-
水在纳米通道中会表现出与体相水不一样的独特结构和动力学性质, 但现有实验技术依然无法有效地进行探测和表征. 光谱是用于研究和鉴别物质成分及其特性的有效技术手段, 因此本文利用分子动力学模拟计算了受限在(6, 6)单壁碳纳米管中一维有序单链状水(single-file water, SW)的红外吸收光谱, 研究发现SW在0—35 THz区域内的主峰相对于体相水有明显的蓝移和增强, 分析表明是由于SW的有序性导致分子间的摆转(libration)振动(包括rock, twist, wag三种模式)耦合权重发生变化引起的, 即频率较高的twist和wag模式在SW中相对体相水中束缚能减小, 振动相对容易发生, 从而导致谱峰发生蓝移和增强. 与此同时, 研究表明SW光谱分量特性能很好地预测和解释SW的结构和动力学性质. 进一步地, 太赫兹电场效应模拟实验验证了SW的红外吸收能力基本符合光谱的分布特性.Compared with bulk water (BW), the water in nanochannels usually shows unique structural and dynamic properties, which is still unable to be effectively detected and characterized by existing experimental techniques. The spectrum is an effective technical means for studying and identifying the material composition and characteristics. In this study, the infrared absorption spectra of one-dimensional ordered single-file water (SW) confined in (6, 6) single-walled carbon nanotubes are calculated by molecular dynamics simulation. It is found that the ordered arrangement of SW results in an obvious blue shift and enhancement of the spectral peak in the 0–35 THz range relative to the bulk water. The analysis shows that this phenomenon is caused by the change of coupling weight of libration vibrations (including rock, twist and wag modes) of SW. The twist vibration mode and wag vibration mode with higher frequency are relatively easy to occur because the binding energy decreases under the single chain structure of water, which results in the blue shift and enhancement of the spectral peak. Meanwhile, the present study shows that the spectral component characteristics of SW can well predict and explain the structural and dynamic properties of SW. Further, terahertz simulation experiments show that the infrared absorption capacity of SW basically conforms with the spectral distribution characteristics.
-
Keywords:
- single-file water /
- infrared absorption spectrum /
- molecular dynamics /
- terahertz electric field
[1] Ball P 2008 Chem. Rev. 108 74
 Google Scholar
Google Scholar
[2] 方海平 2016 65 186101
 Google Scholar
Google Scholar
Fang H P 2016 Acta Phys. Sin. 65 186101
 Google Scholar
Google Scholar
[3] 叶树集, 李传召, 张佳慧, 谈军军, 罗毅 2019 68 013101
 Google Scholar
Google Scholar
Ye S J, Li C Z, Zhang J H, Tan J J, Luo Y 2019 Acta. Phys. Sin. 68 013101
 Google Scholar
Google Scholar
[4] Heyden M, Tobias D J 2013 Phys. Rev. Lett. 111 218101
 Google Scholar
Google Scholar
[5] 王强, 曹则贤 2019 68 015101
 Google Scholar
Google Scholar
Wang Q, Cao Z X 2019 Acta Phys. Sin. 68 015101
 Google Scholar
Google Scholar
[6] Hassan J, Diamantopoulos G, Homouz D, Papavassiliou G 2016 Nanotechnol. Rev. 5 341
[7] Wang C L, Lu H J, Wang Z G, Xiu P, Zhou B, Zuo G H, Wan R Z, Hu J, Fang H P 2009 Phys. Rev. Lett. 103 137801
 Google Scholar
Google Scholar
[8] Laage D, Elsaesser T, Hynes J T 2017 Chem. Rev. 117 10694
 Google Scholar
Google Scholar
[9] Groot B L, Grubmuller H 2001 Science 294 2353
 Google Scholar
Google Scholar
[10] Jensen M, Borhani D W, Lindorff-Larsen K, Maragakis P, Jogini V, Eastwood M P, Dror R O, Shaw D E 2002 Proc. Natl. Acad. Sci. U.S.A. 99 6731
 Google Scholar
Google Scholar
[11] Akeson M, Deamer D W, Branton D, Kasianowicz J J, Brandin E 1999 Biophys. J. 60 101
[12] Pomès R, Roux B 1996 Biophys. J. 71 19
 Google Scholar
Google Scholar
[13] Jiang Y X, Lee A, Chen J, Cadene M, Chait B T, MacKinnon R 2002 Nature 417 515
 Google Scholar
Google Scholar
[14] Hummer G, Jayendran C, Rasaiah, Noworyta J P 2001 Nature 414 188
 Google Scholar
Google Scholar
[15] Holt J K, Park H G, Wang Y M, Stadermann M, Artyukhin A, Grigoropoulos C, Noy A, Bakajin O 2006 Science 312 1034
 Google Scholar
Google Scholar
[16] Xue M M, Hu Z L, Qiu H, Shen C, Guo W, Zhang Z 2021 Natl. Sci. Rev. 9 9
[17] Zhang Q L, Jiang W Z, Liu J, Miao R D, Sheng N 2013 Phys. Rev. Lett. 110 254501
 Google Scholar
Google Scholar
[18] Kalra A, Garde S, Hummer G 2003 Proc. Natl. Acad. Sci. U.S.A. 100 10175
 Google Scholar
Google Scholar
[19] Tu Y, Xiu P, Wan R, Hu J, Zhou R, Fang H 2009 Proc. Natl. Acad. Sci. U.S.A. 106 18120
 Google Scholar
Google Scholar
[20] García F R, Sansom M S 2012 Proc. Natl. Acad. Sci. U.S.A. 109 6939
 Google Scholar
Google Scholar
[21] Oyarzua E, Walther J H, Megaridis C M, Koumoutsakos P, Zambrano H A 2017 ACS Nano. 11 9997
 Google Scholar
Google Scholar
[22] Fang H P, Wan R Z, Gong X J, Lu H J, Li J C 2008 J. Phys. D: Appl. Phys. 41 103002
 Google Scholar
Google Scholar
[23] Fan W, Chen J G 2020 Phys. Rev. E 101 010101
[24] Jin L, Zhang D P, Zhu Y, Yang X R, Gao Y, Wang Z G 2020 J. Phys. Chem. Lett. 12 350
[25] Du Q, Freysz E, Shen Y R 1994 Phys. Rev. Lett. 72 238
 Google Scholar
Google Scholar
[26] Gan W, Wu D, Zhang Z, Feng R R, Wang H F 2006 J. Chem. Phys. 124 114705
 Google Scholar
Google Scholar
[27] Zhang J, Tan J, Pei R, Ye S, Luo Y 2021 J. Am. Chem. Soc. 143 13074
 Google Scholar
Google Scholar
[28] Alfarano S R, Pezzotti S, Stein C J, et al. 2021 Proc. Natl. Acad. Sci. U.S.A. 118 47
[29] Praprotnik M, Janežic D, Mavri J 2004 J. Phys. Chem. A 108 11056
 Google Scholar
Google Scholar
[30] Al-Abadleh H A, Grassian V 2005 Langmuir 19 341
[31] Bezerra D M, Rodrigues J E, Assaf E 2017 Mater. Charact. 125 29
 Google Scholar
Google Scholar
[32] Tayal V P, Srivastava B K, Khandelwal D P, Bist H D 1980 Appl. Spectrosc. Rev. 16 43
 Google Scholar
Google Scholar
[33] Carey D M, Korenowski G M 1996 J. Chem. Phys. 108 2669
[34] Lepodise L M, Horvat J, Lewis R A 2013 Phys. Chem. Chem. Phys. 15 20252
[35] Dalla B S, Paineau E, Brubach J B, Judeinstein P, Rouzière S, Launois P, Roy P 2016 J. Am. Chem. Soc. 15 20252
[36] Nihonyanagi S, Yamaguchi S, Tahara T 2010 J. Am. Chem. Soc. 132 6867
 Google Scholar
Google Scholar
[37] Praprotnik M, Janezic D, Merzel F 2005 J. Chem. Phys. 122 174101
 Google Scholar
Google Scholar
[38] Nagata Y, Mukamel S 2010 J. Am. Chem. Soc. 132 6434
 Google Scholar
Google Scholar
[39] Imoto S, Xantheas S S, Saito S 2015 J. Phys. Chem. B. 119 11068
 Google Scholar
Google Scholar
[40] Yang R Y, Jiang W Z, Huo P Y 2022 J. Mol. Liq. 366 120286
 Google Scholar
Google Scholar
[41] William H, Andrew D, Klaus S 1996 J. Mol. Graphics Modell. 14 33
 Google Scholar
Google Scholar
[42] Phillips J C, Braun R, Wang W, et al. 2005 J. Comput. Chem. 26 1781
 Google Scholar
Google Scholar
[43] MacKerell A D, Bashford D, Bellott M, et al. 1998 J. Phys. Chem. B 102 3586
 Google Scholar
Google Scholar
[44] Darden T, York D, Pedersen L 1993 J. Chem. Phys. 98 10089
 Google Scholar
Google Scholar
[45] Jorgensen W L, Chandrasekhar J, Madura J D, Impey R W, Klein M L 1983 J. Chem. Phys. 79 926
 Google Scholar
Google Scholar
[46] Kou J, Yao J, Lu H J, Zhang B, Li A F, Sun Z X, Zhang J G, Fang Y Z, Wu F M, Fan J T 2015 Angew. Chem. Int. Ed. Engl. 54 2351
 Google Scholar
Google Scholar
[47] Lu Y, Wang Y, Xu C, Xie C, Li W, Ding J, Zhou W, Qin Z, Shen X, Luo L 2021 Nanoscale 13 1000
 Google Scholar
Google Scholar
[48] Zhu Z, Sheng N, Wan R, Fang H J 2014 Phys. Chem. A 118 8936
 Google Scholar
Google Scholar
[49] Guo Y W, Qin J Y, Hu J H, Cao J H, Zhu Z, Wang C L 2020 Nucl. Sci. Tech. 31 53
 Google Scholar
Google Scholar
[50] Perakis F, Marco L D, Shalit A, et al. 2016 Chem. Rev. 116 7590
 Google Scholar
Google Scholar
[51] Cygan R T, Daemen L L, Ilgen A G, Krumhansl J L, Nenoff T M 2015 J. Phys. Chem. 11 9
[52] Maréchal Y 2011 J. Mol. Struct. 1004 146
 Google Scholar
Google Scholar
[53] Heyden M, Sun J, Funkner S, Mathias G, Forbert H, Havenith M, Marxb D 2010 Proc. Natl. Acad. Sci. U.S.A. 107 12068
 Google Scholar
Google Scholar
[54] 段铜川, 闫韶健, 赵妍, 孙庭钰, 李阳梅, 朱智 2021 70 248702
 Google Scholar
Google Scholar
Duan T C, Yan S J, Zhao Y, Sun T Y, Li Y M, Zhu Z 2021 Acta Phys. Sin. 70 248702
 Google Scholar
Google Scholar
[55] Zhang Q L, Wu Y X, Yang R Y, Zhang J L, Wang R F 2021 Chem. Phys. Lett. 762 138139
 Google Scholar
Google Scholar
[56] Sun Q 2009 Vib. Spectrosc. 51 213
 Google Scholar
Google Scholar
[57] Zhang Q L, Yang R Y, Jiang W Z, Huang Z Q 2016 Nanoscale 28 1886
[58] Zhu Z, Chang C, Shu Y, Song B 2020 J. Phys. Chem. Lett. 11 256
 Google Scholar
Google Scholar
[59] Conti N V, Havenith M 2014 J. Am. Chem. Soc. 136 12800
 Google Scholar
Google Scholar
[60] Yang R Y, Huang Z Q, Wei S N, Zhang Q L, Jiang W Z 2017 J. Mol. Liq. 229 148
 Google Scholar
Google Scholar
[61] Saitta A M, Saija F, Giaquinta P V 2012 Phys. Rev. Lett. 108 207801
 Google Scholar
Google Scholar
[62] Ma M, Grey F, Shen L, Urbakh M, Wu S, Liu J Z, Liu Y L, Zheng Q S 2015 Nat. Nanotechnol. 10 692
 Google Scholar
Google Scholar
[63] Zhang Q L, Yang R Y, Wang C L, Hu J 2022 Phys. Rev. Fluids 7 114202
 Google Scholar
Google Scholar
[64] 彭晓昱, 周欢 2021 70 240701
 Google Scholar
Google Scholar
Peng X Y, Zhou H 2021 Acta Phys. Sin. 70 240701
 Google Scholar
Google Scholar
[65] Sun T Y, Zhu Z 2022 J. Membr. Sci. 662 121026
 Google Scholar
Google Scholar
-
图 1 模拟系统示意图. 一个长10 nm无盖的(6, 6) SWNT夹在两片石墨烯之间, 里面填充了一条由37个水分子组成的水链, 石墨烯墙两侧是水库. 图中棕褐色、红色和白色小球分别代表C, O和H原子. 下侧小图为碳管中水分子的局部放大图, 绿色为水分子间的氢键
Fig. 1. Schematic diagram of the simulation system. A 10 nm uncovered (6, 6) SWNT is sandwiched between two graphene sheets. A water chain consisting of 37 water molecules is inside the SWNT, and reservoirs are located on both sides of the graphene wall. The brown, red and white spheres represent C, O and H atoms respectively. The insert shows a partial magnified view of water molecules with the H-bond (green) inside the SWNT
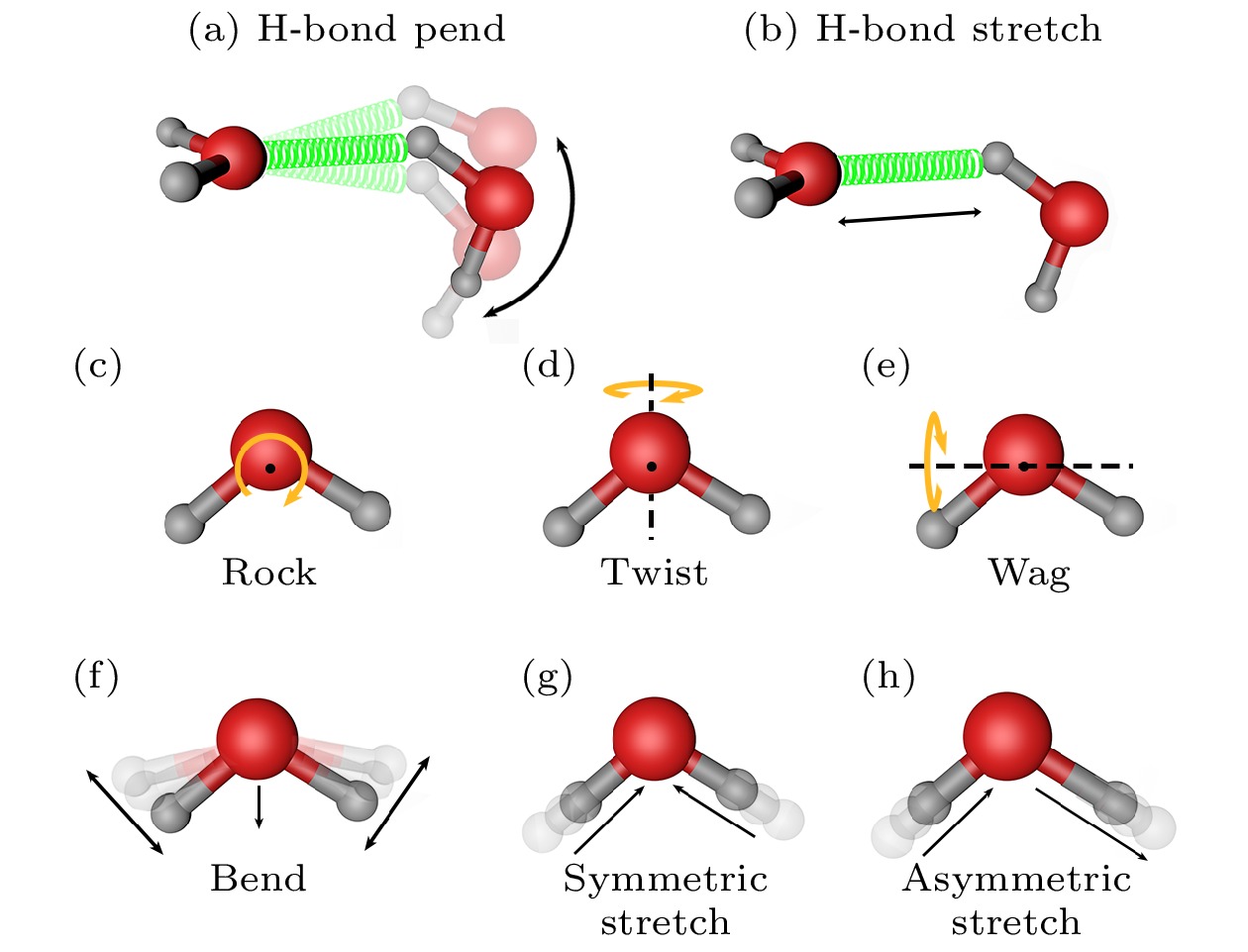
图 2 水分子各种振动模式示意图. 图中红色和灰色小球分别代表O和H原子, 绿色弹簧代表水分子间的氢键, 箭头表示振动或转动方向. 子图(a)−(h)分别为水分子的H-bond pend (悬摆), H-bond stretch (伸缩), rock (旋摆), twist (扭摆), wag (摇摆), bend (弯曲), symmetric stretch (对称伸缩) 和 asymmetric stretch (不对称伸缩) 振动模式, 其中子图(c), (d), (e)中的黑点表示水分子的质心, 黑色虚线代表绕轴
Fig. 2. Schematic diagram of various vibration modes of water molecules. The red and grey balls represent O and H atoms respectively, and the arrows indicate the direction of vibration or rotation. The (a)−(h) subgraphs represent the H-bond pend, H-bond stretch, rock, twist, wag, bend, symmetric stretch and asymmetric stretch vibration modes of water molecules, respectively. The black dots in (c), (d) and (e) represent the center of mass of water molecules, and the black dotted lines represent the axis of rotation
图 3 (a) 体相水(BW)和单链水(SW)的全域红外光谱图,
$ I^{{\rm{BW}}}_ {\rm{total}} $ 和$ I^{{\rm{SW}}}_ {\rm{total}} $ 分别表示BW和SW的光谱强度, 虚线表示峰的中心位置频率; (b) SW 光谱的0—35 THz放大图Fig. 3. (a) Total infrared spectra of BW and SW,
$ I^{{\rm{BW}}}_ {\rm{total}} $ and$ I^{{\rm{SW}}}_ {\rm{total}} $ represents the spectral intensity of BW and SW respectively, and the dotted line represents the cental position frequency of the peak; (b) magnification of SW spectrum in 0–35 THz range图 4 BW(上层)和SW(下层)的红外光谱强度在
$ x $ ,$ y $ , z三个方向的分量(蓝, 绿, 红)分别为$ I^{{\rm{BW}}}_{x} $ ,$ I^{{\rm{BW}}}_{y} $ ,$ I^{{\rm{BW}}}_{z} $ 和$ I^{{\rm{SW}}}_{x} $ ,$ I^{{\rm{SW}}}_{y} $ ,$ I^{{\rm{SW}}}_{z} $ . 下层图中$ I^{{\rm{SW}}}_{\rm{total}} $ (灰色)为SW的总光强Fig. 4. Intensity components of IR of BW (upper) and SW (lower) in the three directions of
$ x $ (blue),$ y $ (green), and$ z $ (red). The$ I^{{\rm{SW}}}_{\rm{total}} $ (grey) is the total spectral intensity图 5
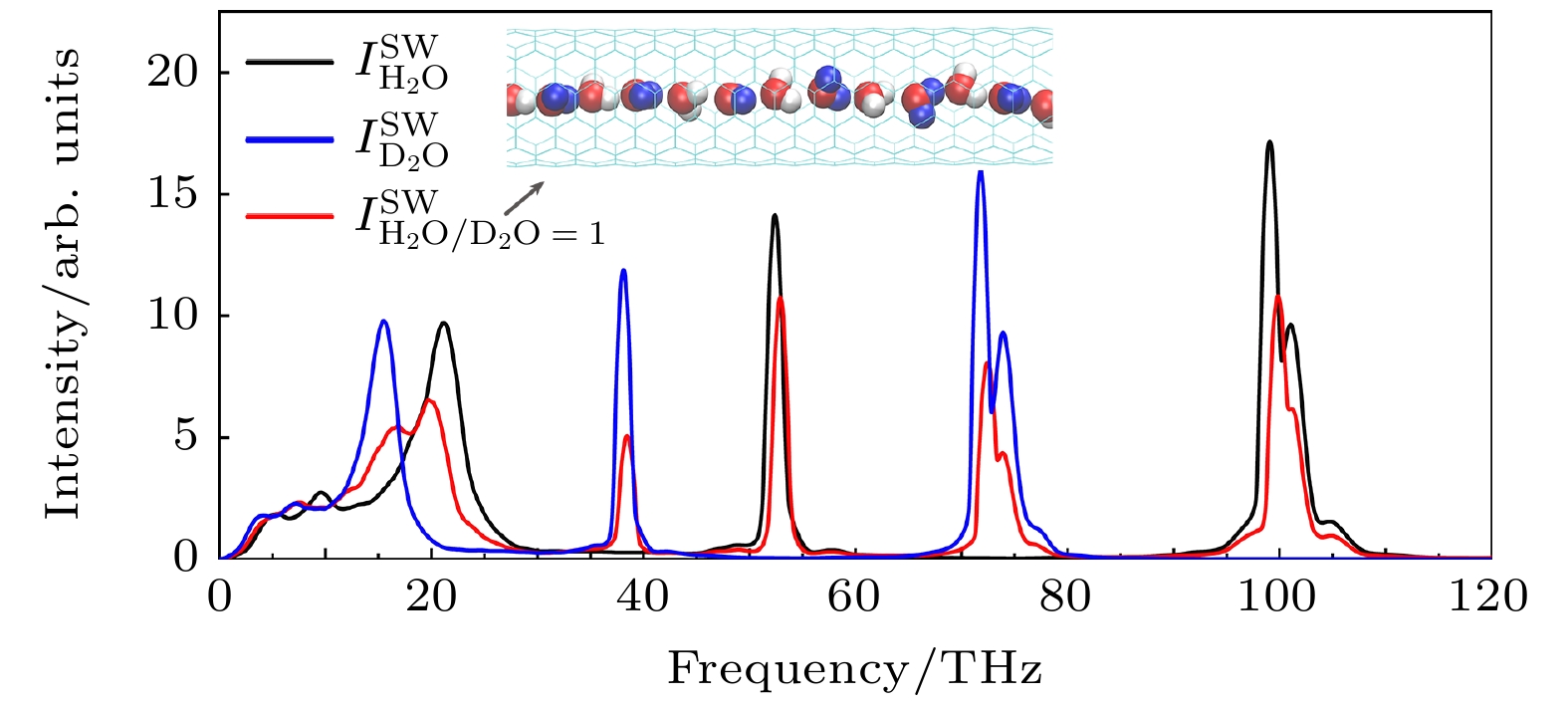
$I_{\rm{H_{2}O} }^{\rm{SW}} $ ,$ I_{ {{\rm{D}}_{\rm{2}}}{\rm{O}} }^{\rm{SW}} $ 和$ I_{\rm{H_{2}O/D_{2}O=1} }^{\rm{SW}} $ 分别表示(6, 6) SWNT中普通水($ \rm{H_2 O} $ )、重水($ \rm{D_2 O} $ )及二者按照1∶1混合水的红外光谱强度. 插图为普通水和重水按照1∶1间隔混合后的示意图, 其中红色、白色和蓝色小球分别代表O, H和D原子Fig. 5.
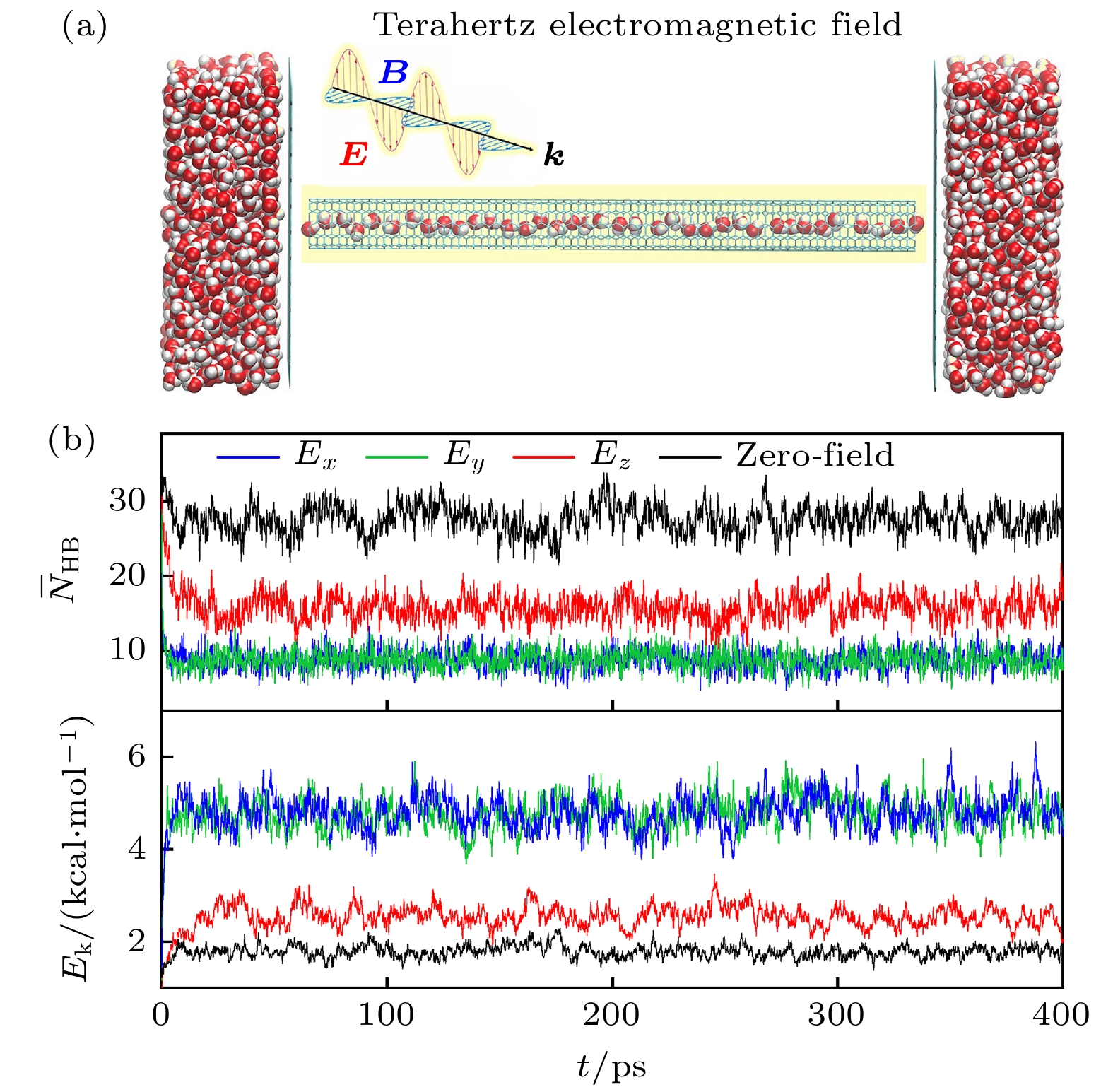
$I_{\rm{H_{2}O} }^{\rm{SW}}$ ,$I_{ {{\rm{D}}_{\rm{2}}}{\rm{O}} }^{\rm{SW}}$ and$ I_{\rm{H_{2}O/D_{2}O=1} }^{\rm{SW}} $ represent the light water, heavy water and mixed water with$ {{\rm{H}}_{\rm{2}}}{\rm{O/}}{{\rm{D}}_{\rm{2}}}{\rm{O}}= $ 1 inside the (6, 6) SWNT, respectively. The insert shows the diagram of the mixed water at a 1 to 1 intervals, where the red, white and blue balls stand for O, H and D atoms, respectively.图 6 (a)水的太赫兹效应模拟示意图, 其中纳米管中的水被施加了太赫兹电磁场 (terahertz electromagnetic field), 其中电场E, 磁场B, 传播k方向构成右手螺旋关系; (b)碳纳米管中水的平均氢键数
$\overline{N}_{{\rm{HB}}}$ 和平均动能$\overline{E}_{{\rm{k}}}$ 随模拟时间t的变化, 图中蓝色、绿色、红色和黑色曲线分别代表$E_x$ ,$E_y$ ,$E_z$ 和zero-field (${{E}}_0$ = 0) 情况Fig. 6. Schematic diagram of water terahertz effect simulation. The water inside the SWNT are applied with the terahertz electric field. The E, B, k form a right-hand spiral relationship. (b) The average H-bond number (
$\overline{N}_{{\rm{HB}}}$ ) and kinetic energy ($\overline{E}_{{\rm{k}}}$ ) of water and inside the SWNT with time t. The blue, green, red and black curves represent the cases of$E_x$ ,$E_y$ ,$E_z$ and zero-field (${{E}}_0$ = 0)表 1 柔性TIP3P水模型力场参数.
$r_{_{\rm{OH}}}$ 表示H—O 键的平衡距离;$\theta_{_{\rm{HOH}}}$ 表示角H—O—H的平衡角度;$q_{\rm{H}}$ ,$q_{\rm{O}}$ 为氢原子、氧原子的电荷量; k,$k_{{\theta}}$ 为伸缩振动和弯曲振动的弹性系数Table 1. Force field parameters of flexible TIP3P water model. The
$r_{\rm{{OH}}}$ represents the equilibrium distance of O—H bond;$\theta_{_{\rm{HOH}}}$ is the equilibrium angle of angle H—O—H;$q_{\rm{H}}$ ,$q_{\rm{{O}}}$ are the charge amount of hydrogen and oxygen atoms; k,$k_{{\theta}}$ are the elastic coefficients of stretching and bending vibrationsParameter Value $\sigma_{{\rm{OO} }}/ $Å 3.1506 $\epsilon_{ {\rm{OO} } }/(\rm{kcal {\cdot} mol^{-1} })$ 0.1521 $r_{{\rm{OH}}}/$Å 0.9572 $q_{\rm{H} } / {{e_0} }$ 0.4170 $q_{ {\rm{O} } }/ {{e_0} }$ –0.8340 $\theta_{ {\rm{HOH} } }/(^\circ)$ 104.52 kθ/(kcal·mol–1·rad–2) 55 k/(kcal·mol–1·Å–2) 450 -
[1] Ball P 2008 Chem. Rev. 108 74
 Google Scholar
Google Scholar
[2] 方海平 2016 65 186101
 Google Scholar
Google Scholar
Fang H P 2016 Acta Phys. Sin. 65 186101
 Google Scholar
Google Scholar
[3] 叶树集, 李传召, 张佳慧, 谈军军, 罗毅 2019 68 013101
 Google Scholar
Google Scholar
Ye S J, Li C Z, Zhang J H, Tan J J, Luo Y 2019 Acta. Phys. Sin. 68 013101
 Google Scholar
Google Scholar
[4] Heyden M, Tobias D J 2013 Phys. Rev. Lett. 111 218101
 Google Scholar
Google Scholar
[5] 王强, 曹则贤 2019 68 015101
 Google Scholar
Google Scholar
Wang Q, Cao Z X 2019 Acta Phys. Sin. 68 015101
 Google Scholar
Google Scholar
[6] Hassan J, Diamantopoulos G, Homouz D, Papavassiliou G 2016 Nanotechnol. Rev. 5 341
[7] Wang C L, Lu H J, Wang Z G, Xiu P, Zhou B, Zuo G H, Wan R Z, Hu J, Fang H P 2009 Phys. Rev. Lett. 103 137801
 Google Scholar
Google Scholar
[8] Laage D, Elsaesser T, Hynes J T 2017 Chem. Rev. 117 10694
 Google Scholar
Google Scholar
[9] Groot B L, Grubmuller H 2001 Science 294 2353
 Google Scholar
Google Scholar
[10] Jensen M, Borhani D W, Lindorff-Larsen K, Maragakis P, Jogini V, Eastwood M P, Dror R O, Shaw D E 2002 Proc. Natl. Acad. Sci. U.S.A. 99 6731
 Google Scholar
Google Scholar
[11] Akeson M, Deamer D W, Branton D, Kasianowicz J J, Brandin E 1999 Biophys. J. 60 101
[12] Pomès R, Roux B 1996 Biophys. J. 71 19
 Google Scholar
Google Scholar
[13] Jiang Y X, Lee A, Chen J, Cadene M, Chait B T, MacKinnon R 2002 Nature 417 515
 Google Scholar
Google Scholar
[14] Hummer G, Jayendran C, Rasaiah, Noworyta J P 2001 Nature 414 188
 Google Scholar
Google Scholar
[15] Holt J K, Park H G, Wang Y M, Stadermann M, Artyukhin A, Grigoropoulos C, Noy A, Bakajin O 2006 Science 312 1034
 Google Scholar
Google Scholar
[16] Xue M M, Hu Z L, Qiu H, Shen C, Guo W, Zhang Z 2021 Natl. Sci. Rev. 9 9
[17] Zhang Q L, Jiang W Z, Liu J, Miao R D, Sheng N 2013 Phys. Rev. Lett. 110 254501
 Google Scholar
Google Scholar
[18] Kalra A, Garde S, Hummer G 2003 Proc. Natl. Acad. Sci. U.S.A. 100 10175
 Google Scholar
Google Scholar
[19] Tu Y, Xiu P, Wan R, Hu J, Zhou R, Fang H 2009 Proc. Natl. Acad. Sci. U.S.A. 106 18120
 Google Scholar
Google Scholar
[20] García F R, Sansom M S 2012 Proc. Natl. Acad. Sci. U.S.A. 109 6939
 Google Scholar
Google Scholar
[21] Oyarzua E, Walther J H, Megaridis C M, Koumoutsakos P, Zambrano H A 2017 ACS Nano. 11 9997
 Google Scholar
Google Scholar
[22] Fang H P, Wan R Z, Gong X J, Lu H J, Li J C 2008 J. Phys. D: Appl. Phys. 41 103002
 Google Scholar
Google Scholar
[23] Fan W, Chen J G 2020 Phys. Rev. E 101 010101
[24] Jin L, Zhang D P, Zhu Y, Yang X R, Gao Y, Wang Z G 2020 J. Phys. Chem. Lett. 12 350
[25] Du Q, Freysz E, Shen Y R 1994 Phys. Rev. Lett. 72 238
 Google Scholar
Google Scholar
[26] Gan W, Wu D, Zhang Z, Feng R R, Wang H F 2006 J. Chem. Phys. 124 114705
 Google Scholar
Google Scholar
[27] Zhang J, Tan J, Pei R, Ye S, Luo Y 2021 J. Am. Chem. Soc. 143 13074
 Google Scholar
Google Scholar
[28] Alfarano S R, Pezzotti S, Stein C J, et al. 2021 Proc. Natl. Acad. Sci. U.S.A. 118 47
[29] Praprotnik M, Janežic D, Mavri J 2004 J. Phys. Chem. A 108 11056
 Google Scholar
Google Scholar
[30] Al-Abadleh H A, Grassian V 2005 Langmuir 19 341
[31] Bezerra D M, Rodrigues J E, Assaf E 2017 Mater. Charact. 125 29
 Google Scholar
Google Scholar
[32] Tayal V P, Srivastava B K, Khandelwal D P, Bist H D 1980 Appl. Spectrosc. Rev. 16 43
 Google Scholar
Google Scholar
[33] Carey D M, Korenowski G M 1996 J. Chem. Phys. 108 2669
[34] Lepodise L M, Horvat J, Lewis R A 2013 Phys. Chem. Chem. Phys. 15 20252
[35] Dalla B S, Paineau E, Brubach J B, Judeinstein P, Rouzière S, Launois P, Roy P 2016 J. Am. Chem. Soc. 15 20252
[36] Nihonyanagi S, Yamaguchi S, Tahara T 2010 J. Am. Chem. Soc. 132 6867
 Google Scholar
Google Scholar
[37] Praprotnik M, Janezic D, Merzel F 2005 J. Chem. Phys. 122 174101
 Google Scholar
Google Scholar
[38] Nagata Y, Mukamel S 2010 J. Am. Chem. Soc. 132 6434
 Google Scholar
Google Scholar
[39] Imoto S, Xantheas S S, Saito S 2015 J. Phys. Chem. B. 119 11068
 Google Scholar
Google Scholar
[40] Yang R Y, Jiang W Z, Huo P Y 2022 J. Mol. Liq. 366 120286
 Google Scholar
Google Scholar
[41] William H, Andrew D, Klaus S 1996 J. Mol. Graphics Modell. 14 33
 Google Scholar
Google Scholar
[42] Phillips J C, Braun R, Wang W, et al. 2005 J. Comput. Chem. 26 1781
 Google Scholar
Google Scholar
[43] MacKerell A D, Bashford D, Bellott M, et al. 1998 J. Phys. Chem. B 102 3586
 Google Scholar
Google Scholar
[44] Darden T, York D, Pedersen L 1993 J. Chem. Phys. 98 10089
 Google Scholar
Google Scholar
[45] Jorgensen W L, Chandrasekhar J, Madura J D, Impey R W, Klein M L 1983 J. Chem. Phys. 79 926
 Google Scholar
Google Scholar
[46] Kou J, Yao J, Lu H J, Zhang B, Li A F, Sun Z X, Zhang J G, Fang Y Z, Wu F M, Fan J T 2015 Angew. Chem. Int. Ed. Engl. 54 2351
 Google Scholar
Google Scholar
[47] Lu Y, Wang Y, Xu C, Xie C, Li W, Ding J, Zhou W, Qin Z, Shen X, Luo L 2021 Nanoscale 13 1000
 Google Scholar
Google Scholar
[48] Zhu Z, Sheng N, Wan R, Fang H J 2014 Phys. Chem. A 118 8936
 Google Scholar
Google Scholar
[49] Guo Y W, Qin J Y, Hu J H, Cao J H, Zhu Z, Wang C L 2020 Nucl. Sci. Tech. 31 53
 Google Scholar
Google Scholar
[50] Perakis F, Marco L D, Shalit A, et al. 2016 Chem. Rev. 116 7590
 Google Scholar
Google Scholar
[51] Cygan R T, Daemen L L, Ilgen A G, Krumhansl J L, Nenoff T M 2015 J. Phys. Chem. 11 9
[52] Maréchal Y 2011 J. Mol. Struct. 1004 146
 Google Scholar
Google Scholar
[53] Heyden M, Sun J, Funkner S, Mathias G, Forbert H, Havenith M, Marxb D 2010 Proc. Natl. Acad. Sci. U.S.A. 107 12068
 Google Scholar
Google Scholar
[54] 段铜川, 闫韶健, 赵妍, 孙庭钰, 李阳梅, 朱智 2021 70 248702
 Google Scholar
Google Scholar
Duan T C, Yan S J, Zhao Y, Sun T Y, Li Y M, Zhu Z 2021 Acta Phys. Sin. 70 248702
 Google Scholar
Google Scholar
[55] Zhang Q L, Wu Y X, Yang R Y, Zhang J L, Wang R F 2021 Chem. Phys. Lett. 762 138139
 Google Scholar
Google Scholar
[56] Sun Q 2009 Vib. Spectrosc. 51 213
 Google Scholar
Google Scholar
[57] Zhang Q L, Yang R Y, Jiang W Z, Huang Z Q 2016 Nanoscale 28 1886
[58] Zhu Z, Chang C, Shu Y, Song B 2020 J. Phys. Chem. Lett. 11 256
 Google Scholar
Google Scholar
[59] Conti N V, Havenith M 2014 J. Am. Chem. Soc. 136 12800
 Google Scholar
Google Scholar
[60] Yang R Y, Huang Z Q, Wei S N, Zhang Q L, Jiang W Z 2017 J. Mol. Liq. 229 148
 Google Scholar
Google Scholar
[61] Saitta A M, Saija F, Giaquinta P V 2012 Phys. Rev. Lett. 108 207801
 Google Scholar
Google Scholar
[62] Ma M, Grey F, Shen L, Urbakh M, Wu S, Liu J Z, Liu Y L, Zheng Q S 2015 Nat. Nanotechnol. 10 692
 Google Scholar
Google Scholar
[63] Zhang Q L, Yang R Y, Wang C L, Hu J 2022 Phys. Rev. Fluids 7 114202
 Google Scholar
Google Scholar
[64] 彭晓昱, 周欢 2021 70 240701
 Google Scholar
Google Scholar
Peng X Y, Zhou H 2021 Acta Phys. Sin. 70 240701
 Google Scholar
Google Scholar
[65] Sun T Y, Zhu Z 2022 J. Membr. Sci. 662 121026
 Google Scholar
Google Scholar
计量
- 文章访问数: 8474
- PDF下载量: 157
- 被引次数: 0














 下载:
下载: