-
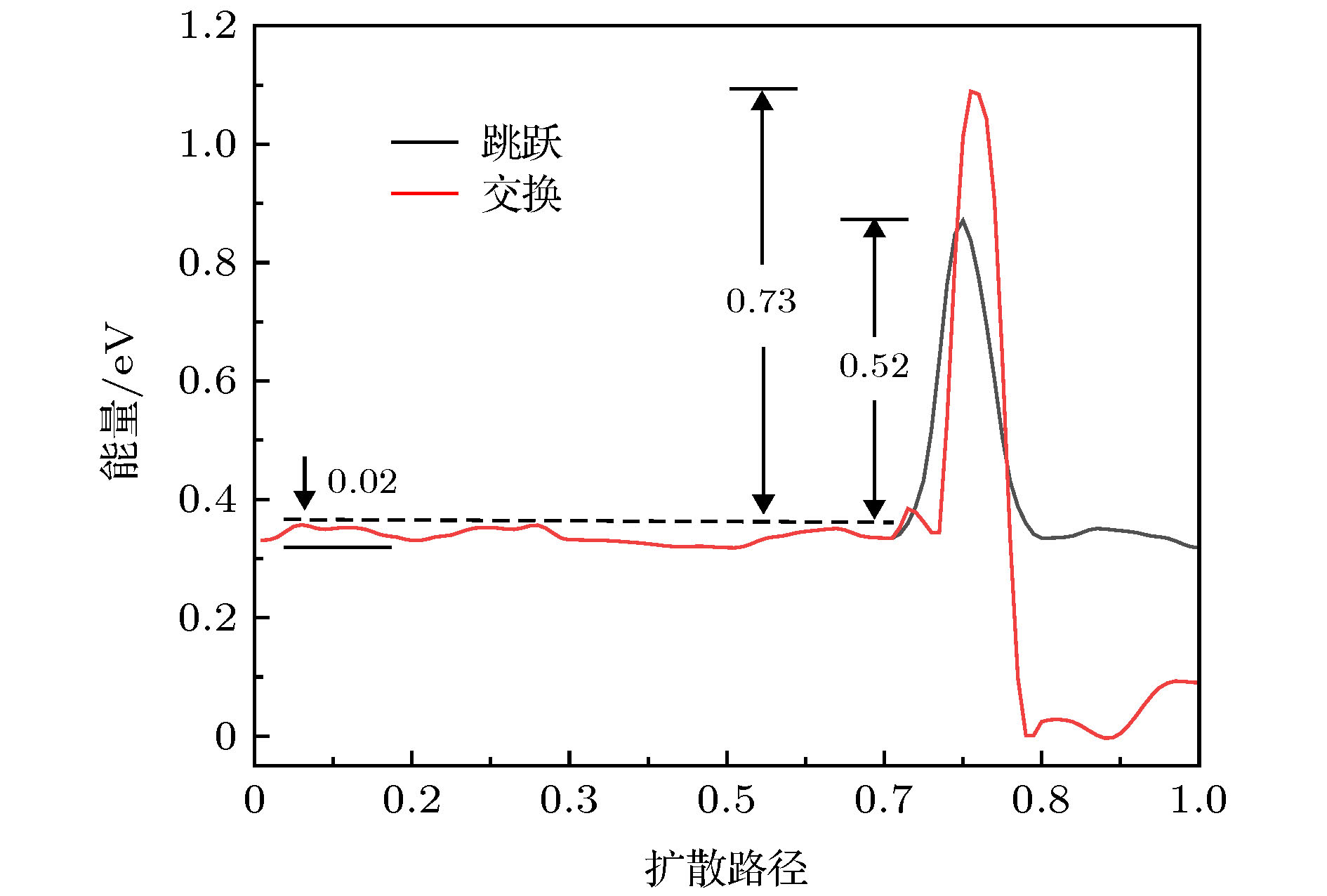
NiAl纳米颗粒具有较高的能量密度和良好的高温力学性能, 铝吸附原子在不同镍基表面上的扩散行为与不同扩散机制对铝在镍基表面沉积生长的影响有待进一步阐明. 本文通过采用肘弹性带和分子动力学结合嵌入原子势的方法, 系统地研究了单个铝吸附原子在镍基表面的扩散行为和纳米颗粒团簇在十面体(DEC)、立方八面体(CUB)和二十面体(ICO)结构上的生长. 研究结果表明: Al吸附原子在三种Ni基底上表面扩散的交换与跳跃两种机制, 最低的Ehrlich-Schwoebel(ES)势垒为0.38 eV (交换CUB{111} → 100})、0.52 eV (交换DEC{111} → 100})和0.52 e V (跳跃ICO{111} → 111}), 从{111}面扩散到{100}面主要以交换机制为主, 而相邻两个{111}面之间的扩散则以跳跃机制为主. 沉积的铝原子首先倾向于扩散到台阶边缘和顶点附近. 随着Al原子数量的增加, 沉积的Al原子开始聚集. 对于Ni团簇上的Al原子, 在较低温度下在镍基底表面沉积Al原子, 可以获得良好的Ni核/Al壳结构. 对于二十面体结构基底, 其对应的核壳团簇的缺陷数最小, 随后为十面体结构和八面体结构. 随着生长温度的增加NiAl纳米粒子的表面逐渐开始合金化.
-
关键词:
- Ni基衬底 /
- 表面扩散 /
- 纳米粒子生长 /
- 核壳结构 /
- 嵌入原子势(EAM)
NiAl nanoparticles possess high-energy density and good mechanical properties at elevated temperatures, and are considered as an important material. However, the differences in the diffusion behavior of Al adsorbed atoms on different Ni substrate surfaces and the effects of different diffusion mechanisms on the deposition growth of Al atoms on the Ni substrate surface are highly desired to be clarified. Therefore, in the present work, the diffusion behavior of single Al adsorbed atoms and nanoparticle cluster growth on the Ni substrate surface of decahedral (DEC), cuboctahedral(CUB) and icosahedral(ICO) structures are systematically studied by molecular dynamics (MD) throuh analyzing the embedded atom potentialand using the nudged elastic band method. The diffusion barriers of Al adsorbed atoms on three different Ni substrates are calculated by nudged elastic band methodand analyzed, showing that the diffusion barrier is greatly affected by the smoothness of the step edge and the atomic coordination number of substrate as well. The diffusions of Al adsorption atoms on the surfaces of three Ni substrates are realized by two mechanisms, namely exchanging or hoping, and the lowest Ehrlich-Schwoebel (ES) barrier is 0.38 eV for exchange CUB{111} → {100}, 0.52 eV for exchange DEC{111} → {100}, and 0.52 eV for hoping ICO {111} → {111}. The exchanging mechanismsupports Al adatoms diffusing from {111} to {100} facet on the three Ni substrates, while the diffusion between two adjacent {111} facets is mainly driven by the hoping mechanism. On this basis, atom-by-atom growth MD simulation is used to study the structure of the Ni-Al cluster. The deposited Al atoms first tend to diffuse near the edges of the steps and the vertices. The deposited Al atoms begin to aggregate into islands with the increase of their number. For Al atoms on the Ni cluster, a good Ni-core/Al-shell structure can be obtained by depositing Al atoms on the surface of Ni substrate at lower temperatures. In this core-shell structure, Al atoms have a larger surface energy and atom radius compared with Ni atoms. For the ICO substrate, the corresponding defect number of core-shell clusters is smaller than for the CUB and the DEC substrate, which is in good agreement with the diffusion behavior of Al adsorbed atoms on the Ni substrate cluster surface. The surface of Ni-Al bimetal is gradually alloyed with the increase of growth temperature. This study provides a good insight into the diffusion and growth of Al adsorbed atoms on Ni substrates surface on an atomic scale.-
Keywords:
- Ni substrate /
- surface diffusion /
- nanoparticle growth /
- core-shell structure /
- Embedded atomic potential (EAM)
[1] Li T T, He C, Zhang W X, Cheng M 2018 J. Alloys Compd. 752 76
 Google Scholar
Google Scholar
[2] Riccardo F, Julius J, Johnston R L 2008 Chem. Rev. 108 845
 Google Scholar
Google Scholar
[3] Yang J Y, Hu W Y, Wu Y R, Dai X Y 2012 Surf. Sci. 606 971
 Google Scholar
Google Scholar
[4] Yang J Y, Hu W Y, Wu Y R, Dai X Y 2012 Cryst. Growth Des. 12 2978
 Google Scholar
Google Scholar
[5] Baletto F, Mottet C, Rapallo A, Rossi G, Ferrando R 2004 Surf. Sci. 566 192
[6] Song P X, Wen D S 2010 J. Phys. Chem. C 114 8688
 Google Scholar
Google Scholar
[7] Ferrer D, Torres-Castro A, Gao X, Sepúlveda-Guzmán S, Ortiz-Méndez U, José-Yacamán M 2007 Nano Lett. 7 1701
 Google Scholar
Google Scholar
[8] Baletto F, Mottet C, Ferrando R 2003 Eur. Phys. J. D 24 233
 Google Scholar
Google Scholar
[9] Deng L, Hu W Y, Deng H Q, Xiao S F 2010 J. Phys. Chem. C 114 11026
 Google Scholar
Google Scholar
[10] Deng L, Hu W Y, Deng H Q, Xiao S F, Tang J F 2011 J. Phys. Chem. C 115 11355
[11] Rapallo A, Rossi G, Ferrando R, et al. 2005 J. Chem. Phys. 122 194308
 Google Scholar
Google Scholar
[12] Henglein A 2000 J. Phys. Chem. B 104 2201
 Google Scholar
Google Scholar
[13] Dai X Y, Hu W Y, Yang J Y, Yi G J 2017 Thin Solid Films 626 178
 Google Scholar
Google Scholar
[14] Yang J Y, Hu W Y, Tang J F, Dai X Y 2013 Comput. Mater. Sci. 74 160
 Google Scholar
Google Scholar
[15] De S, Zhang J G, Luque R, Yan N 2016 Energy Environ Sci. 9 3314
 Google Scholar
Google Scholar
[16] Baletto F, Mottet C, Ferrando R 2003 Phys. Rev. Lett. 90 135504
 Google Scholar
Google Scholar
[17] Yang J Y, Hu W Y, Tang J F 2013 RSC Adv. 4 2155
[18] Shyrokorad D, Kornich G, Buga S 2019 Comput. Mater. Sci. 159 110
 Google Scholar
Google Scholar
[19] Mottet C, Rossi G, Baletto F, Ferrando R 2005 Phys. Rev. Lett. 95 035501
 Google Scholar
Google Scholar
[20] Purja Pun G P, Mishin Y 2009 Philos. Mag. 89 3245
 Google Scholar
Google Scholar
[21] 邓永和, 文大东, 彭超, 韦彦丁, 赵瑞, 彭平 2016 65 066401
 Google Scholar
Google Scholar
Deng Y H, Wen D D, Peng C, Wei Y D, Zhao R, Peng P 2016 Acta Phys. Sin. 65 066401
 Google Scholar
Google Scholar
[22] 彭超, 李媛, 邓永和, 彭平 2017 金属学报 53 1659
 Google Scholar
Google Scholar
Peng C, Li Y, Deng Y H, Peng P 2017 Acta Metal. Sin. 53 1659
 Google Scholar
Google Scholar
[23] Deng Y H, Wen D D, Li Y, Liu J, Peng P 2018 Philos. Mag. 98 2861
 Google Scholar
Google Scholar
[24] Wu B, Zhou J Q, Xue C, Liu H X 2015 Appl. Surf. Sci. 355 1145
 Google Scholar
Google Scholar
[25] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[26] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[27] Yanting W, Teitel S, Christoph D 2005 J. Chem. Phys. 122 9673
[28] Vitos L, Ruban A V, Skriver H L, Kollar J 1998 Surf. Sci. 411 186
 Google Scholar
Google Scholar
[29] Abbaspour M, Akbarzadeh H, Lotfi S 2018 J. Alloys Compd. 764 323
 Google Scholar
Google Scholar
[30] Wang H, Hu T, Qin J Y, Zhang T 2012 J. Appl. Phys. 112 073520
 Google Scholar
Google Scholar
[31] 高明, 邓永和, 文大东, 田泽安, 赵鹤平, 彭平 2020 69 046401
 Google Scholar
Google Scholar
Gao M, Deng Y H, Wen D D, Tian Z A, Zhao H P, Peng P 2020 Acta Phys. Sin. 69 046401
 Google Scholar
Google Scholar
[32] Wang Y, Liu Z K, Chen L Q 2004 Acta Mater. 52 2665
 Google Scholar
Google Scholar
[33] Mishin Y, Mehl M J, Papaconstantopoulos D A 2002 Phys. Rev. B 65 392
[34] Ashcroft N W, Mermin N D 1976 Solid State Physics. (Saunders, Philadelphia) pp216–217, 228–229
[35] Pearson W B, Villars P, Calvert L D 1985 ASM 3 258
[36] Rzyman K, Moser Z 2004 Prog. Mater. Sci. 49 581
 Google Scholar
Google Scholar
[37] Ayrault G, Ehrlich G 1974 J. Chem. Phys. 60 281
 Google Scholar
Google Scholar
[38] Ehrlich G, Hudda F G 1966 J. Chem. Phys. 44 1039
 Google Scholar
Google Scholar
[39] Yildirim H, Rahman T S 2009 Phys. Rev. B: Condens. Matter 80 235413
 Google Scholar
Google Scholar
[40] Yang L Y, Gan X L, Xu C, et al. 2019 Comput. Mater. Sci. 156 47
 Google Scholar
Google Scholar
-
图 1 含有923个原子的CUB (a), DEC (b)和ICO (c)结构示意图, 三种结构是以壳层组成的非晶结构, 其中DEC和CUB由{111}和{100}面组成, 而ICO仅由{111}面组成
Fig. 1. CUB (a), DEC (b) and ICO (c) microstructure with 923 atoms. The three clusters are a non-crystalline structure organized in shells. The DEC and CUB consist of {111} and {100} facets, and the ICO has only {111} facets.
图 3 对于Al 吸附原子在CUB923, DEC923和ICO923结构的Ni基底表面上, 通过跳跃和交换机制, 吸附原子从{111}面向{100}面或向{111}面扩散的路径 (a) Hopping CUB{111} → {100}; (b) Exchange CUB{111} → {100}; (c) Exchange CUB{111} → {111}; (d) Exchange DEC{111} → {100}; (e) Hopping DEC{111} → {100}; (f) Exchange DEC{111} → {111}; (g) Hoping DEC{111} → {111}; (h) Exchange ICO{111} → {111}; (i) Hoping ICO{111} → {111}. 橙色的球表示铝原子, 灰色的球表示镍原子
Fig. 3. For the Al adatoms on the surfaces of the Ni CUB923, DEC923, and ICO923, the diffusion path of the adatom from the {111} facet to the {100} facet or to the {111} facet via the hopping and exchange mechanisms: (a) Hopping CUB{111} → {100}; (b) Exchange CUB{111} → {100}; (c) Exchange CUB{111} → {111}; (d) Exchange DEC{111} → {100}; (e) Hopping DEC{111} → {100}; (f) Exchange DEC{111} → {111}; (g) Hoping DEC{111} → {111}; (h) Exchange ICO{111} → {111}; (i) Hoping ICO{111} → {111}. The orange balls show the Al adatoms and the gray balls show the Ni atoms.
图 4 Al吸附原子在Ni CUB923基底表面扩散对应的能量势垒曲线 (a) {111} → {100}; (b) {111} → {111}. 扩散路径如图3(a)—图3(c)所示
Fig. 4. For the Al adatom on the surface of the Ni CUB923, the system energies as a function of reaction coordinate corresponding to the diffusion path shown in Fig.3(a)-Fig.3(c): (a) {111} → {100}; (b) {111} → {111}.
图 5 Al吸附原子在Ni DEC923基底表面扩散对应的能量势垒曲线 (a) {111} → {100}; (b) {111} → {111}. 扩散路径如图3(d)—图3(g)所示
Fig. 5. For the Al adatom on the surface of the NiDEC923, the system energies as a function of reaction coordinate corresponding to the diffusion path shown in Fig.3(d)-Fig.3(g): (a) {111} → {100}; (b) {111} → {111}.
图 8 T = 300 k, Al原子在Ni ICO923基底上的生长序列 (a) Ndep = 100; (b) Ndep = 200; (c) Ndep = 300; (d) Ndep = 400; (e) Ndep = 500. 橙色和灰色的球分别表示Al原子和Ni原子
Fig. 8. Growth sequence of Al atoms growth on the ICO923 of Fe at T = 300 k: (a) Ndep = 100; (b) Ndep = 200; (c) Ndep = 300; (d) Ndep = 400; (e) Ndep = 500. The orange and gray balls show the Al atoms and the Ni atoms, respectively.
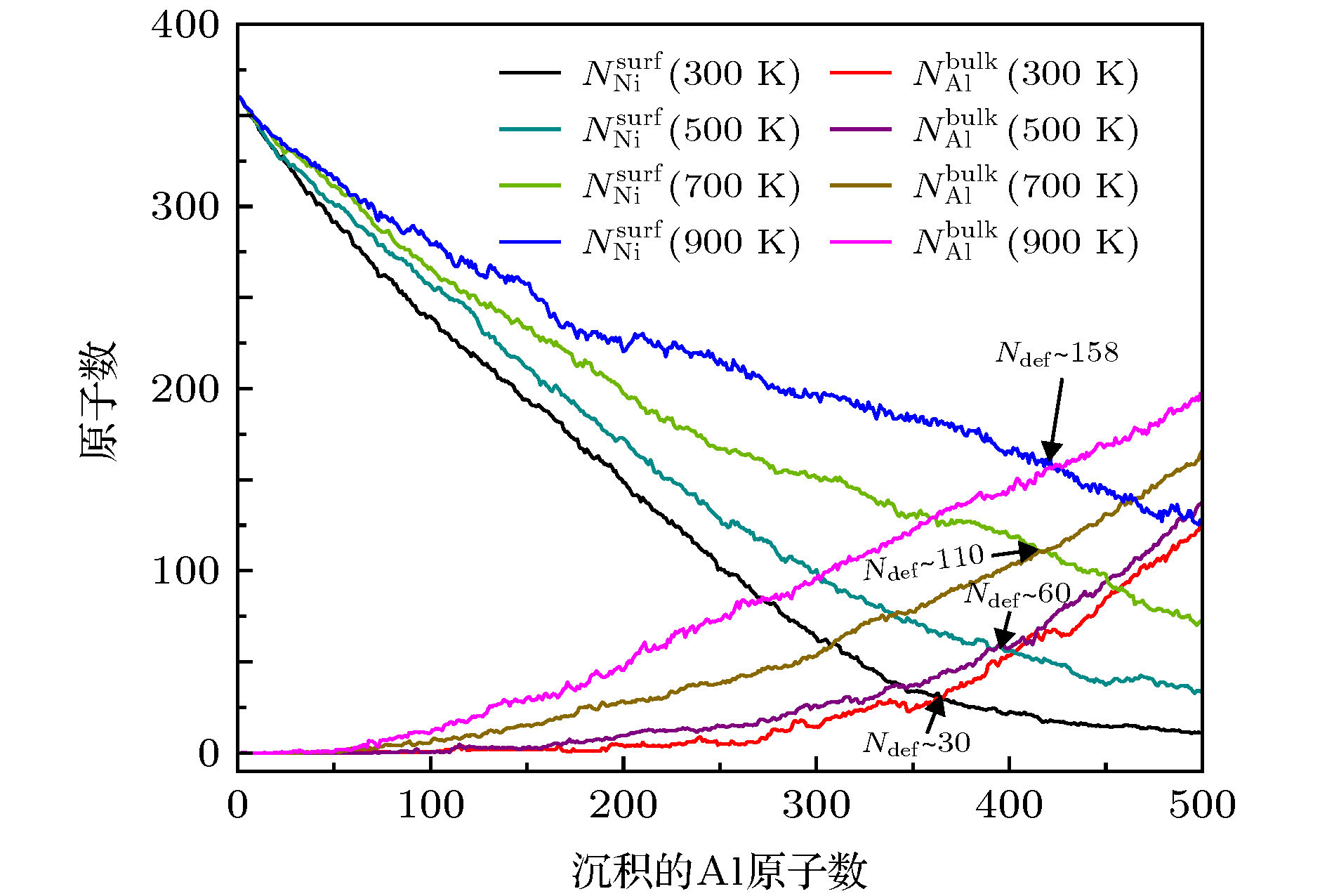
图 9 在T = 300, 500, 700和900 K, Al原子在Ni ICO923基底表面生长,
$ N_{\rm Ni}^{\rm surf} $ 和$ N_{\rm Al}^{\rm bulk} $ 作为沉积的Al原子数的函数Fig. 9. At T = 300, 500, 700 and 900 K, for the growth of Al atoms on the ICO923 of Ni, the
$ N_{\rm Ni}^{\rm surf} $ and$ N_{\rm Al}^{\rm bulk} $ as functions of the deposited Al atoms.图 10 异质配位数(NAl-Ni)随温度的变化, 插图对应于每个生长温度下最终构型. 橙色的球表示Al原子, 灰色的球表示Ni原子
Fig. 10. The variation of hetero-coordination number (NAl-Ni) with temperature, and the inset correspond to the final configuration at each growth temperature. The orange and gray balls show the Al atoms and the Ni atoms, respectively.
表 1 金属Ni和金属Al的表面能(Esurf). 列出了对应的第一性原理(FP)[28]数据
Table 1. The surface energy (Esurf) for the two different material of Ni and Al. For comparison, the first principle (FP) calculations[28] are listed.
元素 晶体结构 Esurf/mJ·m–2 晶面 本工作 FP[25] Ni FCC (111) 1875 2011 (100) 1964 2426 (110) 2148 2368 Al FCC (111) 933 939 (100) 994 1081 (110) 1063 1090 表 2 NiAl不同化合物的形成热(∆H). 为了对比列出了对应的第一性原理[32](FP)和其他理论方法[33] (EMP)以及对应实验结果[34-36] (EXP)
Table 2. The calculated heat of formation (∆H), structural of NiAl in varying crystal structures. For comparison, the first-principle (FP)[32] and other calculations with empirical methods (EMP)[33] and available experimental values (EXP)[34-36] are also listed.
化合物 结构 ∆H/eV·atom–1 本工作 FP EMP EXP NiAl B2 –0.61 –0.67 –0.67 NiAl3 L12 –0.26 Ni3Al L12 –0.45 –0.44 –0.46, -0.48 –0.49, -0.43 -
[1] Li T T, He C, Zhang W X, Cheng M 2018 J. Alloys Compd. 752 76
 Google Scholar
Google Scholar
[2] Riccardo F, Julius J, Johnston R L 2008 Chem. Rev. 108 845
 Google Scholar
Google Scholar
[3] Yang J Y, Hu W Y, Wu Y R, Dai X Y 2012 Surf. Sci. 606 971
 Google Scholar
Google Scholar
[4] Yang J Y, Hu W Y, Wu Y R, Dai X Y 2012 Cryst. Growth Des. 12 2978
 Google Scholar
Google Scholar
[5] Baletto F, Mottet C, Rapallo A, Rossi G, Ferrando R 2004 Surf. Sci. 566 192
[6] Song P X, Wen D S 2010 J. Phys. Chem. C 114 8688
 Google Scholar
Google Scholar
[7] Ferrer D, Torres-Castro A, Gao X, Sepúlveda-Guzmán S, Ortiz-Méndez U, José-Yacamán M 2007 Nano Lett. 7 1701
 Google Scholar
Google Scholar
[8] Baletto F, Mottet C, Ferrando R 2003 Eur. Phys. J. D 24 233
 Google Scholar
Google Scholar
[9] Deng L, Hu W Y, Deng H Q, Xiao S F 2010 J. Phys. Chem. C 114 11026
 Google Scholar
Google Scholar
[10] Deng L, Hu W Y, Deng H Q, Xiao S F, Tang J F 2011 J. Phys. Chem. C 115 11355
[11] Rapallo A, Rossi G, Ferrando R, et al. 2005 J. Chem. Phys. 122 194308
 Google Scholar
Google Scholar
[12] Henglein A 2000 J. Phys. Chem. B 104 2201
 Google Scholar
Google Scholar
[13] Dai X Y, Hu W Y, Yang J Y, Yi G J 2017 Thin Solid Films 626 178
 Google Scholar
Google Scholar
[14] Yang J Y, Hu W Y, Tang J F, Dai X Y 2013 Comput. Mater. Sci. 74 160
 Google Scholar
Google Scholar
[15] De S, Zhang J G, Luque R, Yan N 2016 Energy Environ Sci. 9 3314
 Google Scholar
Google Scholar
[16] Baletto F, Mottet C, Ferrando R 2003 Phys. Rev. Lett. 90 135504
 Google Scholar
Google Scholar
[17] Yang J Y, Hu W Y, Tang J F 2013 RSC Adv. 4 2155
[18] Shyrokorad D, Kornich G, Buga S 2019 Comput. Mater. Sci. 159 110
 Google Scholar
Google Scholar
[19] Mottet C, Rossi G, Baletto F, Ferrando R 2005 Phys. Rev. Lett. 95 035501
 Google Scholar
Google Scholar
[20] Purja Pun G P, Mishin Y 2009 Philos. Mag. 89 3245
 Google Scholar
Google Scholar
[21] 邓永和, 文大东, 彭超, 韦彦丁, 赵瑞, 彭平 2016 65 066401
 Google Scholar
Google Scholar
Deng Y H, Wen D D, Peng C, Wei Y D, Zhao R, Peng P 2016 Acta Phys. Sin. 65 066401
 Google Scholar
Google Scholar
[22] 彭超, 李媛, 邓永和, 彭平 2017 金属学报 53 1659
 Google Scholar
Google Scholar
Peng C, Li Y, Deng Y H, Peng P 2017 Acta Metal. Sin. 53 1659
 Google Scholar
Google Scholar
[23] Deng Y H, Wen D D, Li Y, Liu J, Peng P 2018 Philos. Mag. 98 2861
 Google Scholar
Google Scholar
[24] Wu B, Zhou J Q, Xue C, Liu H X 2015 Appl. Surf. Sci. 355 1145
 Google Scholar
Google Scholar
[25] Henkelman G, Uberuaga B P, Jónsson H 2000 J. Chem. Phys. 113 9901
 Google Scholar
Google Scholar
[26] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[27] Yanting W, Teitel S, Christoph D 2005 J. Chem. Phys. 122 9673
[28] Vitos L, Ruban A V, Skriver H L, Kollar J 1998 Surf. Sci. 411 186
 Google Scholar
Google Scholar
[29] Abbaspour M, Akbarzadeh H, Lotfi S 2018 J. Alloys Compd. 764 323
 Google Scholar
Google Scholar
[30] Wang H, Hu T, Qin J Y, Zhang T 2012 J. Appl. Phys. 112 073520
 Google Scholar
Google Scholar
[31] 高明, 邓永和, 文大东, 田泽安, 赵鹤平, 彭平 2020 69 046401
 Google Scholar
Google Scholar
Gao M, Deng Y H, Wen D D, Tian Z A, Zhao H P, Peng P 2020 Acta Phys. Sin. 69 046401
 Google Scholar
Google Scholar
[32] Wang Y, Liu Z K, Chen L Q 2004 Acta Mater. 52 2665
 Google Scholar
Google Scholar
[33] Mishin Y, Mehl M J, Papaconstantopoulos D A 2002 Phys. Rev. B 65 392
[34] Ashcroft N W, Mermin N D 1976 Solid State Physics. (Saunders, Philadelphia) pp216–217, 228–229
[35] Pearson W B, Villars P, Calvert L D 1985 ASM 3 258
[36] Rzyman K, Moser Z 2004 Prog. Mater. Sci. 49 581
 Google Scholar
Google Scholar
[37] Ayrault G, Ehrlich G 1974 J. Chem. Phys. 60 281
 Google Scholar
Google Scholar
[38] Ehrlich G, Hudda F G 1966 J. Chem. Phys. 44 1039
 Google Scholar
Google Scholar
[39] Yildirim H, Rahman T S 2009 Phys. Rev. B: Condens. Matter 80 235413
 Google Scholar
Google Scholar
[40] Yang L Y, Gan X L, Xu C, et al. 2019 Comput. Mater. Sci. 156 47
 Google Scholar
Google Scholar
计量
- 文章访问数: 14795
- PDF下载量: 154
- 被引次数: 0














 下载:
下载: