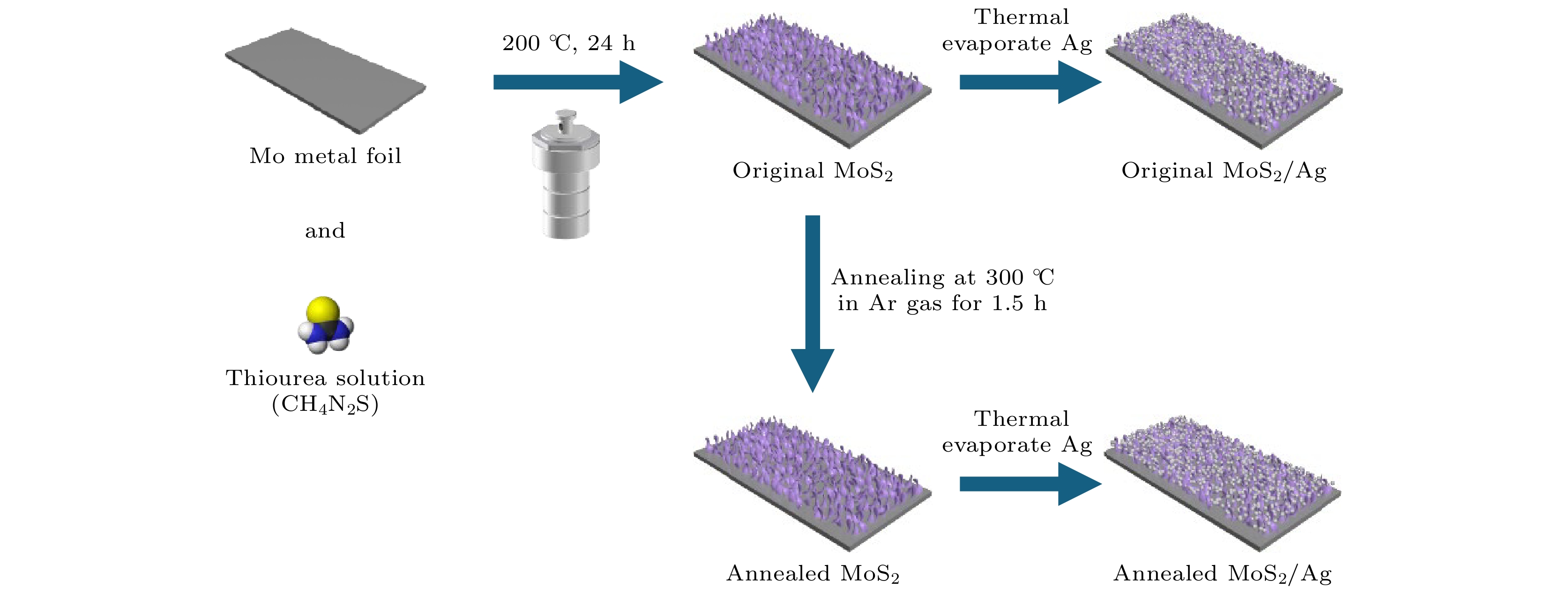
结合金属和二维纳米材料的优点, 研究人员提出了多种二维材料/金属复合结构作为表面增强拉曼光谱(SERS)基底, 然而, 复合结构中的二维纳米材料通常对总增强的作用较小. 本文提出了一种竖直排列的二硫化钼(MoS2)纳米片, 并将其与银纳米颗粒(Ag NPs)复合, 制备MoS2/Ag基底, 用于SERS检测. 竖直排列MoS2纳米片可有效提高对分子的吸附, 增强光吸收, 提升电磁和化学双机制增强. 实验结果表明, MoS2/Ag基底表现出优异的SERS性能, 其对罗丹明6G (R6G)分子的检测极限达到了10–12 mol/L, 接近单分子检测水平, 增强因子约为1.08×109. 同时该基底表现出优异的信号重现性, 最终实现了对水产品中抗菌剂残留的超灵敏检测.
-
关键词:
- 表面增强拉曼光谱 /
- 竖直排列MoS2纳米片 /
- 银纳米颗粒 /
- 电荷转移
Surface enhanced Raman spectroscopy (SERS) can provide rich molecular structure information about ultra-sensitive, non-destructive, and rapid detection, with accuracy down to the single-molecule level. It has been widely applied to physics, chemistry, biomedicine, environmental science, materials science and other fields. Combining the advantages of metals and two-dimensional (2D) nanomaterials, various 2D metal composite structures have been proposed for SERS. However, the contribution of 2D nanomaterials in Raman enhancement is often limited. In this work, vertically aligned MoS2 nanosheet composite with silver nanoparticles (Ag NPs) is proposed for SERS detection. Large-area vertically aligned MoS2 nanosheets, which are grown directly on molybdenum (Mo) foil by using hydrothermal method, can effectively enhance molecular adsorption, light absorption, and provide dual electromagnetic and chemical enhancement. Furthermore, annealing treatment of the MoS2 nanosheets significantly improves the efficiency of charge transfer between Ag NPs and MoS2, thereby increasing the chemical contribution to SERS. The results demonstrate that the annealed MoS2/Ag substrate exhibits outstanding SERS performance, with a detection limit for R6G molecules as low as 10–12 mol/L, which is four orders of magnitude lower than that of the unannealed substrate. The enhancement factor (EF) is calculated to be approximately 1.08×109, approaching the sensitivity required for single-molecule detection. Additionally, the substrate has high signal reproducibility at low concentrations, enabling ultra-sensitive detection of pesticide residues in aquatic products.-
Keywords:
- surface-enhanced Raman spectroscopy /
- vertically aligned molybdenum disulfide nanosheets /
- silver nanoparticles /
- charge transfer
[1] Brosseau C L, Colina A, Perales-Rondon J V, Wilson A J, Joshi P B, Ren B, Wang X 2023 Nat. Rev. Methods Primers 3 79
 Google Scholar
Google Scholar
[2] Hu H F, Tian Y, Chen P P, Chu W G 2024 Adv. Mater. 36 2303001
 Google Scholar
Google Scholar
[3] Peng Y S, Lin C L, Li Y Y, Gao Y, Wang J, He J, Huang Z R, Liu J J, Luo X Y, Yang Y 2022 Matter 5 694
 Google Scholar
Google Scholar
[4] Logan N, Cao C, Freitag S, Haughey S A, Krska R, Elliott C T 2024 Adv. Mater. 36 2309625
 Google Scholar
Google Scholar
[5] Itoh T, Prochazka M, Dong Z C, Ji W, Yamamoto Y S, Zhang Y, Ozaki Y 2023 Chem. Rev. 123 1552
 Google Scholar
Google Scholar
[6] Li L H, Jiang R T, Shan B B, Lu Y X, Zheng C, Li M 2022 Nat. Commun. 13 5249
 Google Scholar
Google Scholar
[7] Jensen L, Aikens C M, Schatz G C 2008 Chem. Soc. Rev. 37 1061
 Google Scholar
Google Scholar
[8] Feng E D, Zheng T T, He X X, Chen J Q, Gu Q Y, He X, Hu F H, Li J H, Tian Y 2023 Angew. Chem. Int. Ed. 62 e202309249
 Google Scholar
Google Scholar
[9] Tang X, Fan X C, Zhou J, Wang S, Li M Z, Hou X Y, Jiang K W, Ni Z H, Zhao B, Hao Q, Qiu T 2023 Nano Lett. 23 7037
 Google Scholar
Google Scholar
[10] Yang L, Kim T H, Cho H Y, Luo J, Lee J M, Chueng S T D, Hou Y N, Yin P T T, Han J Y, Kim J H, Chung B G, Choi J W, Lee K B 2021 Adv. Funct. Mater. 31 2006918
 Google Scholar
Google Scholar
[11] Jiang Y, Wang X C, Zhao G, Shi Y Y, Wu Y, Yang H L, Zhao F Y 2024 Water Res. 255 121444
 Google Scholar
Google Scholar
[12] Hao N J, Liu P Z, Bachman H, Pei Z C, Zhang P R, Rufo J, Wang Z Y, Zhao S G, Huang T J 2020 ACS Nano 14 6150
 Google Scholar
Google Scholar
[13] Butmee P, Samphao A, Tumcharern G 2022 J. Hazard. Mater. 437 129344
 Google Scholar
Google Scholar
[14] Zhou L, Zhou J, Lai W, Yang X D, Meng J, Su L B, Gu C J, Jiang T, Pun E Y B, Shao L Y, Petti L, Sun X W, Jia Z H, Li Q X, Han J G, Mormile P 2020 Nat. Commun. 11 1785
 Google Scholar
Google Scholar
[15] Pan H M, Dong Y, Gong L B, Zhai J Y, Song C Y, Ge Z L, Su Y, Zhu D, Chao J, Su S, Wang L H, Wan Y, Fan C H 2022 Biosens. Bioelectron. 215 114553
 Google Scholar
Google Scholar
[16] Zhou P Y, Cheng S Y, Li Q, Pang Y F, Xiao R 2023 Chem. Eng. J. 471 144514
 Google Scholar
Google Scholar
[17] Jalali M, Mata C D, Montermini L, Jeanne O, Hosseini, II, Gu Z L, Spinelli C, Lu Y, Tawil N, Guiot M C, He Z, Wachsmann-Hogiu S, Zhou R H, Petrecca K, Reisner W W, Rak J, Mahshid S 2023 ACS Nano 17 12052
 Google Scholar
Google Scholar
[18] Wang X Y, Zhang Y Q, Yu J H, Xie X, Deng R P, Min C J, Yuan X C 2022 ACS Nano 16 18621
 Google Scholar
Google Scholar
[19] Choi J H, Kim T H, El-said W A, Lee J H, Yang L T, Conley B, Choi J W, Lee K B 2020 Nano Lett. 20 7670
 Google Scholar
Google Scholar
[20] Lin C L, Liang S S, Peng Y S, Long L, Li Y Y, Huang Z R, Long N V, Luo X Y, Liu J J, Li Z Y, Yang Y 2022 Nanomicro Lett. 14 75
 Google Scholar
Google Scholar
[21] Son W K, Choi Y S, Han Y W, Shin D W, Min K Y H, Shin J, Lee M J, Son H, Jeong D H, Kwak S Y 2023 Nat. Nanotechnol. 18 205
 Google Scholar
Google Scholar
[22] Ge Y C, Yang Y, Zhu Y J, Yuan M L, Sun L B, Jiang D F, Liu X H, Zhang Q W, Zhang J Y, Wang Y 2024 Small 20 2302410
[23] Yuan H, Yu S, Kim M, Lee J E, Kang H, Jang D, Ramasamy M S, Kim D H 2022 Sens. Actuators B Chem. 371 132453
 Google Scholar
Google Scholar
[24] Yu L L, Lu L, Zeng L H, Yan X H, Ren X F, Wu J 2021 J. Phys. Chem. C 125 1940
 Google Scholar
Google Scholar
[25] Zhai Y J, Yang H, Zhang S N, Li J H, Shi K X, Jin F J 2021 J. Mater. Chem. C 9 6823
 Google Scholar
Google Scholar
[26] Li H, Zhang Q, Yap C C R, Tay B K, Edwin T H T, Olivier A, Baillargeat D 2012 Adv. Funct. Mater. 22 1385
 Google Scholar
Google Scholar
[27] Niu Y, Gonzalez-Abad S, Frisenda R, Marauhn P, Drüppel M, Gant P, Schmidt R, Taghavi N S, Barcons D, Molina-Mendoza A J, de Vasconcellos S M, Bratschitsch R, De Lara D P, Rohlfing M, Castellanos-Gomez A 2018 Nanomaterials 8 725
 Google Scholar
Google Scholar
[28] Liu H Q, Yao C B, Li J, Sun W J, Jiang C H 2022 Appl. Surf. Sci. 571 151176
 Google Scholar
Google Scholar
[29] Yu D H, Yu X D, Wang C H, Liu X C, Xing Y 2012 ACS Appl. Mater. Interfaces 4 2781
 Google Scholar
Google Scholar
[30] Wang P, Liang O, Zhang W, Schroeder T, Xie Y H 2013 Adv. Mater. 25 4918
 Google Scholar
Google Scholar
[31] Jones L A H, Xing Z D, Swallow J E N, Shiel H, Featherstone T J, Smiles M J, Fleck N, Thakur P K, Lee T L, Hardwick L J, Scanlon D O, Regoutz A, Veal T D, Dhanak V R 2022 J. Phys. Chem. C 126 21022
 Google Scholar
Google Scholar
[32] Choi S, Shaolin Z, Yang W 2014 J. Korean Phys. Soc. 64 1550
 Google Scholar
Google Scholar
[33] Dieringer J A, Wustholz K L, Masiello D J, Camden J P, Kleinman S L, Schatz G C, Van Duyne R P 2009 J. Am. Chem. Soc. 131 849
 Google Scholar
Google Scholar
[34] Kaushik A, Singh J, Soni R, Singh J P 2023 ACS Appl. Nano Mater. 6 9236
 Google Scholar
Google Scholar
[35] Giovannetti G, Khomyakov P A, Brocks G, Karpan V M, van den Brink J, Kelly P J 2008 Phys. Rev. Lett. 101 026803
 Google Scholar
Google Scholar
[36] Chenal C, Birke R L, Lombardi J R 2008 ChemPhysChem 9 1617
 Google Scholar
Google Scholar
[37] Lombardi J R, Birke R L 2008 J. Phys. Chem. C 112 5605
 Google Scholar
Google Scholar
-
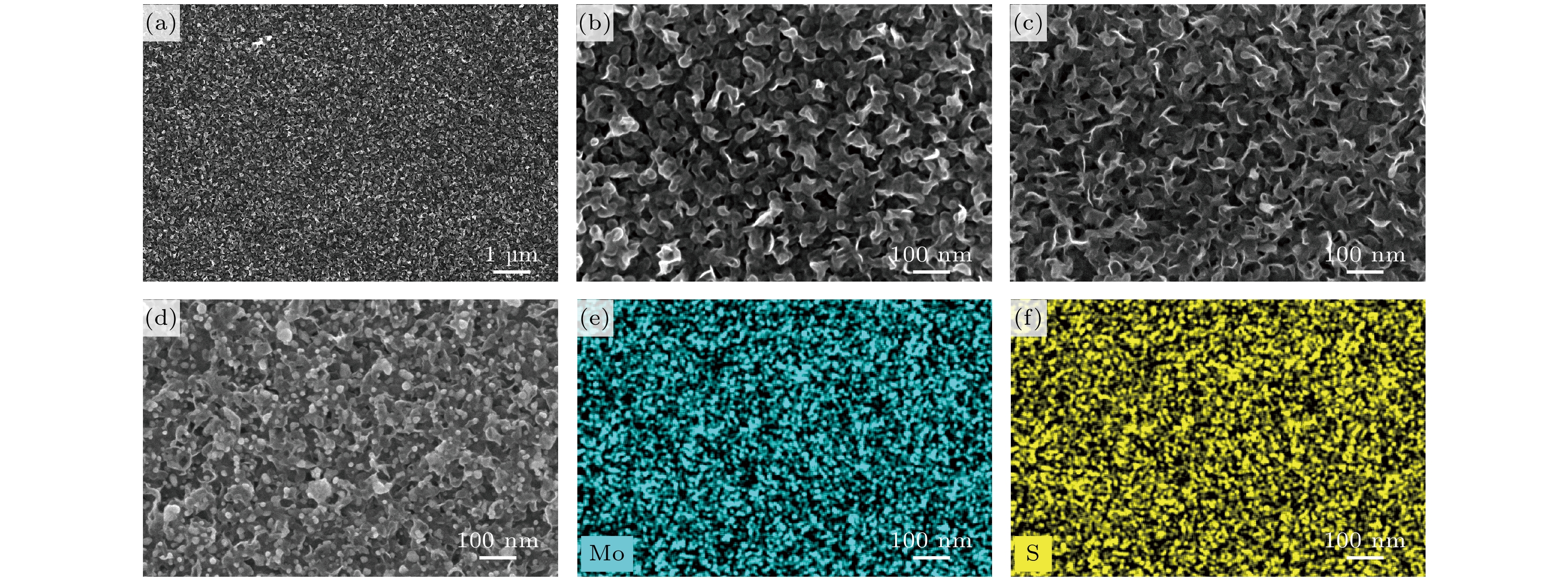
图 2 基底的SEM和EDS图 (a), (b)不同放大倍数下退火后大面积竖直MoS2 纳米片SEM图; (c)未退火MoS2 纳米片SEM图; (d)退火后MoS2/Ag SERS基底的SEM图; (e), (f) 退火后MoS2 SERS基底上Mo元素和S元素的EDS图
Fig. 2. SEM and EDS images of substrates: (a), (b) SEM images of large-area vertical MoS2 nanosheets after annealing at different magnifications; (c) SEM image of MoS2 nanosheets before annealing; (d) SEM image of annealed MoS2/Ag SERS substrate; (e), (f) EDS images of Mo and S elements on MoS2 SERS substrate after annealing.
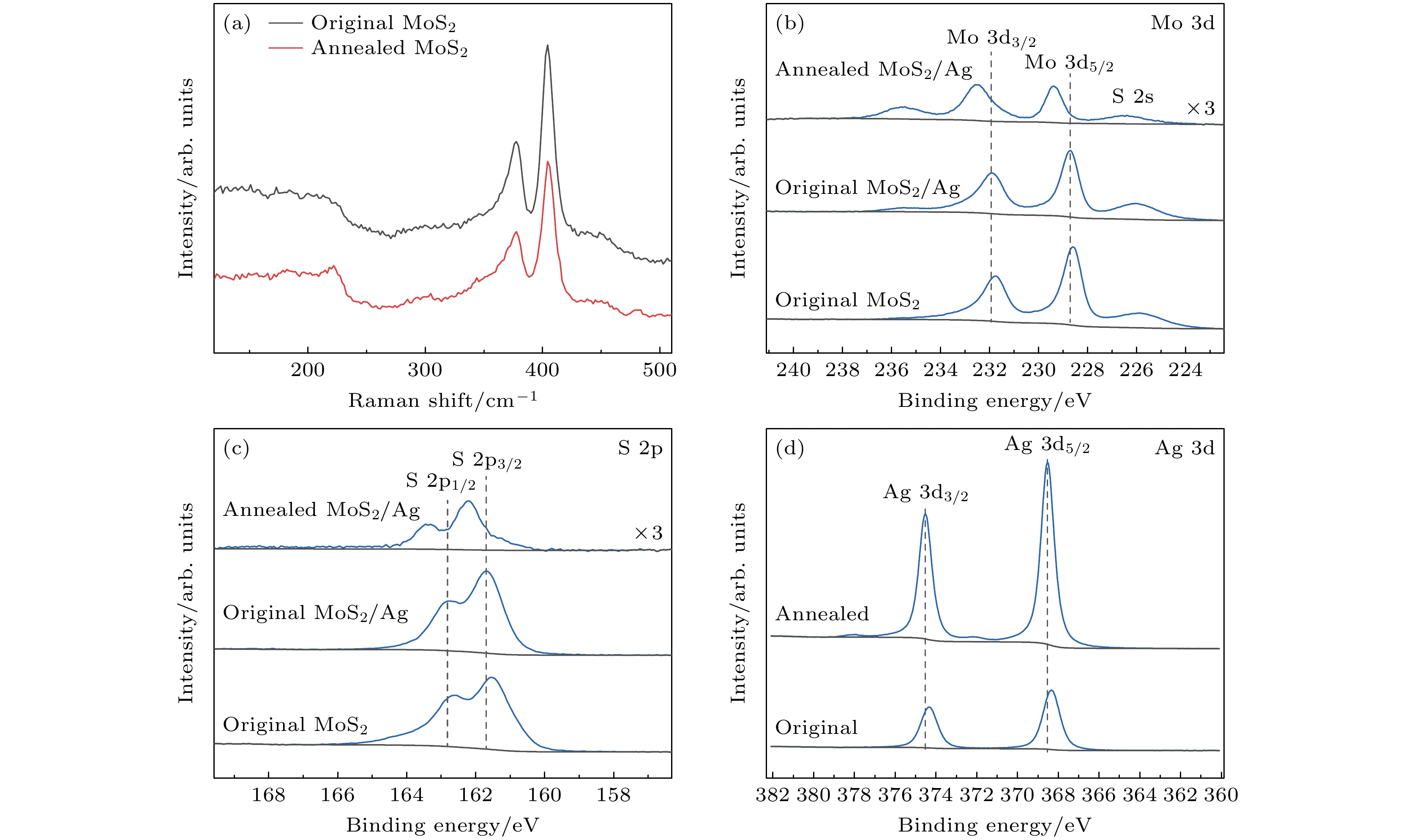
图 3 基底的拉曼光谱和XPS光谱 (a)未退火和退火后MoS2纳米片的拉曼光谱; 退火后MoS2/Ag、未退火MoS2/Ag和未退火MoS2 SERS基底的(b) Mo 3d和(c) S 2p XPS光谱; (d)未退火和退火后MoS2/Ag SERS基底的Ag 3d XPS光谱
Fig. 3. Raman spectra and XPS spectra of substrates: (a) Raman spectra of MoS2 nanosheets before and after annealing; (b) Mo 3d and (c) S 2p XPS spectra of annealed MoS2/Ag, original MoS2/Ag and unannealed MoS2 SERS substrate; (d) Ag 3d XPS spectra of MoS2/Ag SERS substrate before and after annealing.
图 4 MoS2和MoS2/Ag基底的SERS性能 (a)不同浓度R6G分子在竖直MoS2纳米片基底上的SERS光谱; (b) R6G分子(10–6 mol/L)在不同厚度Ag复合未退火MoS2基底上的SERS光谱
Fig. 4. SERS performance of MoS2 and MoS2/Ag substrates: (a) SERS spectra of different concentrations of R6G molecules on vertically aligned MoS2 nanosheet substrates; (b) SERS spectra of R6G molecules at a concentration of 10–6 mol/L on unannealed MoS2 substrates composite with Ag of different thicknesses.
图 5 MoS2/Ag基底的SERS性能 (a) 经过退火和未经退火MoS2/Ag基底采集R6G (10–5 mol/L)分子的SERS光谱; (b)不同浓度R6G分子在未退火MoS2/Ag基底上的SERS光谱; (c) 不同浓度R6G分子在退火后MoS2/Ag基底上的SERS光谱; (d) 双对数坐标下, 吸附在退火后MoS2/Ag基底上的R6G分子在613 cm–1处的拉曼峰强度与R6G分子浓度的关系
Fig. 5. SERS performance of MoS2/Ag substrates: (a) Comparison of SERS spectra of R6G (10–5 mol/L) molecules collected on annealed and original MoS2/Ag substrates; (b) SERS spectra of R6G molecules with different concentrations on original MoS2/Ag substrate; (c) SERS spectra of R6G molecules with different concentrations on annealed MoS2/Ag substrate; (d) in double logarithmic coordinates, the relationship between the Raman peak intensity of R6G molecules adsorbed on the annealed MoS2/Ag substrate at 613 cm–1 and the concentration of R6G molecules.
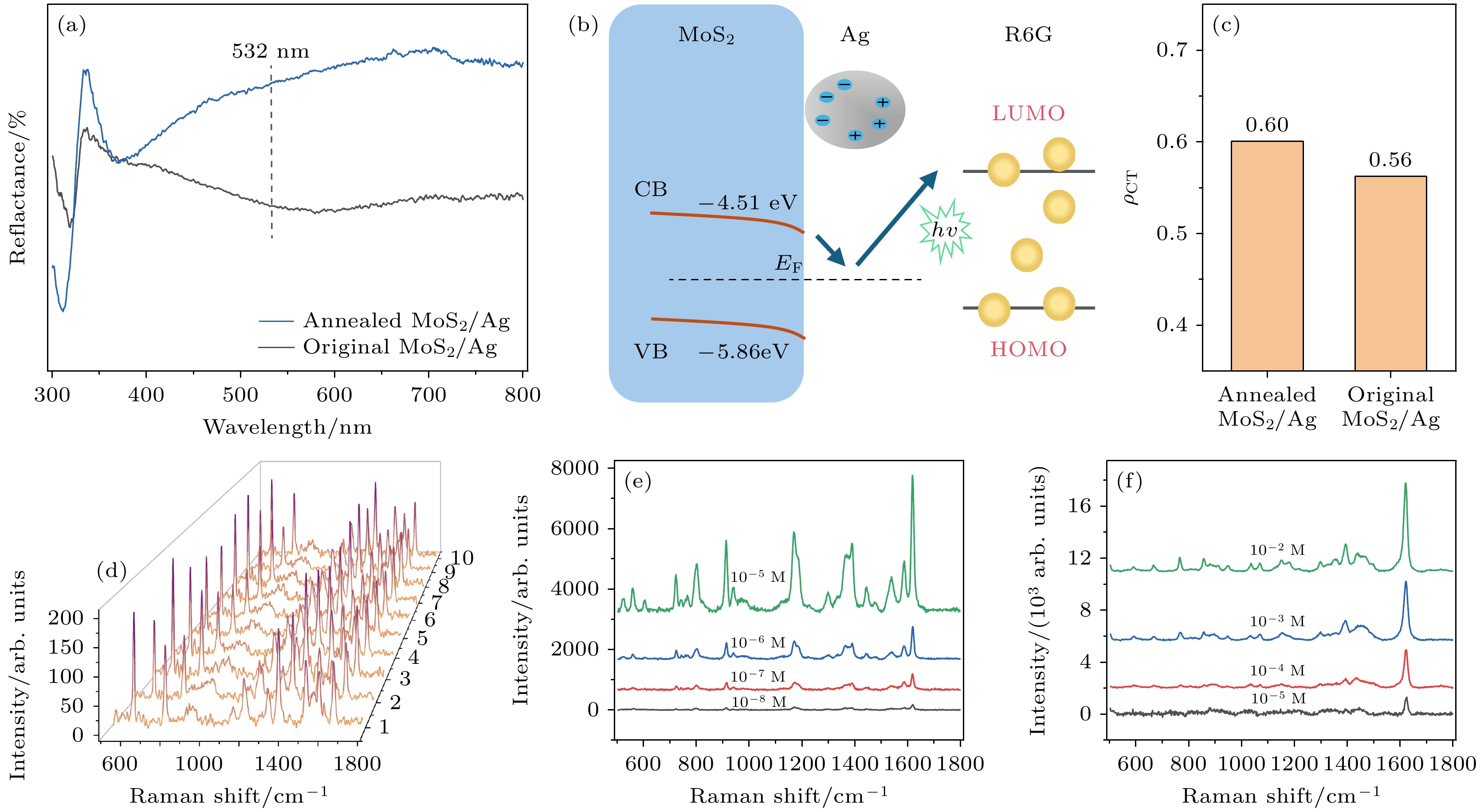
图 6 基底的SERS增强机制分析和检测性能 (a)经过退火和未经退火MoS2/Ag基底的反射光谱; (b) MoS2/Ag基底电荷转移分析; (c)经过退火和未经退火MoS2/Ag基底上R6G分子的电荷转移度(ρCT); (d) R6G (10–12 mol/L)分子在退火后MoS2/Ag 基底的多个随机位置上的SERS光谱; (e)不同浓度CV分子在MoS2/Ag上的SERS光谱; (f)不同浓度MB分子在MoS2/Ag上的SERS光谱
Fig. 6. Analysis of SERS enhancement mechanism and detection performance of substrates: (a) Diffuse reflectance spectra of annealed and original MoS2/Ag substrates; (b) charge transfer analysis of MoS2/Ag substrate; (c) charge transfer degree (ρCT) of R6G molecules on annealed MoS2/Ag and original MoS2/Ag substrate; (d) SERS spectra of R6G (10–12 mol/L) molecules at multiple random positions on annealed MoS2/Ag substrate; (e) SERS spectra of CV molecules with different concentrations on annealed MoS2/Ag substrate; (f) SERS spectra of MB molecules with different concentrations on annealed MoS2/Ag substrate.
-
[1] Brosseau C L, Colina A, Perales-Rondon J V, Wilson A J, Joshi P B, Ren B, Wang X 2023 Nat. Rev. Methods Primers 3 79
 Google Scholar
Google Scholar
[2] Hu H F, Tian Y, Chen P P, Chu W G 2024 Adv. Mater. 36 2303001
 Google Scholar
Google Scholar
[3] Peng Y S, Lin C L, Li Y Y, Gao Y, Wang J, He J, Huang Z R, Liu J J, Luo X Y, Yang Y 2022 Matter 5 694
 Google Scholar
Google Scholar
[4] Logan N, Cao C, Freitag S, Haughey S A, Krska R, Elliott C T 2024 Adv. Mater. 36 2309625
 Google Scholar
Google Scholar
[5] Itoh T, Prochazka M, Dong Z C, Ji W, Yamamoto Y S, Zhang Y, Ozaki Y 2023 Chem. Rev. 123 1552
 Google Scholar
Google Scholar
[6] Li L H, Jiang R T, Shan B B, Lu Y X, Zheng C, Li M 2022 Nat. Commun. 13 5249
 Google Scholar
Google Scholar
[7] Jensen L, Aikens C M, Schatz G C 2008 Chem. Soc. Rev. 37 1061
 Google Scholar
Google Scholar
[8] Feng E D, Zheng T T, He X X, Chen J Q, Gu Q Y, He X, Hu F H, Li J H, Tian Y 2023 Angew. Chem. Int. Ed. 62 e202309249
 Google Scholar
Google Scholar
[9] Tang X, Fan X C, Zhou J, Wang S, Li M Z, Hou X Y, Jiang K W, Ni Z H, Zhao B, Hao Q, Qiu T 2023 Nano Lett. 23 7037
 Google Scholar
Google Scholar
[10] Yang L, Kim T H, Cho H Y, Luo J, Lee J M, Chueng S T D, Hou Y N, Yin P T T, Han J Y, Kim J H, Chung B G, Choi J W, Lee K B 2021 Adv. Funct. Mater. 31 2006918
 Google Scholar
Google Scholar
[11] Jiang Y, Wang X C, Zhao G, Shi Y Y, Wu Y, Yang H L, Zhao F Y 2024 Water Res. 255 121444
 Google Scholar
Google Scholar
[12] Hao N J, Liu P Z, Bachman H, Pei Z C, Zhang P R, Rufo J, Wang Z Y, Zhao S G, Huang T J 2020 ACS Nano 14 6150
 Google Scholar
Google Scholar
[13] Butmee P, Samphao A, Tumcharern G 2022 J. Hazard. Mater. 437 129344
 Google Scholar
Google Scholar
[14] Zhou L, Zhou J, Lai W, Yang X D, Meng J, Su L B, Gu C J, Jiang T, Pun E Y B, Shao L Y, Petti L, Sun X W, Jia Z H, Li Q X, Han J G, Mormile P 2020 Nat. Commun. 11 1785
 Google Scholar
Google Scholar
[15] Pan H M, Dong Y, Gong L B, Zhai J Y, Song C Y, Ge Z L, Su Y, Zhu D, Chao J, Su S, Wang L H, Wan Y, Fan C H 2022 Biosens. Bioelectron. 215 114553
 Google Scholar
Google Scholar
[16] Zhou P Y, Cheng S Y, Li Q, Pang Y F, Xiao R 2023 Chem. Eng. J. 471 144514
 Google Scholar
Google Scholar
[17] Jalali M, Mata C D, Montermini L, Jeanne O, Hosseini, II, Gu Z L, Spinelli C, Lu Y, Tawil N, Guiot M C, He Z, Wachsmann-Hogiu S, Zhou R H, Petrecca K, Reisner W W, Rak J, Mahshid S 2023 ACS Nano 17 12052
 Google Scholar
Google Scholar
[18] Wang X Y, Zhang Y Q, Yu J H, Xie X, Deng R P, Min C J, Yuan X C 2022 ACS Nano 16 18621
 Google Scholar
Google Scholar
[19] Choi J H, Kim T H, El-said W A, Lee J H, Yang L T, Conley B, Choi J W, Lee K B 2020 Nano Lett. 20 7670
 Google Scholar
Google Scholar
[20] Lin C L, Liang S S, Peng Y S, Long L, Li Y Y, Huang Z R, Long N V, Luo X Y, Liu J J, Li Z Y, Yang Y 2022 Nanomicro Lett. 14 75
 Google Scholar
Google Scholar
[21] Son W K, Choi Y S, Han Y W, Shin D W, Min K Y H, Shin J, Lee M J, Son H, Jeong D H, Kwak S Y 2023 Nat. Nanotechnol. 18 205
 Google Scholar
Google Scholar
[22] Ge Y C, Yang Y, Zhu Y J, Yuan M L, Sun L B, Jiang D F, Liu X H, Zhang Q W, Zhang J Y, Wang Y 2024 Small 20 2302410
[23] Yuan H, Yu S, Kim M, Lee J E, Kang H, Jang D, Ramasamy M S, Kim D H 2022 Sens. Actuators B Chem. 371 132453
 Google Scholar
Google Scholar
[24] Yu L L, Lu L, Zeng L H, Yan X H, Ren X F, Wu J 2021 J. Phys. Chem. C 125 1940
 Google Scholar
Google Scholar
[25] Zhai Y J, Yang H, Zhang S N, Li J H, Shi K X, Jin F J 2021 J. Mater. Chem. C 9 6823
 Google Scholar
Google Scholar
[26] Li H, Zhang Q, Yap C C R, Tay B K, Edwin T H T, Olivier A, Baillargeat D 2012 Adv. Funct. Mater. 22 1385
 Google Scholar
Google Scholar
[27] Niu Y, Gonzalez-Abad S, Frisenda R, Marauhn P, Drüppel M, Gant P, Schmidt R, Taghavi N S, Barcons D, Molina-Mendoza A J, de Vasconcellos S M, Bratschitsch R, De Lara D P, Rohlfing M, Castellanos-Gomez A 2018 Nanomaterials 8 725
 Google Scholar
Google Scholar
[28] Liu H Q, Yao C B, Li J, Sun W J, Jiang C H 2022 Appl. Surf. Sci. 571 151176
 Google Scholar
Google Scholar
[29] Yu D H, Yu X D, Wang C H, Liu X C, Xing Y 2012 ACS Appl. Mater. Interfaces 4 2781
 Google Scholar
Google Scholar
[30] Wang P, Liang O, Zhang W, Schroeder T, Xie Y H 2013 Adv. Mater. 25 4918
 Google Scholar
Google Scholar
[31] Jones L A H, Xing Z D, Swallow J E N, Shiel H, Featherstone T J, Smiles M J, Fleck N, Thakur P K, Lee T L, Hardwick L J, Scanlon D O, Regoutz A, Veal T D, Dhanak V R 2022 J. Phys. Chem. C 126 21022
 Google Scholar
Google Scholar
[32] Choi S, Shaolin Z, Yang W 2014 J. Korean Phys. Soc. 64 1550
 Google Scholar
Google Scholar
[33] Dieringer J A, Wustholz K L, Masiello D J, Camden J P, Kleinman S L, Schatz G C, Van Duyne R P 2009 J. Am. Chem. Soc. 131 849
 Google Scholar
Google Scholar
[34] Kaushik A, Singh J, Soni R, Singh J P 2023 ACS Appl. Nano Mater. 6 9236
 Google Scholar
Google Scholar
[35] Giovannetti G, Khomyakov P A, Brocks G, Karpan V M, van den Brink J, Kelly P J 2008 Phys. Rev. Lett. 101 026803
 Google Scholar
Google Scholar
[36] Chenal C, Birke R L, Lombardi J R 2008 ChemPhysChem 9 1617
 Google Scholar
Google Scholar
[37] Lombardi J R, Birke R L 2008 J. Phys. Chem. C 112 5605
 Google Scholar
Google Scholar
计量
- 文章访问数: 1732
- PDF下载量: 93
- 被引次数: 0













 下载:
下载: