-
制备了单个颗粒(domain)组成的纳米金刚石薄膜, 薄膜中单个颗粒由尺寸超过100 nm的金刚石晶粒与非晶碳复合而成. 对薄膜进行氧化处理后, 其硅空位色心的光致发光强度增强了22.7倍. 扫描电镜及拉曼光谱测试结果表明, 不同时间氧化后的样品中存在由尺寸超过100 nm的晶粒组成的花瓣状金刚石聚集体, 这些金刚石在较长氧化时间下仍能保持稳定. 氧化后的薄膜内非晶碳大大减少, 金刚石含量增大, 纳米金刚石晶粒充分暴露引起了薄膜发光强度大幅增强, 其发光半峰宽为5.6—6.0 nm. 继续增加氧化时间, 薄膜的光致发光会因为部分细小纳米金刚石晶粒的损失而略微降低, 但是稳定的大尺寸金刚石晶粒的存在使得薄膜的发光强度依然维持在氧化前的8.3倍以上.In order to increase the oxidation sites for enhancing the Si-V photoluminescence intensity of nanocrytalline diamond films, we prepare nanocrystalline diamond films; these films each are comprised of separated domains and oxidized for different times. Each single domain consists of nanodiamond grains with a size of larger than 100 nm and amorphous carbon. In the gaps between domains of separated domain there is formed a film that allows more sites to contact air to ensure the efficient oxidation of the film. As a result, silicon vacancy photoluminescence intensity of the separated domain forming the film is largely enhanced by about 22.7 times after oxidation. The SEM images and Raman spectra of oxidized samples show that the film contains flower-shaped diamond aggregates, each of which is comprised of radially arranged diamond grains. The mixture of nanodiamond grains and amorphous carbon fills the gaps between diamond petals. These fillers disappear after long-term oxidation, but the diamond petals stay stable. Raman spectra show that the amount of amorphous carbon largely decreases after oxidation, while diamond content apparently rises. Hydrogen is desorbed from the film after short-time oxidation according to Raman spectra, thus the quenching effect on silicon vacancy photoluminescence caused by hydrogen termination of diamond surface state is removed. Diamond petals of large size and nanodiamond grains in the fillers are both silicon vacancy photoluminescence sources of the film; the exposed diamond flats on the surface of unoxidized domains provide limited silicon vacancy photoluminescence for the film. The sufficient exposure of diamond grains after the removal of amorphous carbon leads to the significant enhancement of film’s silicon vacancy photoluminescence. With longer-time oxidation, the photoluminescence of film will slightly decrease due to the disappearance of small-sized nanodiamond grains, but the film photoluminescence almost remains stable in both intensity and property due to the stability of large-sized diamond grains. The film after 140-min oxidation remains photoluminescence enhancement, 8.3 times the photoluminescence of the unoxidized sample. The full width at half maximum of photoluminescence peak declines to merely 5.6-6.0 nm because of diamond petals’ high degree of order, which is advantageous for diamond silicon vacancy photoluminescence.
-
Keywords:
- diamond /
- silicon vacancy center /
- oxidation /
- photoluminescence
[1] Aharonovich I, Englund D, Toth M 2016 Nat. Photon. 10 631
 Google Scholar
Google Scholar
[2] Aharonovich I, Castelletto S, Simpson D A, Su C H, Greentree A D, Prawer S 2011 Rep. Prog. Phys. 740 076501
[3] Aharonovich I, Neu E 2014 Adv. Opt. Mater. 2 911
 Google Scholar
Google Scholar
[4] Schröder T, Mouradian S L, Zheng J, Trusheim M E, Walsh M, Chen E H, Li L, Bayn I, Englund D 2016 J. Opt. Soc. Am. B 33 B65
 Google Scholar
Google Scholar
[5] Schirhagl R, Chang K, Loretz M, Degen C L 2014 Annu. Rev. Phys. Chem. 65 83
 Google Scholar
Google Scholar
[6] Muller T, Hepp C, Pingault B, Neu E, Gsell S, Schreck M, Sternschulte H, Steinmuller-Nethl D, Becher C, Atature M 2014 Nat. Commun. 5 3328
 Google Scholar
Google Scholar
[7] Merson T D, Castelletto S, Aharonovich I, Turbic A, Kilpatrick T J, Turnley A M 2013 Opt. Lett. 38 4170
 Google Scholar
Google Scholar
[8] Doherty M W, Manson N B, Delaney P, Jelezko F, Wrachtrup J, Hollenberg L C L 2013 Phys. Rep. 528 1
 Google Scholar
Google Scholar
[9] Aharonovich I 2014 Nat. Photon. 8 818
 Google Scholar
Google Scholar
[10] Jelezko F, Wrachtrup J 2006 Phys. Status Solidi A 203 3207
 Google Scholar
Google Scholar
[11] Rogers L J, Jahnke K D, Teraji T, Marseglia L, Muller C, Naydenov B, Schauffert H, Kranz C, Isoya J, McGuinness L P, Jelezko F 2014 Nat. Commun. 5 4739
 Google Scholar
Google Scholar
[12] Neu E, Albrecht R, Fischer M, Gsell S, Schreck M, Becher C 2012 Phys. Rev. B 85 245207
 Google Scholar
Google Scholar
[13] Dragounová K, Ižák T, Kromka A, Potůček Z, Bryknar Z, Potocký Š 2018 Appl. Phys. A 124 219
[14] Hu X J, Ye J S, Liu H J, Shen Y G, Chen X H, Hu H 2011 J. Appl. Phys. 109 053524
 Google Scholar
Google Scholar
[15] Klauser F, Steinmüller-Nethl D, Kaindl R, Bertel E, Memmel N 2010 Chem. Vapor Depos. 16 127
 Google Scholar
Google Scholar
[16] Lin S C, Yeh C J, Kurian J, Dong C L, Niu H, Leou K C, Lin I N 2014 J. Appl. Phys. 116 183701
 Google Scholar
Google Scholar
[17] Wilson J I B, Walton J S, Beamson G 2001 J. Electron Spectrosc. Relat. Phenom. 121 183
 Google Scholar
Google Scholar
[18] Osswald S, Yushin G, Mochalin V, Kucheyev S O, Gogotsi Y 2006 J. Am. Chem. Soc. 128 11635
 Google Scholar
Google Scholar
[19] Pu J C, Wang S F, Sung J C 2010 J. Alloy. Compd. 489 638
 Google Scholar
Google Scholar
[20] Huang K, Hu X, Xu H, Shen Y, Khomich A 2014 Appl. Surf. Sci. 317 11
 Google Scholar
Google Scholar
[21] Mei Y, Fan D, Lu S, Shen Y, Hu X 2016 J. Appl. Phys. 120 225107
 Google Scholar
Google Scholar
[22] Nimmagadda R R, Joshi A, Hsu W L 1990 J. Mater. Res. 5 2445
 Google Scholar
Google Scholar
[23] Obraztsov A N, Kopylov P G, Chuvilin A L, Savenko N V 2009 Diamond Relat. Mater. 18 1289
 Google Scholar
Google Scholar
[24] Zolotukhin A, Kopylov P G, Ismagilov R R, Obraztsov A N 2010 Diamond Relat. Mater. 19 1007
 Google Scholar
Google Scholar
[25] Hei L F, Zhao Y, Wei J J, Liu J L, Li C M, Lü F X 2017 Int. J. Miner. Metall. Mater. 24 1424
 Google Scholar
Google Scholar
[26] Mildren R P, Butler J E, Rabeau J R 2008 Opt. Express 16 18950
 Google Scholar
Google Scholar
[27] Reilly S, Savitski V G, Liu H, Gu E, Dawson M D, Kemp A J 2015 Opt. Lett. 40 930
 Google Scholar
Google Scholar
[28] Hu X J, Li N 2013 Chin. Phys. Lett. 30 088102
 Google Scholar
Google Scholar
[29] Dychalska A, Fabisiak K, Paprocki K, Makowiecki J, Iskaliyeva A, Szybowicz M 2016 Mater. Des. 112 320
 Google Scholar
Google Scholar
[30] Prawer S, Nugent K W, Jamieson D N, Orwa J O, Bursill L A, Peng J L 2000 Chem. Phys. Lett. 332 93
 Google Scholar
Google Scholar
[31] Prawer S, Nemanich R J 2004 Philos. Trans. R. Soc. Lond. Ser. A: Math. Phys. Eng. Sci. 362 2537
 Google Scholar
Google Scholar
[32] Ferrari A C, Robertson J 2000 Phys. Rev. B 61 14095
 Google Scholar
Google Scholar
[33] Ferrari A C, Robertson J 2001 Phys. Rev. B 64 075414
 Google Scholar
Google Scholar
[34] Ferrari A C, Robertson J 2001 Phys. Rev. B 63 121405
 Google Scholar
Google Scholar
[35] Ferrari A C, Robertson J 2004 Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 362 2477
 Google Scholar
Google Scholar
[36] Fecher J, Wormser M, Rosiwal S M 2016 Diamond Relat. Mater. 61 41
 Google Scholar
Google Scholar
[37] Sails S R, Gardiner D J, Bowden M, Savage J, Rodway D 1996 Diamond Relat. Mater. 5 589
 Google Scholar
Google Scholar
-
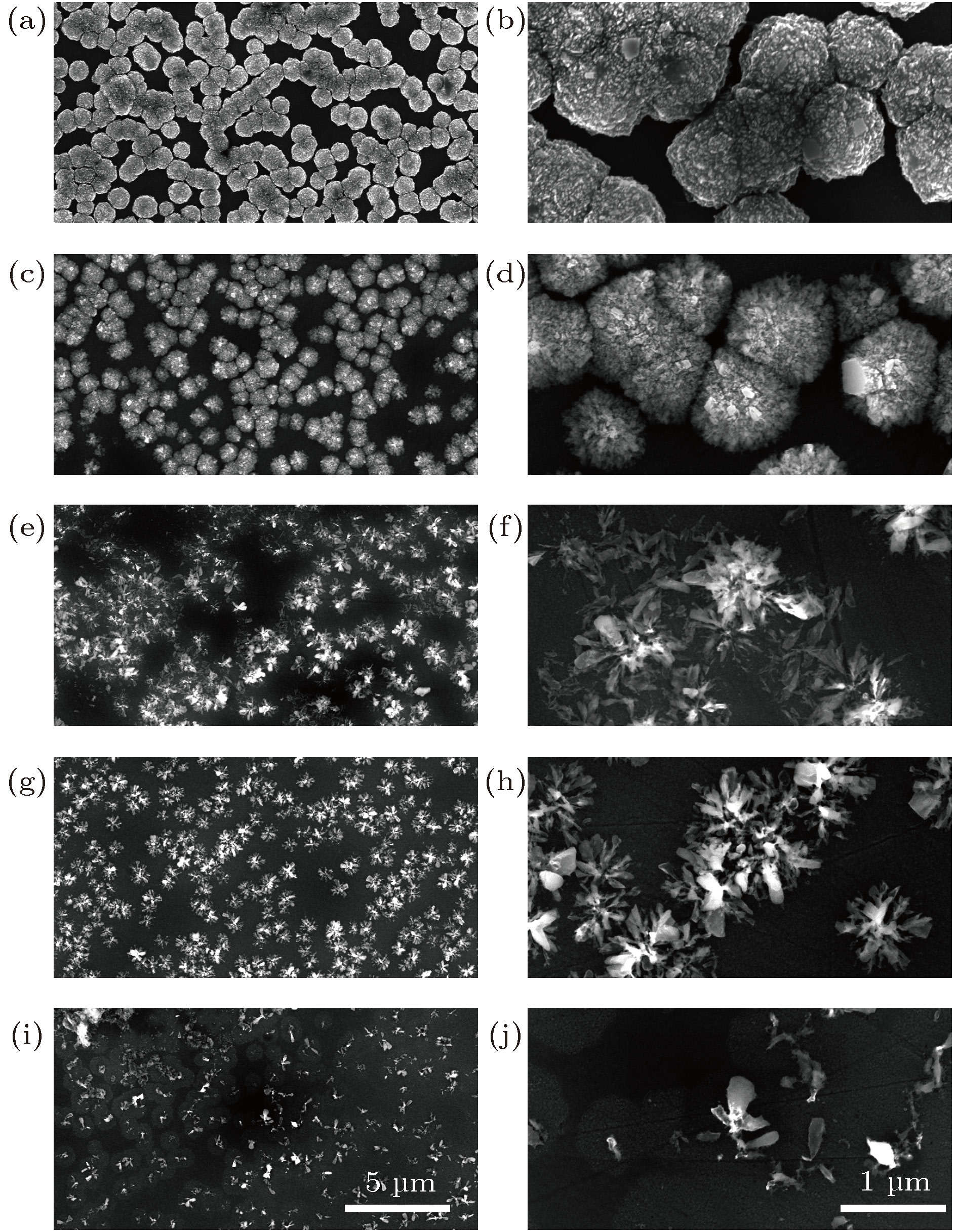
图 1 单颗粒层纳米晶金刚石薄膜的表面形貌 (a) OX-0; (b) OX-0; (c) OX-30; (d) OX-30; (e) OX-40; (f) OX-40; (g) OX-130; (h) OX-130; (i) OX-140; (j) OX-140
Fig. 1. Morphology of separated domains formed nanocrystalline diamond film: (a) OX-0; (b) OX-0; (c) OX-30; (d) OX-30; (e) OX-40; (f) OX-40; (g) OX-130; (h) OX-130; (i) OX-140; (j) OX-140.
图 2 (a) 572 nm处的金刚石的一阶斯托克斯线; (b)单颗粒层纳米晶金刚石薄膜的硅空位光致发光谱; (c) 738 nm波长强度与572 nm强度比值随时间变化
Fig. 2. (a) Diamond first Stokes line at 572 nm; (b) SiV photoluminescence spectra of separated domains formed nanocrystalline diamond film; (c) photoluminescence intensity ratio of 738 nm emission and 572 nm emission with different oxidation time.
图 5 (a)不同氧化时间单颗粒层纳米晶金刚石薄膜的拉曼光谱; (b)金刚石含量与It-PA/ISUM和样品氧化时间的关系; (c)金刚石峰位置与半峰宽随氧化时间的变化关系
Fig. 5. (a) Raman spectra of variety time oxidized separated domains formed nanocrystalline diamond film; (b) diamond content and It-PA/ISUM of films with their oxidation time; (c) diamond peak position and FWHM values of films with their oxidation time.
-
[1] Aharonovich I, Englund D, Toth M 2016 Nat. Photon. 10 631
 Google Scholar
Google Scholar
[2] Aharonovich I, Castelletto S, Simpson D A, Su C H, Greentree A D, Prawer S 2011 Rep. Prog. Phys. 740 076501
[3] Aharonovich I, Neu E 2014 Adv. Opt. Mater. 2 911
 Google Scholar
Google Scholar
[4] Schröder T, Mouradian S L, Zheng J, Trusheim M E, Walsh M, Chen E H, Li L, Bayn I, Englund D 2016 J. Opt. Soc. Am. B 33 B65
 Google Scholar
Google Scholar
[5] Schirhagl R, Chang K, Loretz M, Degen C L 2014 Annu. Rev. Phys. Chem. 65 83
 Google Scholar
Google Scholar
[6] Muller T, Hepp C, Pingault B, Neu E, Gsell S, Schreck M, Sternschulte H, Steinmuller-Nethl D, Becher C, Atature M 2014 Nat. Commun. 5 3328
 Google Scholar
Google Scholar
[7] Merson T D, Castelletto S, Aharonovich I, Turbic A, Kilpatrick T J, Turnley A M 2013 Opt. Lett. 38 4170
 Google Scholar
Google Scholar
[8] Doherty M W, Manson N B, Delaney P, Jelezko F, Wrachtrup J, Hollenberg L C L 2013 Phys. Rep. 528 1
 Google Scholar
Google Scholar
[9] Aharonovich I 2014 Nat. Photon. 8 818
 Google Scholar
Google Scholar
[10] Jelezko F, Wrachtrup J 2006 Phys. Status Solidi A 203 3207
 Google Scholar
Google Scholar
[11] Rogers L J, Jahnke K D, Teraji T, Marseglia L, Muller C, Naydenov B, Schauffert H, Kranz C, Isoya J, McGuinness L P, Jelezko F 2014 Nat. Commun. 5 4739
 Google Scholar
Google Scholar
[12] Neu E, Albrecht R, Fischer M, Gsell S, Schreck M, Becher C 2012 Phys. Rev. B 85 245207
 Google Scholar
Google Scholar
[13] Dragounová K, Ižák T, Kromka A, Potůček Z, Bryknar Z, Potocký Š 2018 Appl. Phys. A 124 219
[14] Hu X J, Ye J S, Liu H J, Shen Y G, Chen X H, Hu H 2011 J. Appl. Phys. 109 053524
 Google Scholar
Google Scholar
[15] Klauser F, Steinmüller-Nethl D, Kaindl R, Bertel E, Memmel N 2010 Chem. Vapor Depos. 16 127
 Google Scholar
Google Scholar
[16] Lin S C, Yeh C J, Kurian J, Dong C L, Niu H, Leou K C, Lin I N 2014 J. Appl. Phys. 116 183701
 Google Scholar
Google Scholar
[17] Wilson J I B, Walton J S, Beamson G 2001 J. Electron Spectrosc. Relat. Phenom. 121 183
 Google Scholar
Google Scholar
[18] Osswald S, Yushin G, Mochalin V, Kucheyev S O, Gogotsi Y 2006 J. Am. Chem. Soc. 128 11635
 Google Scholar
Google Scholar
[19] Pu J C, Wang S F, Sung J C 2010 J. Alloy. Compd. 489 638
 Google Scholar
Google Scholar
[20] Huang K, Hu X, Xu H, Shen Y, Khomich A 2014 Appl. Surf. Sci. 317 11
 Google Scholar
Google Scholar
[21] Mei Y, Fan D, Lu S, Shen Y, Hu X 2016 J. Appl. Phys. 120 225107
 Google Scholar
Google Scholar
[22] Nimmagadda R R, Joshi A, Hsu W L 1990 J. Mater. Res. 5 2445
 Google Scholar
Google Scholar
[23] Obraztsov A N, Kopylov P G, Chuvilin A L, Savenko N V 2009 Diamond Relat. Mater. 18 1289
 Google Scholar
Google Scholar
[24] Zolotukhin A, Kopylov P G, Ismagilov R R, Obraztsov A N 2010 Diamond Relat. Mater. 19 1007
 Google Scholar
Google Scholar
[25] Hei L F, Zhao Y, Wei J J, Liu J L, Li C M, Lü F X 2017 Int. J. Miner. Metall. Mater. 24 1424
 Google Scholar
Google Scholar
[26] Mildren R P, Butler J E, Rabeau J R 2008 Opt. Express 16 18950
 Google Scholar
Google Scholar
[27] Reilly S, Savitski V G, Liu H, Gu E, Dawson M D, Kemp A J 2015 Opt. Lett. 40 930
 Google Scholar
Google Scholar
[28] Hu X J, Li N 2013 Chin. Phys. Lett. 30 088102
 Google Scholar
Google Scholar
[29] Dychalska A, Fabisiak K, Paprocki K, Makowiecki J, Iskaliyeva A, Szybowicz M 2016 Mater. Des. 112 320
 Google Scholar
Google Scholar
[30] Prawer S, Nugent K W, Jamieson D N, Orwa J O, Bursill L A, Peng J L 2000 Chem. Phys. Lett. 332 93
 Google Scholar
Google Scholar
[31] Prawer S, Nemanich R J 2004 Philos. Trans. R. Soc. Lond. Ser. A: Math. Phys. Eng. Sci. 362 2537
 Google Scholar
Google Scholar
[32] Ferrari A C, Robertson J 2000 Phys. Rev. B 61 14095
 Google Scholar
Google Scholar
[33] Ferrari A C, Robertson J 2001 Phys. Rev. B 64 075414
 Google Scholar
Google Scholar
[34] Ferrari A C, Robertson J 2001 Phys. Rev. B 63 121405
 Google Scholar
Google Scholar
[35] Ferrari A C, Robertson J 2004 Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 362 2477
 Google Scholar
Google Scholar
[36] Fecher J, Wormser M, Rosiwal S M 2016 Diamond Relat. Mater. 61 41
 Google Scholar
Google Scholar
[37] Sails S R, Gardiner D J, Bowden M, Savage J, Rodway D 1996 Diamond Relat. Mater. 5 589
 Google Scholar
Google Scholar
计量
- 文章访问数: 9347
- PDF下载量: 59
- 被引次数: 0













 下载:
下载:




