-
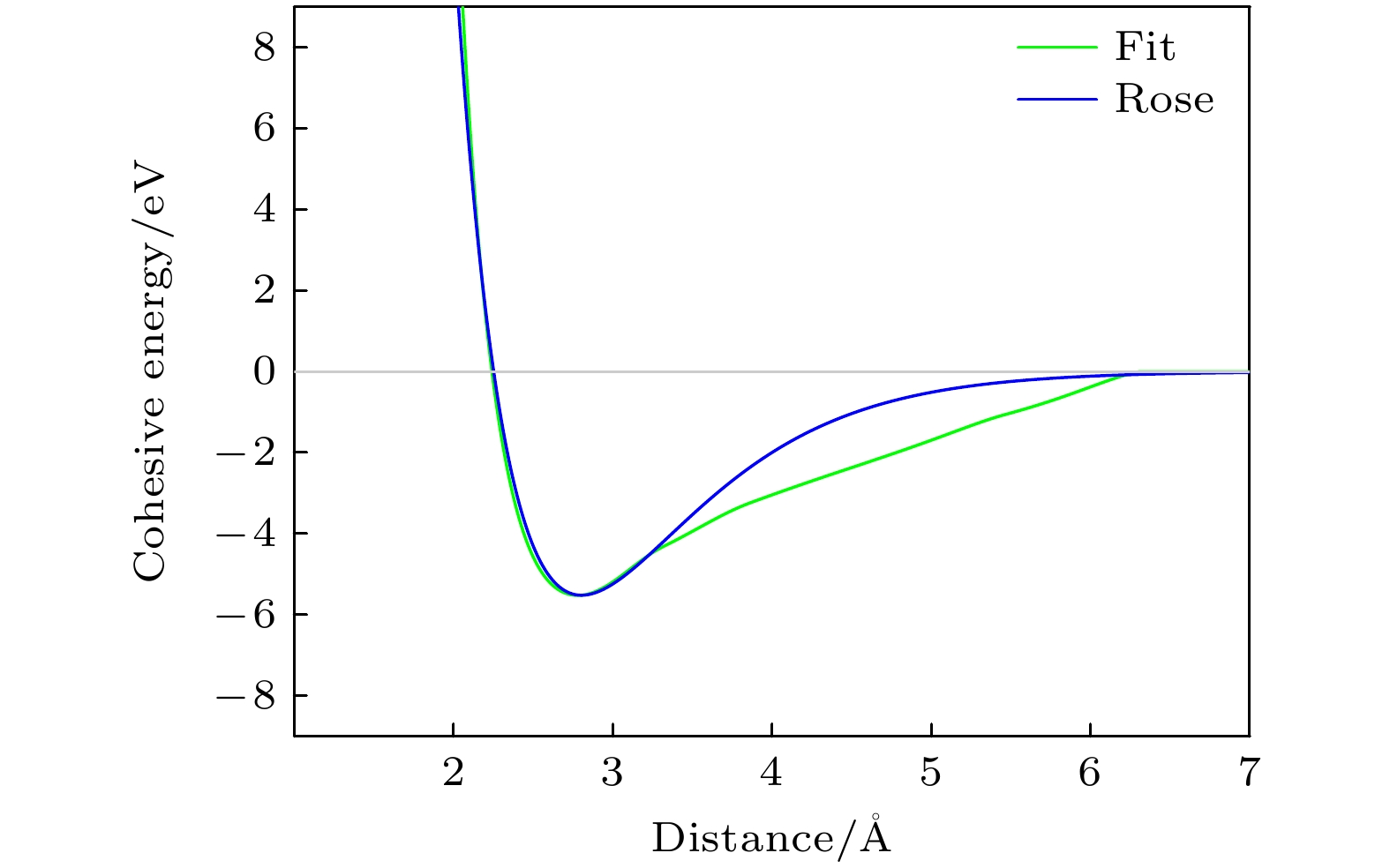
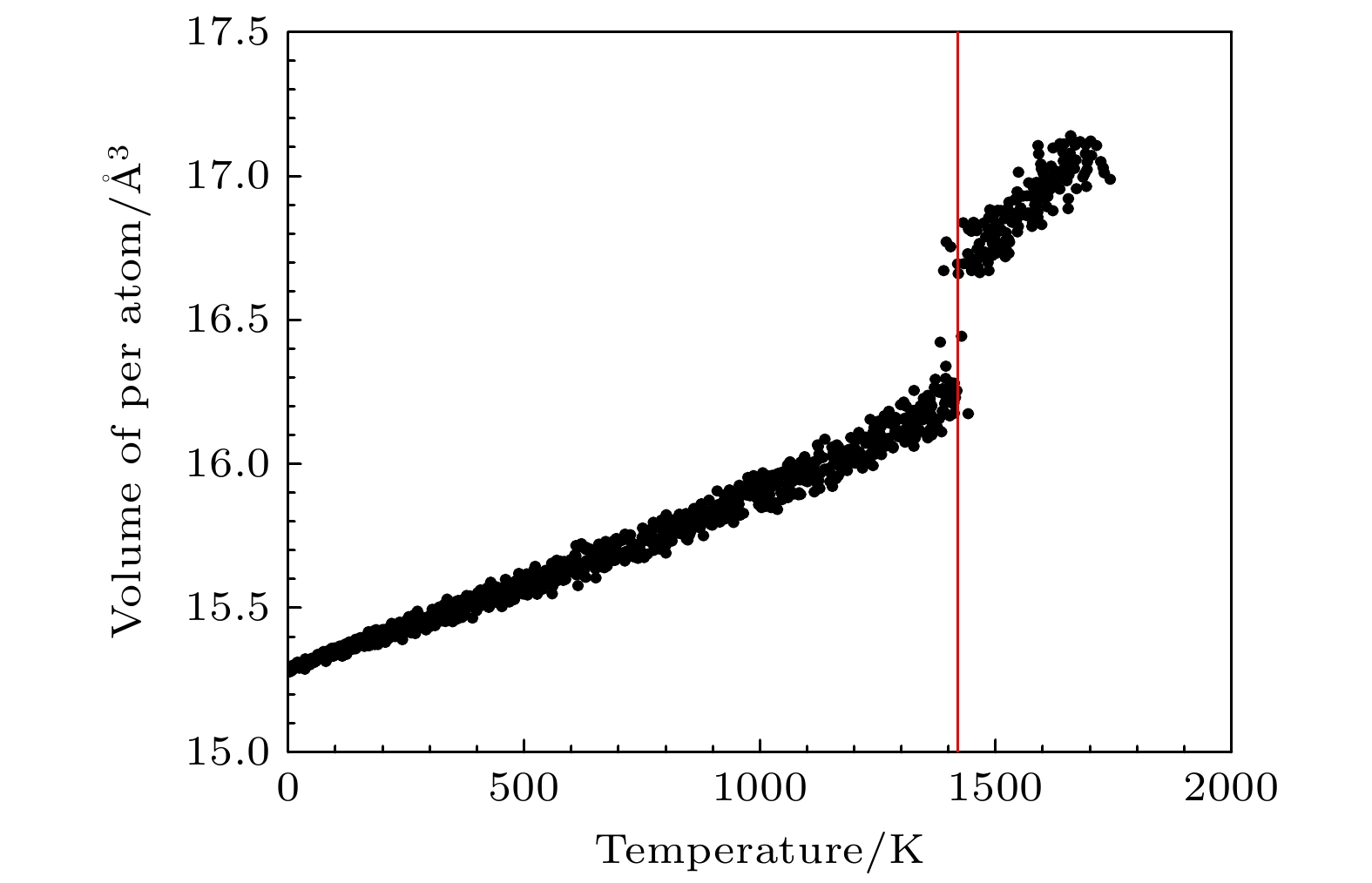
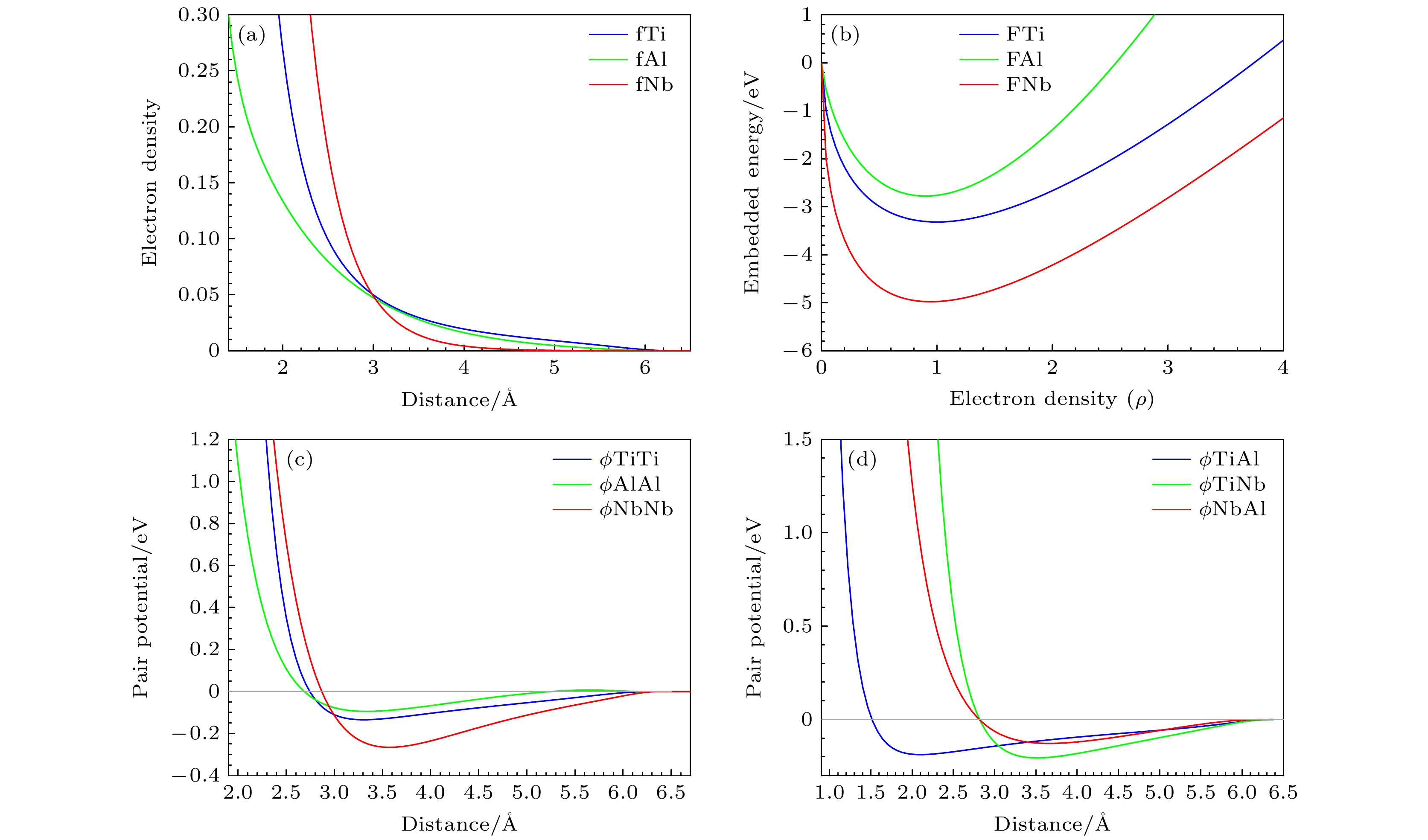
分子动力学模拟是一种行之有效的计算机模拟方法; 然而, 由于缺少合适的多元合金原子间势, 因而限制了分子动力学模拟的应用. 多元合金原子间势的开发一直具有挑战性. 本文在嵌入原子势模型的框架下, 提出一种适用于三元有序合金的原子间势构建方法, 并开发了适用于原子尺度力学行为模拟的Ti2AlNb合金新型原子间势. 该势能够很好地再现B2-Ti2AlNb的弹性常数、未弛豫的空位形成能、置换原子形成能、换位原子形成能、表面能和三种有序构型(B2相、D019相、O相)在不同体积下的内聚能. 为了进一步检验势函数, 计算了B2相的E-V曲线, 结果与Rose曲线符合得很好; 利用分子动力学模拟研究了B2相的熔化转变过程, 结果大致反映了实验情况. 本文的工作一方面为开发多元合金原子间势提供一种途径, 另一方面为模拟计算Ti2AlNb合金的工作者提供一种选择.Molecular dynamics simulation is an effective computer simulation method. However, owing to the lack of suitable interatomic potential of multicomponent alloys, the application of molecular dynamics simulation is limited. The development of interatomic potential of multicomponent alloys has always been challenging. In this work, under the framework of EAM model, a construction method of interatomic potential suitable for ternary ordered alloys is proposed, and a new interatomic potential of Ti2AlNb alloys suitable for atomic-scale mechanical behavior simulation is developed. The potential can well reproduce the elastic constants of B2-Ti2AlNb, unrelaxation vacancy formation energy, substitutional atom formation energy, transposition atom formation energy, surface energy and cohesive energy of three ordered phase (B2, D019 and O phases ) in different volumes. To further test the potential functions, 1) the E-V curve of B2 phase is calculated, and the result is well consistent with Rose curve; 2) the melting transformation process of B2 phase is studied by molecular dynamics simulation, and the results roughly reflect the experimental fact. The present work provides a way to develop the interatomic potential of multicomponent alloys, and a option for the workers who simulate and calculate the Ti2AlNb alloys as well.
-
Keywords:
- embedded atom method potential /
- Ti2AlNb alloys /
- defects
[1] Deringer V, Csanyi G 2017 Phys. Rev. B 95 094203
 Google Scholar
Google Scholar
[2] Alizadeh Z, Mohammadizadeh M R 2019 Physica C 558 7
 Google Scholar
Google Scholar
[3] Jordan M I, Mitchell T M 2015 Science 349 255
 Google Scholar
Google Scholar
[4] Smith J S, Nebgen B, Mathew N, Chen J, Lubbers N, Burakovsky L, Tretiak S, Nam H A, Germann T, Fensin S, Barros K 2021 Nat. Commun. 12 1257
 Google Scholar
Google Scholar
[5] Artrith N, Urban A 2016 Comput. Mater. Sci. 114 135
 Google Scholar
Google Scholar
[6] Kumpfert J 2001 Adv. Eng. Mater. 3 851
 Google Scholar
Google Scholar
[7] Boehlert C J, Majumdar B S 1999 Metall. Mater. Trans. A 30 2305
 Google Scholar
Google Scholar
[8] 冯艾寒, 李渤渤, 沈军 2011 材料与冶金学报 10 30
 Google Scholar
Google Scholar
Feng A H, Li B B, Shen J 2011 J. Mater. Metall. 10 30
 Google Scholar
Google Scholar
[9] Gogia T K, Nandy T K, Banerjee D, Carisey T, Strudel J L, Franchet J M 1998 Intermetallics 6 741
 Google Scholar
Google Scholar
[10] Banerjee D, Gogia A K, Nandi T K, Joshi V A 1988 Acta Metall. 36 871
 Google Scholar
Google Scholar
[11] Pathak A, Singh A K 2015 Solid State Commun. 204 9
 Google Scholar
Google Scholar
[12] Daw M S, Baskes M I 1984 Phys. Rev. B 29 6443
 Google Scholar
Google Scholar
[13] Cheng C, Ma Y L, Bao Q L, Wang X, Sun J X, Zhou G, Wang H, Liu Y X, Xu D S 2019 Comput. Mater. Sci. 173 109432
 Google Scholar
Google Scholar
[14] Johnson R A 1989 Phys. Rev. B 39 12554
 Google Scholar
Google Scholar
[15] Voter A F 1994 Intermetallic Compounds (New York: Wiley) pp77—80
[16] Mishin Y, Mehl M J 2002 Phys. Rev. B 65 224114
 Google Scholar
Google Scholar
[17] Ravi C, Vajeeston P, Mathijaya S, Asokamani R 1999 Phys. Rev. B:Condens. Matter. 60 683
 Google Scholar
Google Scholar
[18] Kittel C 1976 Introduction to Solid State Physics (New Jersey: John Wiley & Sons) pp57–58
[19] Weast R C 1984 Handbook of Chemistry and Physics (Boca Raton: Chemical Rubber) p64
[20] Johnson R A 1972 Phys. Rev. B 6 2094
 Google Scholar
Google Scholar
[21] Oh D J, Johnson R A 2011 Mater. Res. 3 471
 Google Scholar
Google Scholar
[22] Bolef D I 1961 Appl. Phys. 32 100
 Google Scholar
Google Scholar
[23] Agrawal A, Mishra R, Ward L, Flores K M, Windl W 2013 Modell. Simul. Mater. Sci. Eng. 21 085001
 Google Scholar
Google Scholar
[24] Farkas D 1999 Modell. Simul Mater. Sci. Eng. 4 23
 Google Scholar
Google Scholar
[25] Rose J H, Smith J R, Guinea F, Ferrante J 1984 Phys. Rev. B 29 2963
 Google Scholar
Google Scholar
[26] Julius C S, Martin P 2006 J. Phase Equilib. Diffus. 27 255
 Google Scholar
Google Scholar
[27] Vasudevan V K, Yang J, Woodfield A P 1996 Scr. Mater. 35 1033
 Google Scholar
Google Scholar
-
表 1 B2-Ti2AlNb的晶格常数和生成焓(单位: eV)
Table 1. Lattice constants and formation enthalpy for B2-Ti2AlNb (in eV).
表 2 单质Ti, Al, Nb的内聚能(单位: eV)
Table 2. Cohesive energies for Ti, Al and Nb (in eV)
表 3 Ti2AlNb合金EAM势的拟合参数
Table 3. Fitting parameters of EAM potential for Ti2AlNb alloys.
$ \phi \left({r}_{\mathrm{T}\mathrm{i}\mathrm{A}\mathrm{l}}\right) $ $ \phi \left({r}_{\mathrm{T}\mathrm{i}\mathrm{N}\mathrm{b}}\right) $ $ \phi \left({r}_{\mathrm{N}\mathrm{b}\mathrm{A}\mathrm{l}}\right) $ Parameter value Parameter value Parameter value $ \alpha $ –2.1129 $ \alpha $ –4.2151 $ \alpha $ –0.2797 $ \beta $ –1.3742 $ \beta $ –6.6808 $ \beta $ –2.5122 $ \gamma $ 0.2705 $ \gamma $ 1.4339 $ \gamma $ 1.6140 $ \epsilon $ 0.1758 $ \epsilon $ –0.0008 $ \epsilon $ 0.1140 $ \sigma $ –2.3191 $ \sigma $ –1.4062 $ \sigma $ –1.6132 $ \mu $ 0.0516 $ \mu $ 0.2694 $ \mu $ 0.4012 表 4 B2-Ti2AlNb的弹性常数(单位: GPa)
Table 4. Elastic constants for B2-Ti2AlNb (in GPa).
C11 C33 C44 C66 C12 C13 ABINIT[11] 136 — 81 — 100 — DFT 153.20 147.83 69.36 71.86 133.42 97.62 This work 195.82 178.95 64.35 66.83 136.92 127.04 表 5 B2-Ti2AlNb单空位形成能和双空位形成能(单位: eV)
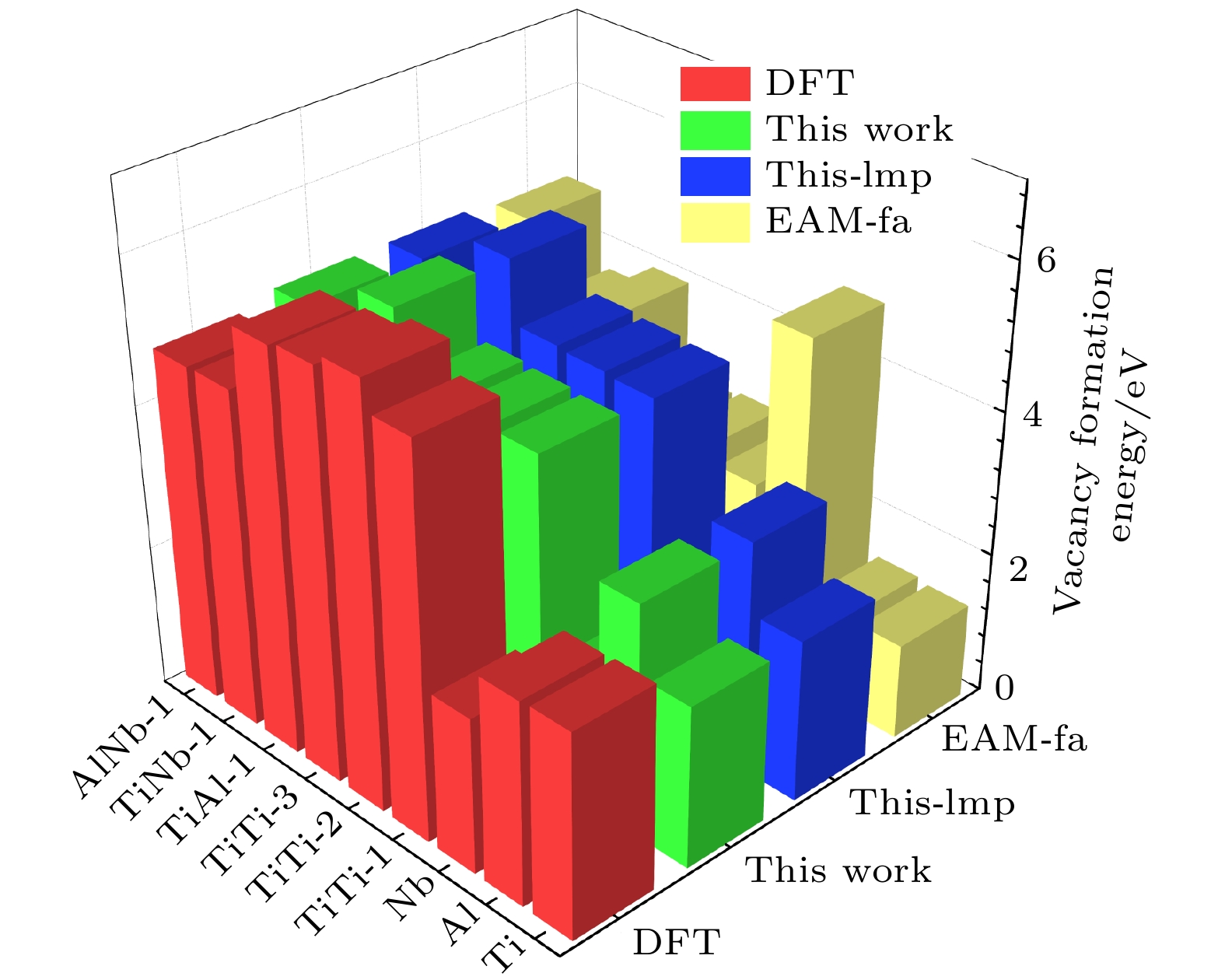
Table 5. Mono-vacancy formation energies and di-vacancy formation energies for B2-Ti2AlNb (in eV).
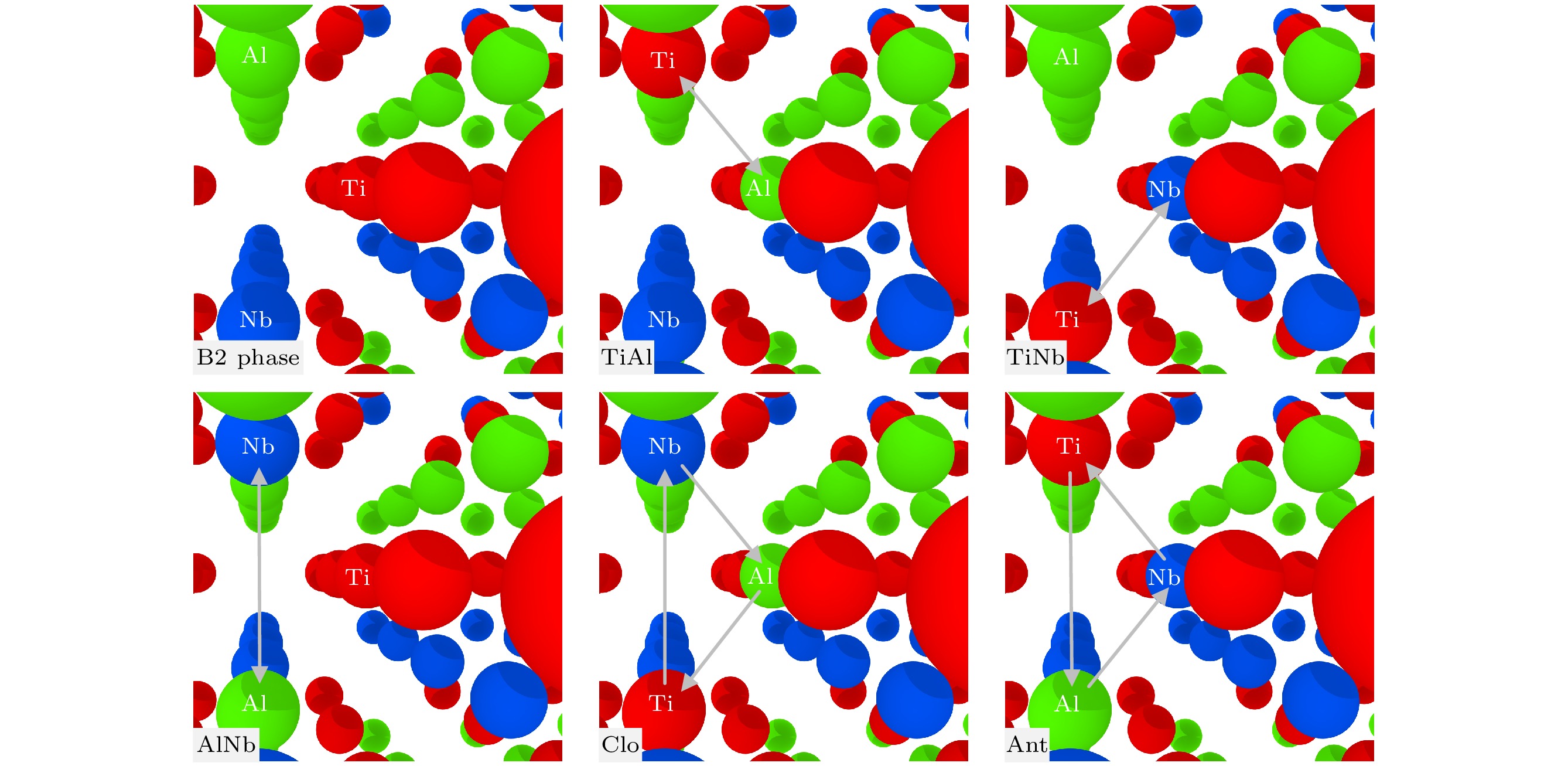
DFT This work This-lmp EAM-fa $ {E}_{\mathrm{v}}^{\mathrm{f}} $ Ti 2.879 2.283 2.283 1.343 Al 2.859 3.265 3.265 1.391 Nb 2.194 1.763 1.657 4.919 $ {E}_{\mathrm{d}}^{\mathrm{f}} $ TiTi-1 5.454 4.503 4.498 2.562 TiTi-2 5.854 4.539 4.533 2.777 TiTi-3 5.688 4.570 4.567 2.724 TiAl-1 5.623 5.434 5.434 4.014 TiNb-1 4.731 3.980 3.980 3.699 AlNb-1 4.710 4.854 4.854 4.777 表 6 B2-Ti2AlNb置换原子和换位原子形成能(单位: eV)
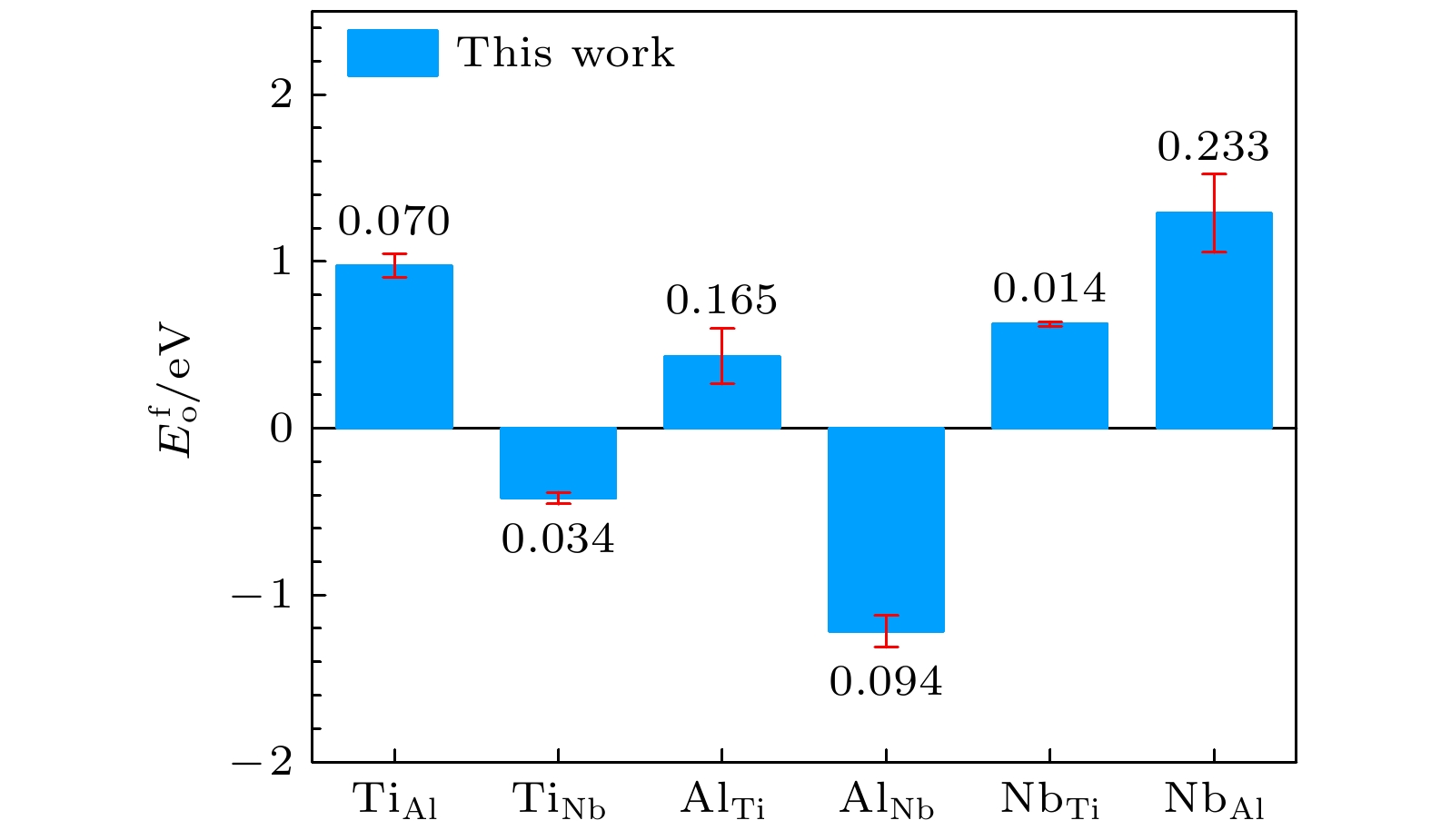
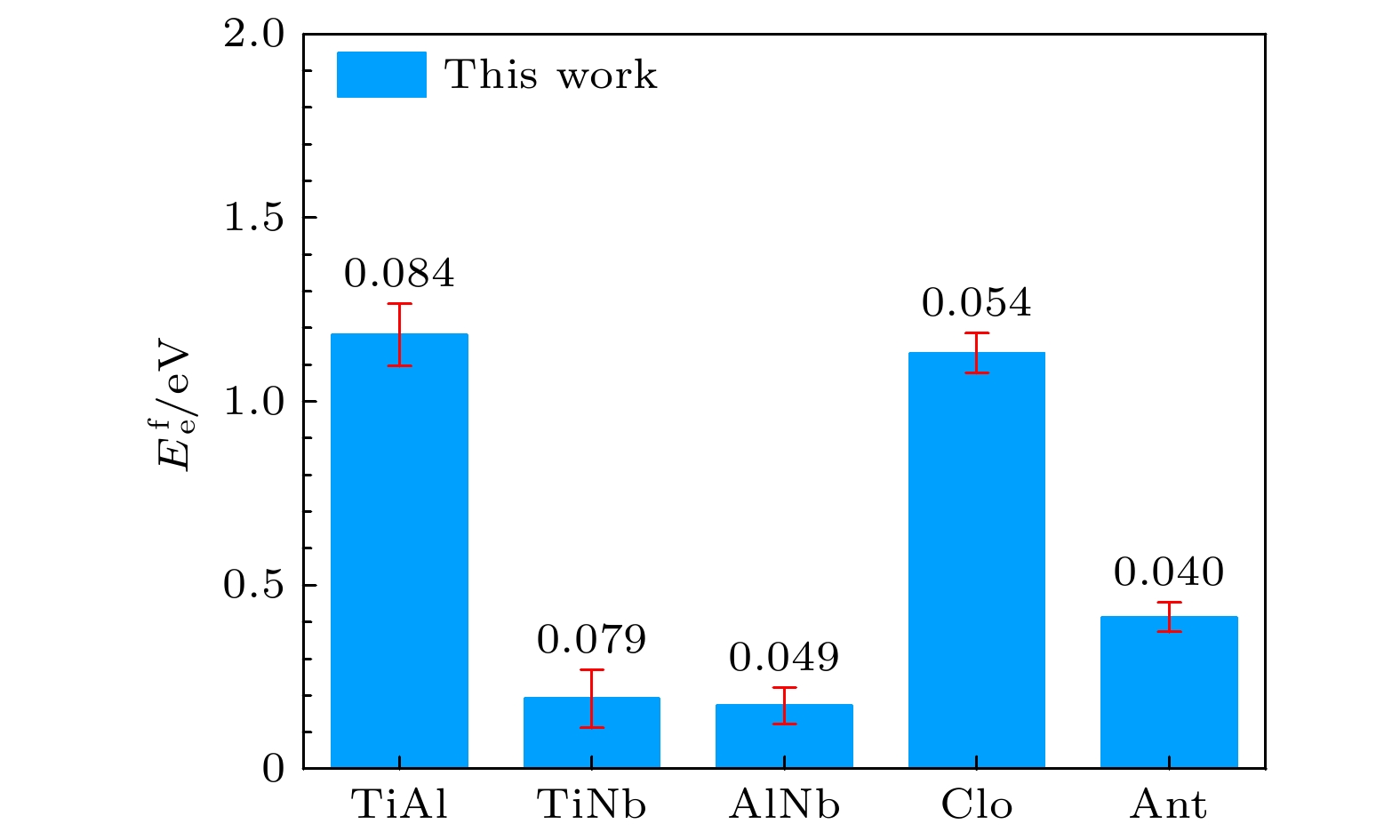
Table 6. Substitutional atom and transposition atom formation energies for B2-Ti2AlNb (in eV).
DFT This work This-lmp EAM-fa $ {E}_{\mathrm{o}}^{\mathrm{f}} $ $ {\mathrm{T}\mathrm{i}}_{\mathrm{A}\mathrm{l}} $ 1.046 0.976 0.976 –2.863 $ {\mathrm{T}\mathrm{i}}_{\mathrm{N}\mathrm{b}} $ –0.384 –0.418 –0.418 –2.440 $ {\mathrm{A}\mathrm{l}}_{\mathrm{T}\mathrm{i}} $ 0.268 0.433 0.433 –1.164 $ {\mathrm{A}\mathrm{l}}_{\mathrm{N}\mathrm{b}} $ –1.310 –1.216 –1.216 –1.113 $ {\mathrm{N}\mathrm{b}}_{\mathrm{T}\mathrm{i}} $ 0.638 0.624 0.624 –7.439 $ {\mathrm{N}\mathrm{b}}_{\mathrm{A}\mathrm{l}} $ 1.522 1.289 1.289 0.911 $ {E}_{\mathrm{e}}^{\mathrm{f}} $ TiAl 1.097 1.181 1.181 –4.142 TiNb 0.270 0.191 0.191 –9.213 AlNb 0.123 0.172 0.172 –0.571 Clo 1.078 1.132 1.132 –3.169 Ant 0.453 0.413 0.413 –10.752 表 7 B2-Ti2AlNb的表面能(单位: eV)
Table 7. Surface energies for
B2-Ti2AlNb (in eV). DFT This work This-lmp EAM-fa $ {E}_{\mathrm{s}\mathrm{u}\mathrm{r}}^{\mathrm{f}} $ (100) 2.056 2.298 2.273 1.705 (110) 1.937 2.194 2.170 1.620 (100)′ 1.844 — 1.923 0.484 (110)′ 1.661 — 1.750 –11.000 表 8 Ti2AlNb合金的内聚能(单位: eV)
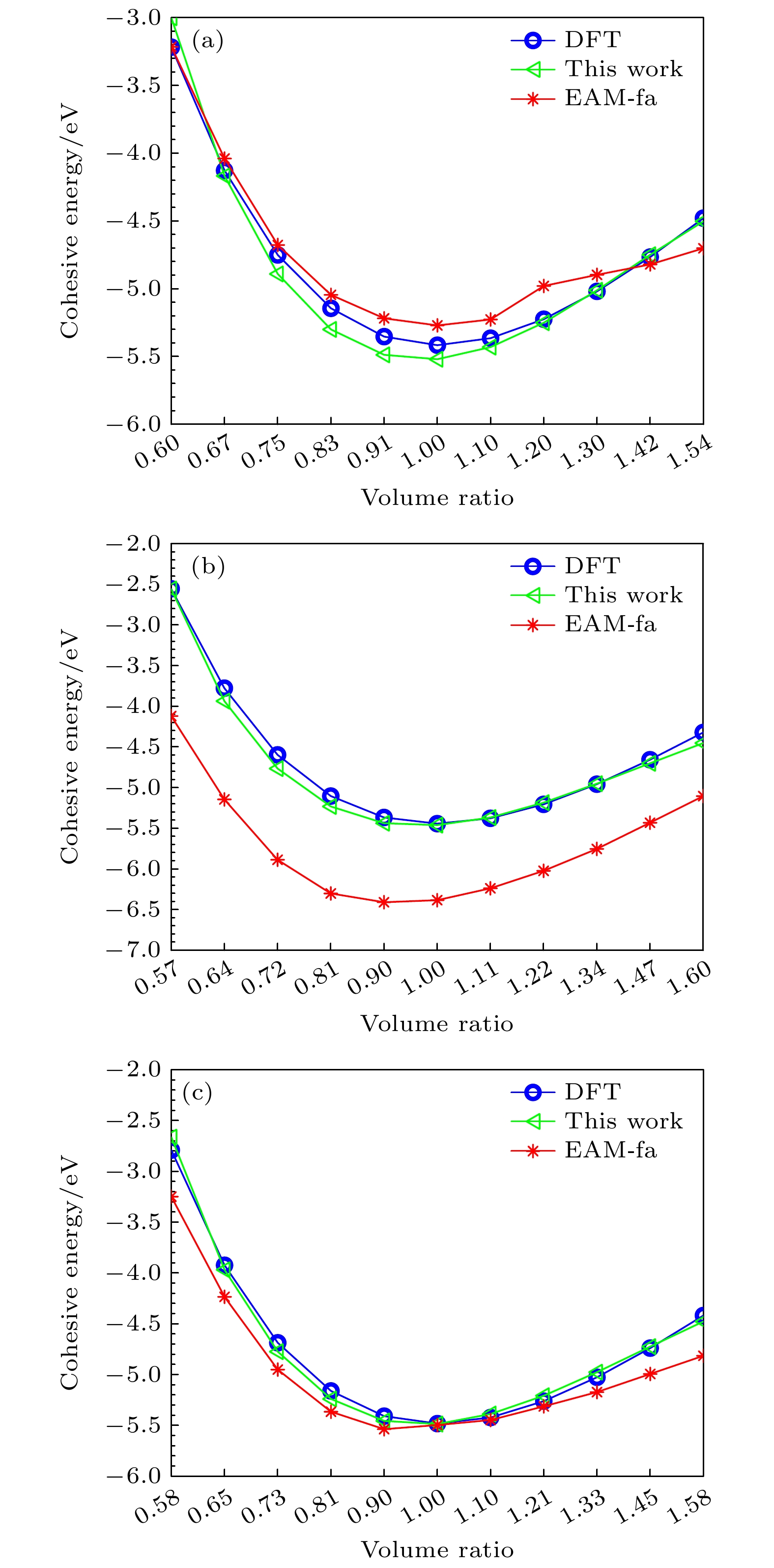
Table 8. Cohesive energies for Ti2AlNb alloys (in eV).
VB2 60% 67% 75% 83% 91% 1 110% 120% 130% 142% 154% E-DFT –3.22 –4.13 –4.75 –5.14 –5.35 –5.42 –5.37 –5.22 –5.02 –4.77 –4.48 $ V_{{\rm D0}_{19}} $ 57% 64% 72% 81% 90% 1 111% 122% 134% 147% 160% E-DFT –2.55 –3.78 –4.60 –5.10 –5.37 –5.44 –5.38 –5.21 –4.96 –4.66 –4.32 VO 58% 65% 73% 81% 90% 1 110% 121% 133% 145% 158% E-DFT –2.79 –3.92 –4.69 –5.16 –5.41 –5.48 –5.42 –5.26 –5.03 –4.74 –4.42 -
[1] Deringer V, Csanyi G 2017 Phys. Rev. B 95 094203
 Google Scholar
Google Scholar
[2] Alizadeh Z, Mohammadizadeh M R 2019 Physica C 558 7
 Google Scholar
Google Scholar
[3] Jordan M I, Mitchell T M 2015 Science 349 255
 Google Scholar
Google Scholar
[4] Smith J S, Nebgen B, Mathew N, Chen J, Lubbers N, Burakovsky L, Tretiak S, Nam H A, Germann T, Fensin S, Barros K 2021 Nat. Commun. 12 1257
 Google Scholar
Google Scholar
[5] Artrith N, Urban A 2016 Comput. Mater. Sci. 114 135
 Google Scholar
Google Scholar
[6] Kumpfert J 2001 Adv. Eng. Mater. 3 851
 Google Scholar
Google Scholar
[7] Boehlert C J, Majumdar B S 1999 Metall. Mater. Trans. A 30 2305
 Google Scholar
Google Scholar
[8] 冯艾寒, 李渤渤, 沈军 2011 材料与冶金学报 10 30
 Google Scholar
Google Scholar
Feng A H, Li B B, Shen J 2011 J. Mater. Metall. 10 30
 Google Scholar
Google Scholar
[9] Gogia T K, Nandy T K, Banerjee D, Carisey T, Strudel J L, Franchet J M 1998 Intermetallics 6 741
 Google Scholar
Google Scholar
[10] Banerjee D, Gogia A K, Nandi T K, Joshi V A 1988 Acta Metall. 36 871
 Google Scholar
Google Scholar
[11] Pathak A, Singh A K 2015 Solid State Commun. 204 9
 Google Scholar
Google Scholar
[12] Daw M S, Baskes M I 1984 Phys. Rev. B 29 6443
 Google Scholar
Google Scholar
[13] Cheng C, Ma Y L, Bao Q L, Wang X, Sun J X, Zhou G, Wang H, Liu Y X, Xu D S 2019 Comput. Mater. Sci. 173 109432
 Google Scholar
Google Scholar
[14] Johnson R A 1989 Phys. Rev. B 39 12554
 Google Scholar
Google Scholar
[15] Voter A F 1994 Intermetallic Compounds (New York: Wiley) pp77—80
[16] Mishin Y, Mehl M J 2002 Phys. Rev. B 65 224114
 Google Scholar
Google Scholar
[17] Ravi C, Vajeeston P, Mathijaya S, Asokamani R 1999 Phys. Rev. B:Condens. Matter. 60 683
 Google Scholar
Google Scholar
[18] Kittel C 1976 Introduction to Solid State Physics (New Jersey: John Wiley & Sons) pp57–58
[19] Weast R C 1984 Handbook of Chemistry and Physics (Boca Raton: Chemical Rubber) p64
[20] Johnson R A 1972 Phys. Rev. B 6 2094
 Google Scholar
Google Scholar
[21] Oh D J, Johnson R A 2011 Mater. Res. 3 471
 Google Scholar
Google Scholar
[22] Bolef D I 1961 Appl. Phys. 32 100
 Google Scholar
Google Scholar
[23] Agrawal A, Mishra R, Ward L, Flores K M, Windl W 2013 Modell. Simul. Mater. Sci. Eng. 21 085001
 Google Scholar
Google Scholar
[24] Farkas D 1999 Modell. Simul Mater. Sci. Eng. 4 23
 Google Scholar
Google Scholar
[25] Rose J H, Smith J R, Guinea F, Ferrante J 1984 Phys. Rev. B 29 2963
 Google Scholar
Google Scholar
[26] Julius C S, Martin P 2006 J. Phase Equilib. Diffus. 27 255
 Google Scholar
Google Scholar
[27] Vasudevan V K, Yang J, Woodfield A P 1996 Scr. Mater. 35 1033
 Google Scholar
Google Scholar
计量
- 文章访问数: 7707
- PDF下载量: 121
- 被引次数: 0













 下载:
下载: