-
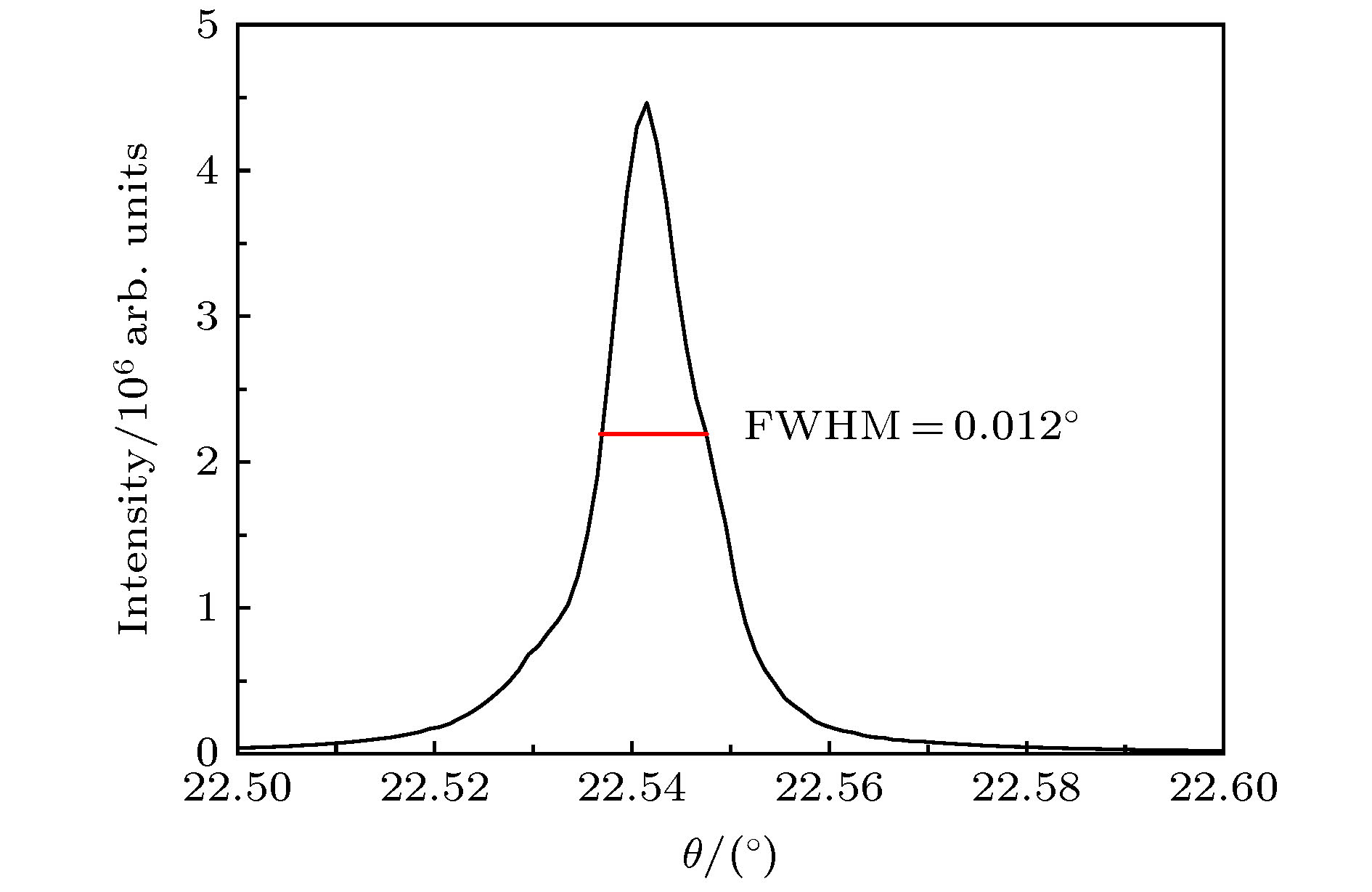
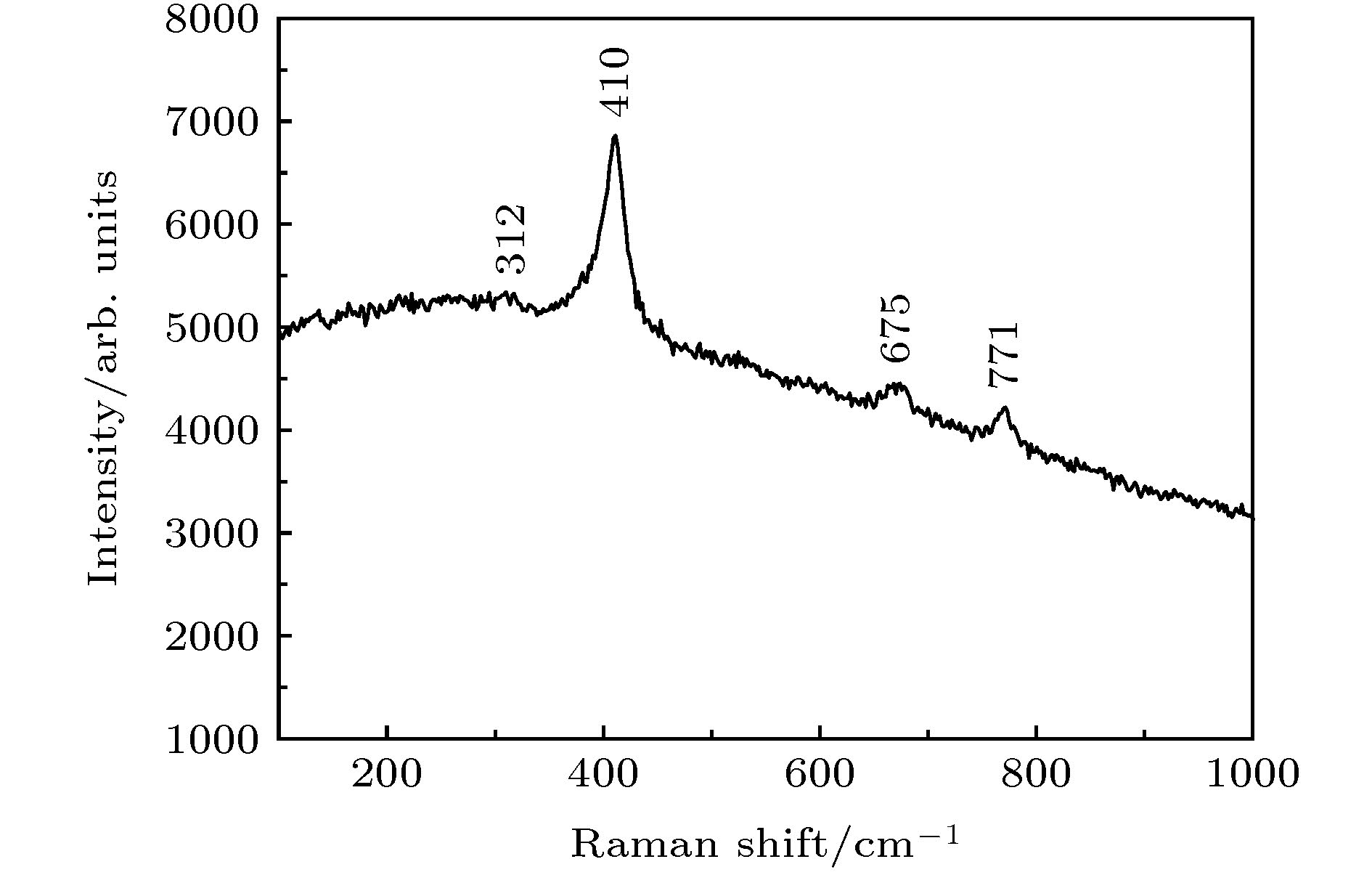
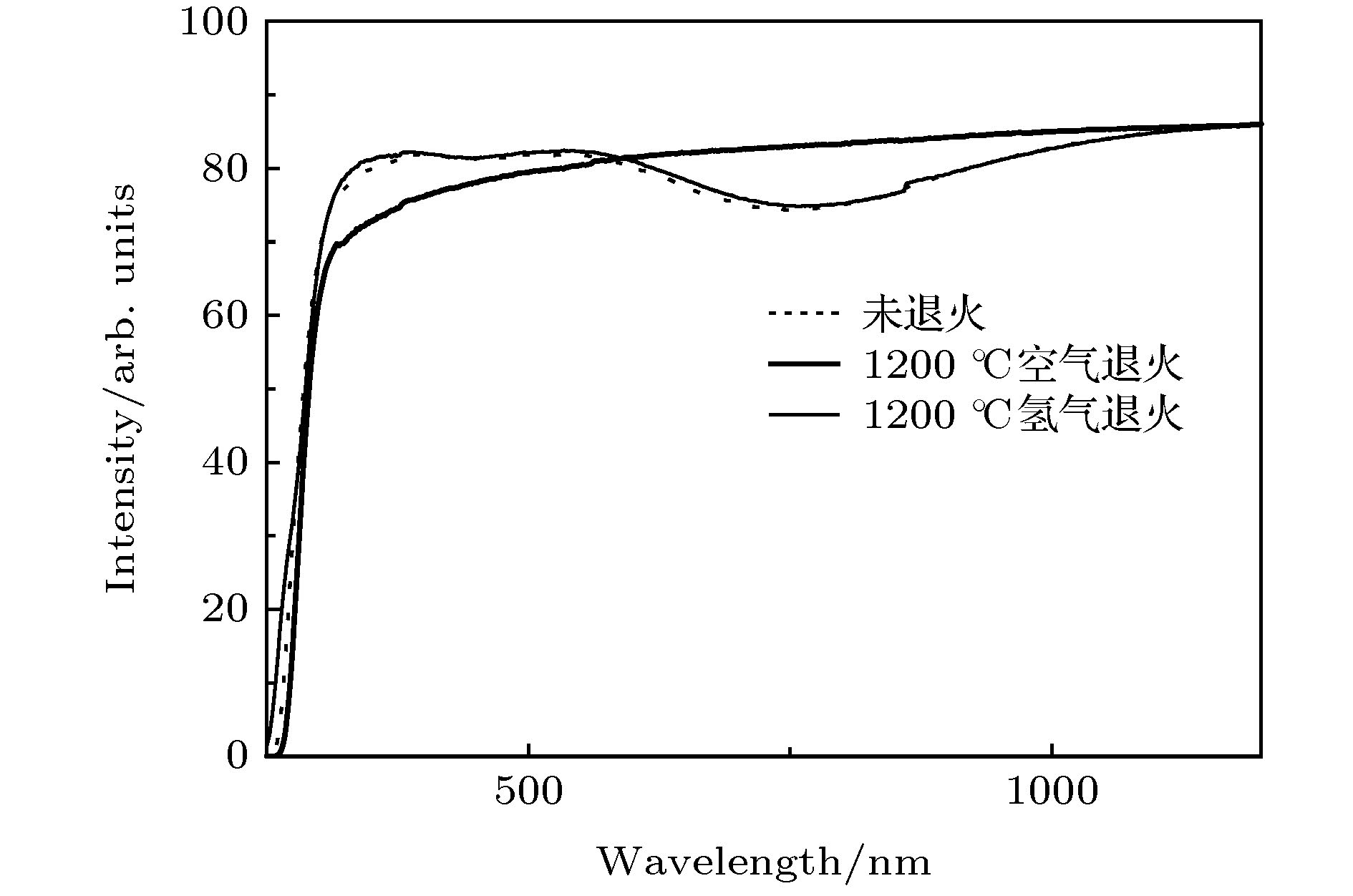
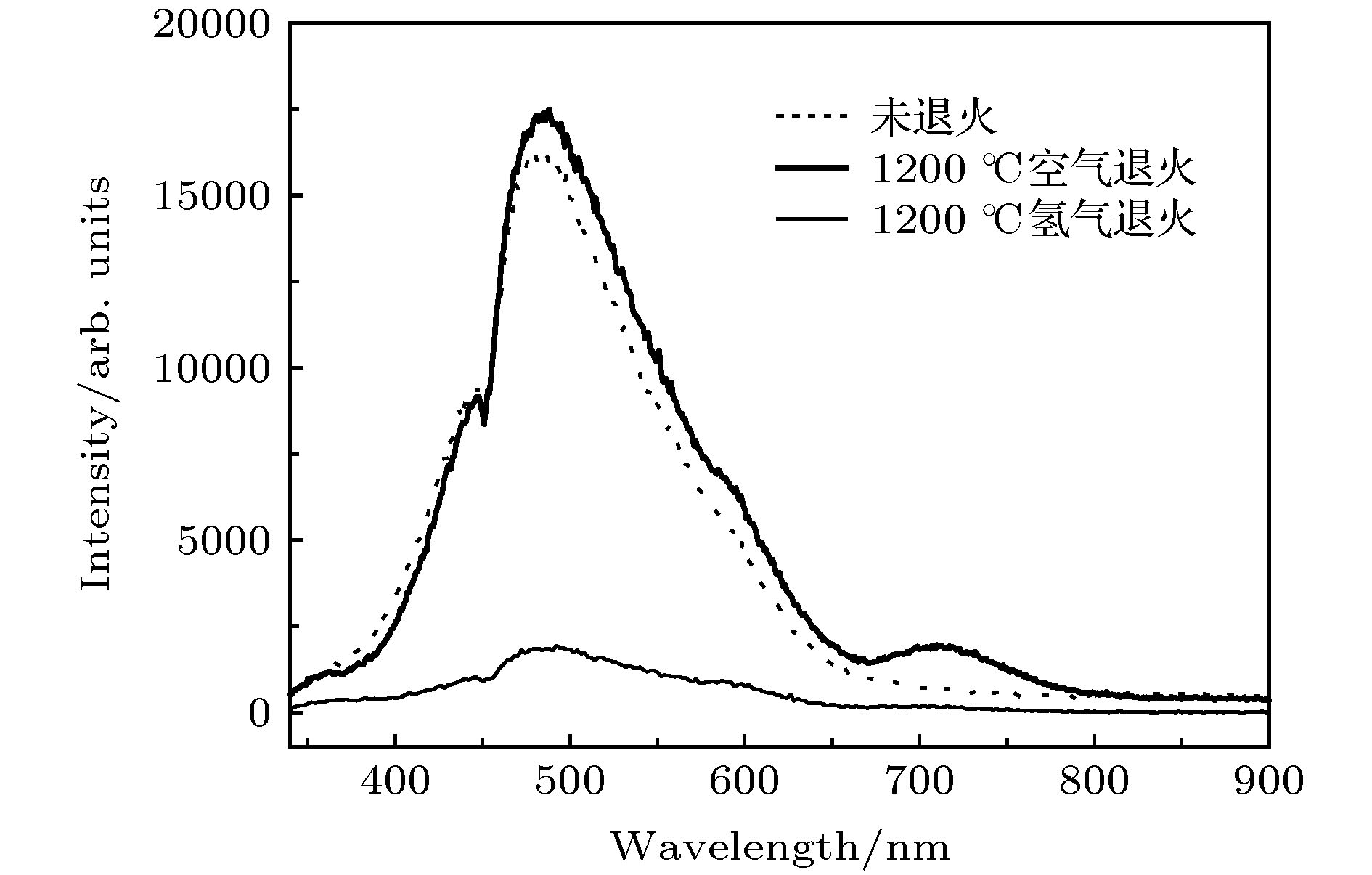
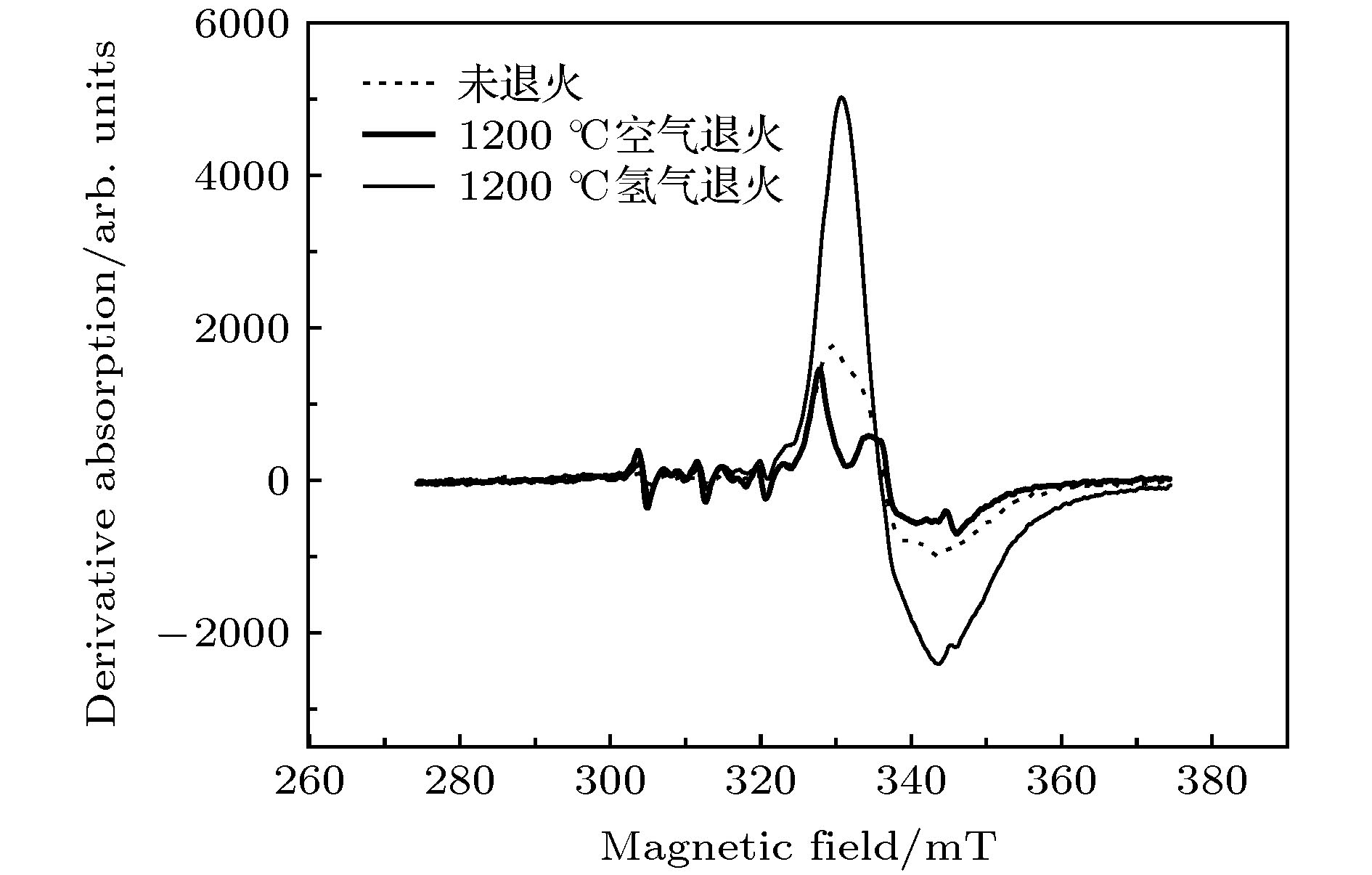
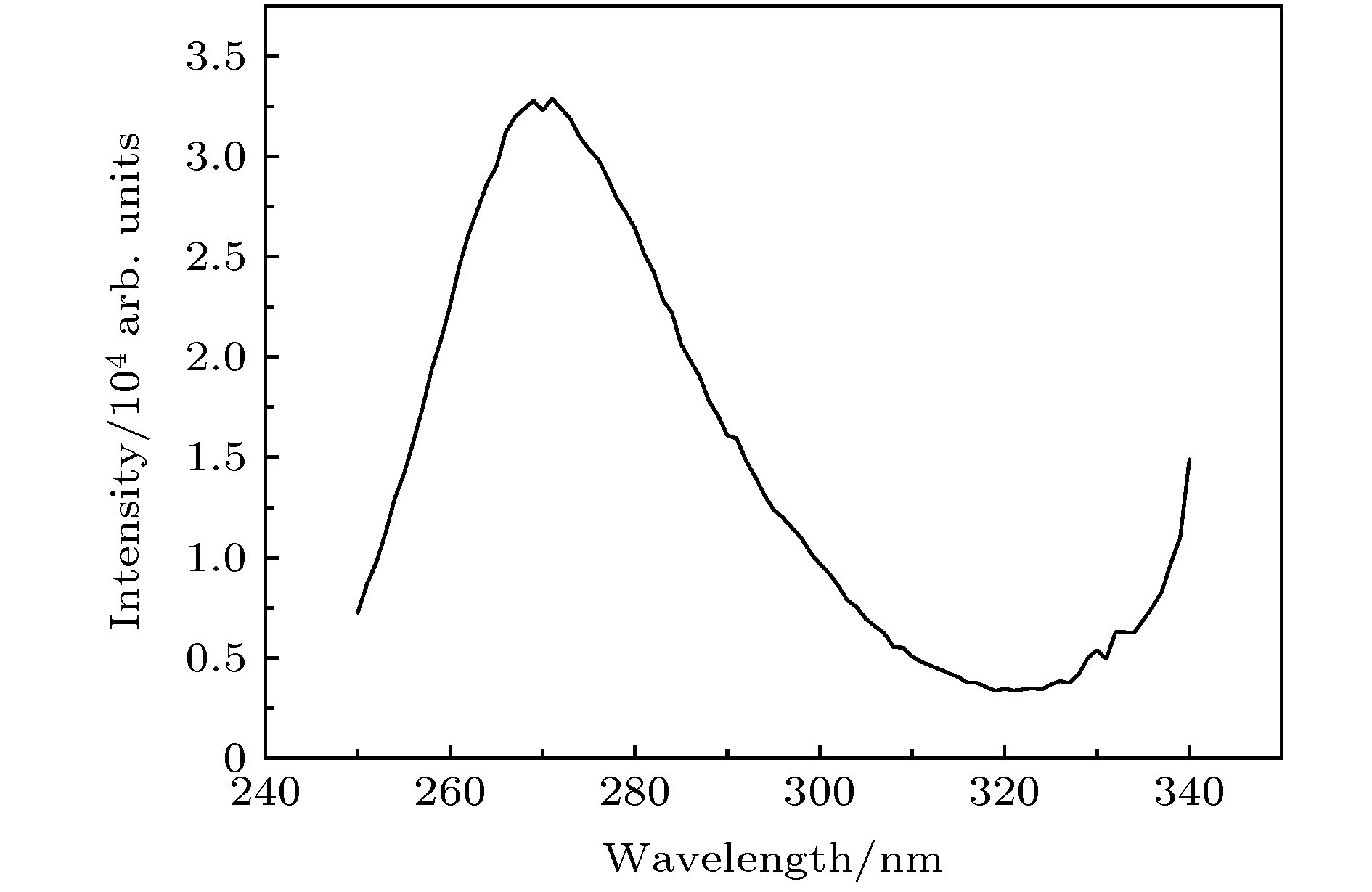
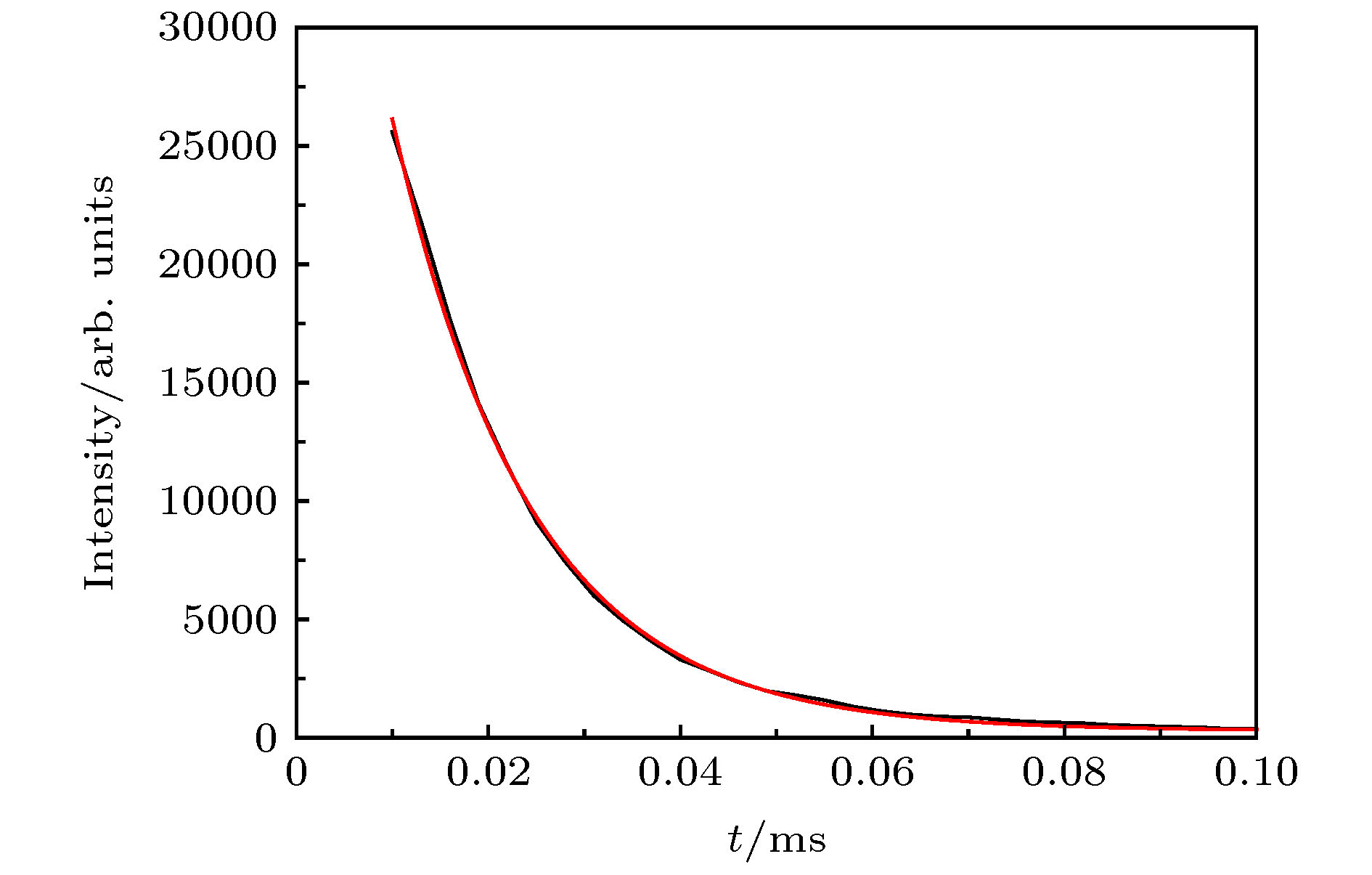
通过构建合适的温度梯度, 优化转速、生长速度等工艺条件, 采用提拉法晶体生长技术, 首次成功地生长出了Ti离子掺杂的Ti:MgAl2O4晶体, 晶体尺寸为Ø30 mm × 70 mm, X射线摇摆曲线表明该晶体的结晶质量良好. 对晶体的拉曼振动峰进行了指认. 测量了Ti:MgAl2O4晶体退火前后的透过和荧光发射光谱, 结合电子自旋共振(ESR)谱图分析了晶体中Ti离子的价态. 室温下Ti:MgAl2O4晶体在480 nm附近有个宽带发射峰, 其荧光寿命为14 μs, 是Ti:Al2O3、Ti:BeAl2O4晶体的4—5倍, 长的荧光寿命有利于储能; 发射截面为2 × 10–20 cm2, 较大的发射截面利于实现激光输出. 因此, Ti:MgAl2O4晶体是潜在的能够实现宽带可调谐蓝光激光输出的晶体材料.
-
关键词:
- 提拉法 /
- 晶体生长 /
- Ti:MgAl2O4晶体 /
- 蓝光发射
The melting point of Ti:MgAl2O4 crystal is as high as 2130 °C, it is a challenge to obtain a large-sized and high-quality laser crystal. By optimizing the crystal growth process, Ti:MgAl2O4 crystal with a size of 30 mm× 70 mm is successfully grown by the Czochralski method under the condition of weak reducing atmosphere. The X-ray diffraction pattern is studied, and the x-ray rocking curve indicates that the grown crystal has a high crystalline quality in terms of the lower full width at half maximum(FWHM) intensity, which provides a material basis for the next laser output experiment. In a range of 100–1000 cm–1, there are four Raman vibration peaks located at 312, 410, 675 cm–1 and 771 cm–1 respectively. The grown crystal has an absorption cutoff range of 250–318 nm and two wide absorption bands of 395–495 nm and 550–1100 nm. Excited by 271 nm, the grown crystal shows a strong broadband emission ina range of 340–650 nm with a peak centered at 480 nm. After annealing in hydrogen atmosphere, shape of the transmittance spectrum and emission spectrum are both unchanged, but the fluorescent emission intensity is significantly reduced. After annealing in air atmosphere, the original two absorption bands disappear while none of the characteristics of fluorescence emission in a 340–650 nm range changes significantly. In addition, a new fluorescence emission peak near 725 nm is observed. Combining with the ESR spectrum, what we canconfirm is that the Ti:MgAl2O4 as-grown crystal contains Ti3+ and Ti4+ ions, and no ESR signal of Ti3+ is observed after annealing in air atmosphere. Moreover, excitationspectrum is also recorded. The fluorescence lifetime is 14 μs at room temperature, which is 4–5 times that of Ti:Al2O3 crystal and Ti:BeAl2O4 crystal. Furthermore, the emission cross section of the grown Ti:MgAl2O4 crystal is calculated from the Füchtbauer-Ladenburg (F-L) formula and its value is 2 × 10–20 cm2, large emission cross section which is beneficial for realizing laser oscillation. All the above results show that the Ti:MgAl2O4 crystal is a potential crystal material for realizing broadband tunable blue laser output.-
Keywords:
- Czochralski method /
- crystal growth /
- Ti:MgAl2O4 crystal /
- blue luminescence
[1] 章佶, 孙真荣, 王祖赓, 司继良, 王静雅, 杭寅, 徐军 2005 人工晶体学报 34 657
 Google Scholar
Google Scholar
Zhang J, Sun Z R, Wang Z G, Si J L, Wang J Y, Hang Y, Xu J 2005 J. Synth. Cryst. 34 657
 Google Scholar
Google Scholar
[2] Gourier D, Colle L, Lejus A M, Vivein D, Moncorge R 1988 J. Appl. Phys. 63 1144
 Google Scholar
Google Scholar
[3] 夏海平, 徐铁峰, 张新民, 王金浩, 章践立 2009 光学技术 35 307
 Google Scholar
Google Scholar
Xia H P, Xu T F, Zhang X M, Wang J H, Zhang J L 2009 Opt. Tech. 35 307
 Google Scholar
Google Scholar
[4] Basun S A, Danger T, Kaplyanskii A A, McClure D S, Petermann K, Wong W C 1996 Phys. Rev. B 54 6141
 Google Scholar
Google Scholar
[5] Bausa l E, Vergara I, Garcia-Sole J, Strek W, Deren P J 1990 J. Appl. Phys. 68 736
 Google Scholar
Google Scholar
[6] Sato T, Shirai M, Tanaka K, Kawabe Y, Hanamura E 2005 J. Lumin. 114 155
 Google Scholar
Google Scholar
[7] Jouini A, Sato H, Yoshikawa A, Fukuda T 2006 J. Mater. Res. 21 2337
 Google Scholar
Google Scholar
[8] Kuleshov N V, Shcherbitsky V G, Mikhailov V P, Kiick S, Koetke J, Petermann K, Huber G 1997 J. Lumin. 71 265
 Google Scholar
Google Scholar
[9] Tomita A, Sato T, Tanaka K, Kawabe Y, Shirai M, Tanaka K, Hanamura E 2004 J. Lumin. 109 19
 Google Scholar
Google Scholar
[10] Jouini A, Yoshikawa A, Fukuda T, Boulon G 2006 J. Cryst. Growth 293 517
 Google Scholar
Google Scholar
[11] Lombard P, Boizot B, Ollier N, Jouini A, Yoshikawa A 2009 J. Cryst. Growth 311 899
 Google Scholar
Google Scholar
[12] Wood D L, Imbusch G F, Macfarlane R M, Kisliuk P, Larkin D M 1968 J. Chem. Phys. 48 5255
 Google Scholar
Google Scholar
[13] 王成思, 沈锡田, 刘云贵, 张倩 2019 光谱学与光谱分析 39 109
Wang C S, Shen X T, Liu Y G, Zhang Q 2019 Spectrosc. Spect. Anal. 39 109
[14] O'Horo M P, Frisillo A L, White W B 1973 J. Phys. Chem. Solids 34 23
 Google Scholar
Google Scholar
[15] Takahashi S, Kan A, Ogawa H 2017 J. Eur. Ceram. Soc. 37 1001
 Google Scholar
Google Scholar
[16] Frass L W, Moore J E, Salzberg J B 1973 J. Chem. Phys. 58 3585
 Google Scholar
Google Scholar
[17] Simeone D, Dodane-Thiriet C, Gosset D, Daniel P, Beauvy M 2002 J. Nucl. Mater. 300 151
 Google Scholar
Google Scholar
[18] Dash S, Sahoo R K, Das A, Bajpai S, Debasish D, Singh S K 2017 J. Alloy. Compd. 726 1186
 Google Scholar
Google Scholar
[19] Watterich A, Hofstaetter A, Wuerz’ R and Scharmann A 1996 Solid State Commun. 100 513
 Google Scholar
Google Scholar
[20] Jiang Y Q, Halliburton L E, Roth M, Tseitlin M, Angert N, 2007 Physica B 400 190
 Google Scholar
Google Scholar
[21] Dong S Y, Wang X Y, Shen L F, Li H S, Wang J, Nie P, Wang J J, Zhang X G 2015 J. Electroanal. Chem. 757 1
 Google Scholar
Google Scholar
-
表 1 几种不同的MgAl2O4的拉曼振动峰
Table 1. Raman vibration peaks of several different MgAl2O4.
-
[1] 章佶, 孙真荣, 王祖赓, 司继良, 王静雅, 杭寅, 徐军 2005 人工晶体学报 34 657
 Google Scholar
Google Scholar
Zhang J, Sun Z R, Wang Z G, Si J L, Wang J Y, Hang Y, Xu J 2005 J. Synth. Cryst. 34 657
 Google Scholar
Google Scholar
[2] Gourier D, Colle L, Lejus A M, Vivein D, Moncorge R 1988 J. Appl. Phys. 63 1144
 Google Scholar
Google Scholar
[3] 夏海平, 徐铁峰, 张新民, 王金浩, 章践立 2009 光学技术 35 307
 Google Scholar
Google Scholar
Xia H P, Xu T F, Zhang X M, Wang J H, Zhang J L 2009 Opt. Tech. 35 307
 Google Scholar
Google Scholar
[4] Basun S A, Danger T, Kaplyanskii A A, McClure D S, Petermann K, Wong W C 1996 Phys. Rev. B 54 6141
 Google Scholar
Google Scholar
[5] Bausa l E, Vergara I, Garcia-Sole J, Strek W, Deren P J 1990 J. Appl. Phys. 68 736
 Google Scholar
Google Scholar
[6] Sato T, Shirai M, Tanaka K, Kawabe Y, Hanamura E 2005 J. Lumin. 114 155
 Google Scholar
Google Scholar
[7] Jouini A, Sato H, Yoshikawa A, Fukuda T 2006 J. Mater. Res. 21 2337
 Google Scholar
Google Scholar
[8] Kuleshov N V, Shcherbitsky V G, Mikhailov V P, Kiick S, Koetke J, Petermann K, Huber G 1997 J. Lumin. 71 265
 Google Scholar
Google Scholar
[9] Tomita A, Sato T, Tanaka K, Kawabe Y, Shirai M, Tanaka K, Hanamura E 2004 J. Lumin. 109 19
 Google Scholar
Google Scholar
[10] Jouini A, Yoshikawa A, Fukuda T, Boulon G 2006 J. Cryst. Growth 293 517
 Google Scholar
Google Scholar
[11] Lombard P, Boizot B, Ollier N, Jouini A, Yoshikawa A 2009 J. Cryst. Growth 311 899
 Google Scholar
Google Scholar
[12] Wood D L, Imbusch G F, Macfarlane R M, Kisliuk P, Larkin D M 1968 J. Chem. Phys. 48 5255
 Google Scholar
Google Scholar
[13] 王成思, 沈锡田, 刘云贵, 张倩 2019 光谱学与光谱分析 39 109
Wang C S, Shen X T, Liu Y G, Zhang Q 2019 Spectrosc. Spect. Anal. 39 109
[14] O'Horo M P, Frisillo A L, White W B 1973 J. Phys. Chem. Solids 34 23
 Google Scholar
Google Scholar
[15] Takahashi S, Kan A, Ogawa H 2017 J. Eur. Ceram. Soc. 37 1001
 Google Scholar
Google Scholar
[16] Frass L W, Moore J E, Salzberg J B 1973 J. Chem. Phys. 58 3585
 Google Scholar
Google Scholar
[17] Simeone D, Dodane-Thiriet C, Gosset D, Daniel P, Beauvy M 2002 J. Nucl. Mater. 300 151
 Google Scholar
Google Scholar
[18] Dash S, Sahoo R K, Das A, Bajpai S, Debasish D, Singh S K 2017 J. Alloy. Compd. 726 1186
 Google Scholar
Google Scholar
[19] Watterich A, Hofstaetter A, Wuerz’ R and Scharmann A 1996 Solid State Commun. 100 513
 Google Scholar
Google Scholar
[20] Jiang Y Q, Halliburton L E, Roth M, Tseitlin M, Angert N, 2007 Physica B 400 190
 Google Scholar
Google Scholar
[21] Dong S Y, Wang X Y, Shen L F, Li H S, Wang J, Nie P, Wang J J, Zhang X G 2015 J. Electroanal. Chem. 757 1
 Google Scholar
Google Scholar
计量
- 文章访问数: 13630
- PDF下载量: 108
- 被引次数: 0














 下载:
下载: