-
在众多实验和理论研究中已经发现自突触通过自反馈电流调节神经元电活动和网络时空行为来实现生理功能. 本文通过理论研究, 发现在一些合适的时滞下, 抑制性自反馈电流能引起放电频率增加, 这是不同于传统结果—抑制性作用引起频率降低的新发现. 进一步, 对于没有自反馈的神经元, 发现在作用相位合适的抑制性脉冲电流的作用下, 放电的相位会提前, 导致放电频率增加, 这就表现出对应Hopf分岔的II型相位响应曲线的特征. 引起放电频率增加的抑制性脉冲刺激的相位与自反馈的时滞相对应, 这也就给出了自反馈能够引起放电频率增强的原因. 最后, 发现抑制性自反馈的时滞较长或耦合强度较大时, 噪声诱发的神经元放电峰-峰间期的变异系数较小, 也就是放电精确性提高, 与实验发现的慢抑制性自突触诱发放电精确性增加的结果相一致. 研究结果揭示了负反馈能增强系统响应这一新现象和相应的非线性动力学机制, 提供了调控神经电活动的新手段, 有助于认识现实神经系统的自突触的潜在功能.It has been found in many experimental and theoretical studies that autapse regulates the electrical activities of single neurons and the spatiotemporal behaviors of neuronal networks through feedback or coupling currents to achieve physiological functions. In the present paper, the effect of inhibitory self-feedback on spiking patterns near Hopf bifurcation point is studied in the deterministic Morris-Lecar model and the stochastic Morris-Lecar model, and the dynamical mechanism is acquired with the phase response curve (PRC) of spiking to the inhibitory square pulse current stimulation. The inhibitory self-feedback current with a suitable time-delay can induce the spiking frequency to increase, which is different from the traditional viewpoint that the inhibitory stimulations often induce the firing frequency to decrease. For the remained time delays, spiking frequency decreases. Furthermore, the changes of spiking frequency, induced by the inhibitory self-feedback current, can be well explained with the dynamical responses of the spiking pattern of a single neuron without autapse to an inhibitory square pulse current stimulation. For the spiking pattern of a neuron without autapse, when an inhibitory square pulse stimulation current resembling to the inhibitory self-feedback current is applied at some suitable phases after an action potential/spike, the phase of the action potential/spike following the square pulse current advances, which leads the interspike intervals (ISIs) to decrease and firing frequency to increase. For the remained stimulation phases of the inhibitory pulse current, the response phase of the following action potential/spike delays. Therefore, the PRC of the action potential/spike shows the characteristics of type-II excitability corresponding to Hopf bifurcation. The stimulation phase of the inhibitory square pulse current that can induce the spiking frequency of single neurons to increase corresponds to the time delay of inhibitory self-feedback that can enhance firing frequency, which shows that the type-II PRC is the cause that the inhibitory self-feedback can induce the spiking frequency to increase. Finally, when noise is introduced into the ML model with inhibitory self-feedback, the coefficient of variation (CV) of the ISIs is smaller for the longer time delay of the self-feedback or the stronger coupling strength of the autapse, that is, the spike-timing precision is improved for the smaller CV of ISIs. Such a result is consistent with the experimental result that slow inhibitory autapse can enhance spike-timing precision. The results present a novel phenomenon that negative self-feedback can enhance the response of the system and the corresponding nonlinear dynamical mechanism, i.e. the PRC, provide a new method of regulating the neural electrical activities, and are helpful in understanding the potential function of inhibitory autapse.
-
Keywords:
- Hopf bifurcation /
- phase response curve /
- inhibitory autapse /
- spike-timing precision
[1] Gu H G, Pan B B, Chen G R, Duan L X 2014 Nonlinear Dyn. 78 391
 Google Scholar
Google Scholar
[2] Gu H G, Chen S G 2014 Sci. China: Technol. Sci. 57 864
 Google Scholar
Google Scholar
[3] Gu H G, Chen S G, Li Y Y 2015 Chin. Phys. B 24 050505
 Google Scholar
Google Scholar
[4] Izhikevich E M 2000 Int. J. Bifurcat. Chaos 10 1171
 Google Scholar
Google Scholar
[5] Tateno T, Pakdaman K 2004 Chaos 14 511
 Google Scholar
Google Scholar
[6] Tateno T, Harsch A, Robinson H P C 2004 J. Neurophysiol. 92 2283
 Google Scholar
Google Scholar
[7] Ermentrout B 1996 Neural Comput. 8 979
 Google Scholar
Google Scholar
[8] Gutkin B S, Ermentrout G B, Reyes A D 2005 J. Neurophysiol. 94 1623
 Google Scholar
Google Scholar
[9] Stiefel K M, Gutkin B S, Sejnowski T J 2009 J. Comput. Neurosci. 26 289
 Google Scholar
Google Scholar
[10] 谢勇, 程建慧 2017 66 090501
 Google Scholar
Google Scholar
Xie Y, Cheng J H 2017 Acta Phys. Sin. 66 090501
 Google Scholar
Google Scholar
[11] van der Loos H, Glaser E M 1972 Brain Res. 48 355
 Google Scholar
Google Scholar
[12] Pouzat C, Marty A 1998 J. Physiol. 509 777
 Google Scholar
Google Scholar
[13] Tamas G, Buhl E H, Somogyi P 1997 J. Neurosci. 17 6352
 Google Scholar
Google Scholar
[14] Saada R, Miller N, Hurwitz I, Susswein A J 2009 Curr. Biol. 19 479
 Google Scholar
Google Scholar
[15] Bacci A, Huguenard J R 2006 Neuron 49 119
 Google Scholar
Google Scholar
[16] Bacci A, Huguenard J R, Prince D A 2003 J. Neurosci. 23 859
 Google Scholar
Google Scholar
[17] Hájos N, Pálhalmi J, Mann E O, Németh B, Paulsen O, Freund T F 2004 J. Neurosci. 24 9127
 Google Scholar
Google Scholar
[18] Vida I, Bartos M, Jonas P 2006 Neuron 49 107
 Google Scholar
Google Scholar
[19] Zhao Z G, Li L, Gu H G 2018 Front. Cell. Neurosci. 12 62
[20] Zhao Z G, Jia B, Gu H G 2016 Nonlinear Dyn. 86 1549
 Google Scholar
Google Scholar
[21] Wang H T, Ma J, Chen Y L, Chen Y 2014 Commun. Nonlinear Sci. Numer. Simul. 19 3242
 Google Scholar
Google Scholar
[22] Wang H T, Wang L F, Chen Y L, Chen Y 2014 Chaos 24 033122
 Google Scholar
Google Scholar
[23] Zhao Z G, Gu H G 2017 Sci. Rep. 7 6760
 Google Scholar
Google Scholar
[24] Han F, Gu X C, Wang Z J, Fan H, Cao J F, Lu Q S 2018 Chaos 28 106324
 Google Scholar
Google Scholar
[25] Dodla R, Svirskis G, Rinzel J 2006 J. Neurophysiol. 95 2664
 Google Scholar
Google Scholar
[26] Dodla R, Rinzel J 2006 Phys. Rev. E 73 010903
 Google Scholar
Google Scholar
[27] 丁学利, 李玉叶 2016 65 210502
 Google Scholar
Google Scholar
Ding X L, Li Y Y 2016 Acta Phys. Sin. 65 210502
 Google Scholar
Google Scholar
[28] Qin H X, Ma J, Wang C N, Wu Y 2014 PloS One 9 e100849
 Google Scholar
Google Scholar
[29] Wu Y A, Gong Y B, Wang Q 2015 Chaos 25 043113
 Google Scholar
Google Scholar
[30] Yilmaz E, Baysal V, Perc M, Ozer M 2016 Sci. China: Technol. Sci. 59 364
 Google Scholar
Google Scholar
[31] Elson R C, Selverston A I, Abarbanel H D I, Rabinovich M I 2002 J. Neurophysiol. 88 1166
 Google Scholar
Google Scholar
[32] Prinz A A, Abbott L F, Marder E 2004 Trends Neurosci. 27 218
 Google Scholar
Google Scholar
[33] Blitz D M, Nusbaum M P 2012 J. Neurosci. 32 9182
 Google Scholar
Google Scholar
[34] Hashemi M, Valizadeh A, Azizi Y 2012 Phys. Rev. E 85 021917
 Google Scholar
Google Scholar
[35] Gu H G, Zhao Z G 2015 PloS One 10 e0138593
 Google Scholar
Google Scholar
[36] Jia B, Wu Y C, He D, Guo B H, Xue L 2018 Nonlinear Dyn. 93 1599
 Google Scholar
Google Scholar
[37] 曹奔, 关利南, 古华光 2018 67 240502
 Google Scholar
Google Scholar
Cao B, Guan L N, Gu H G 2018 Acta Phys. Sin. 67 240502
 Google Scholar
Google Scholar
[38] Achuthan S, Canavier C C 2009 J. Neurosci. 29 5218
 Google Scholar
Google Scholar
[39] Goldberg J A, Atherton J F, Surmeier D J 2013 J. Neurophysiol. 110 2497
 Google Scholar
Google Scholar
[40] Smeal R M, Ermentrout G B, White J A 2010 Philos. Trans. R. Soc. Lond. B: Biol. Sci. 365 2407
 Google Scholar
Google Scholar
-
图 1 当I = 45.5 µA·cm–2, 方波脉冲幅值A = 1.65 µA·cm–2, 宽度d = 4.4 ms时, ML模型的放电序列和PRC (a) 方波脉冲刺激电流(短划线)、没有方波脉冲的放电(点线)和有方波脉冲的放电(实线); (b) PRC
Fig. 1. Spiketrains and PRC of the ML model when I = 45.5 µA·cm–2, A = 1.65 µA·cm–2, and d = 4.4 ms: (a) Square pulse disturbance current (dashed line), and spike trains without (dotted line) and with square pulse disturbance (solid line); (b) PRC.
图 2 (a) ML模型随I的平衡点分岔; (b) 当I = 45.5 µA·cm–2时, ML模型的放电序列图; (c) 当I = 44 µA·cm–2时, ML模型处于静息状态; (d) ISIs和频率随I的变化(实线表示ISIs, 虚线表示频率)
Fig. 2. (a) Bifurcation of ML model with respect to I; (b) spike trains of ML model when I = 45.5 µA·cm–2; (c) resting state of ML model when I = 44 µA·cm–2; (d) the changes of ISIs (solid line) and frequency (dashed line) with respect to I.
图 3 当I = 45.5 µA·cm–2时, 负向方波脉冲电流(虚线)作用在不同相位的放电序列(实线)和无方波脉冲作用的放电序列(红色的点线) (a) ts = 22 ms, A = –0.6 µA·cm–2, d = 4.9 ms; (b) ts = 22 ms, A = –1.65 µA·cm–2, d = 4.8 ms; (c) ts = 40 ms, A = –0.6 µA·cm–2, d = 4.9 ms; (d) ts = 40 ms, A = –1.65 µA·cm–2, d = 4.8 ms
Fig. 3. Spike trains induced by square pulse current applied at different phases when I = 45.5 µA·cm–2. The spike trains (solid line) influenced by negative square pulse current (dashed line) and the trains (red dotted line) without negative square pulse current. (a) ts = 22 ms, A = –0.6 µA·cm–2, d = 4.9 ms; (b) ts = 22 ms, A = –1.65 µA·cm–2, d = 4.8 ms; (c) ts = 40 ms, A = –0.6 µA·cm–2, d = 4.9 ms; (d) ts = 40 ms, A = –1.65 µA·cm–2, d = 4.8 ms.
图 4 当I = 45.5 µA·cm–2时, ML模型在Hopf分岔点附近的负向脉冲刺激诱发的PRC (a) A = –0.6 µA·cm–2, d = 4.9 ms; (b) A = –1.65 µA·cm–2, d = 4.8 ms
Fig. 4. PRC induced by negative square pulse current near the Hopf bifurcation point in the ML model when I = 45.5 µA·cm–2: (a) A = –0.6 µA·cm–2, d = 4.9 ms; (b) A = –1.65 µA·cm–2, d = 4.8 ms.
图 5 当I = 45.5 µA·cm–2, gaut = 0.04 mS·cm–2, 具有抑制性自突触ML模型的放电模式(实线)与抑制性自突触电流(短划线) (a) τ = 0 mS; (b) τ = 10 mS; (c) τ =20 mS; (d) τ = 30 mS; (e) τ = 40 mS; (f) τ =50 mS
Fig. 5. Inhibitory autapse current (dashed line) and spike trains (solid line) of the ML model with inhibitory autapse when I = 45.5 µA·cm–2 and gaut = 0.04 mS·cm–2: (a) τ = 0 mS; (b) τ = 10 mS; (c) τ = 20 mS; (d) τ = 30 mS; (e) τ = 40 mS; (f) τ =50 mS.
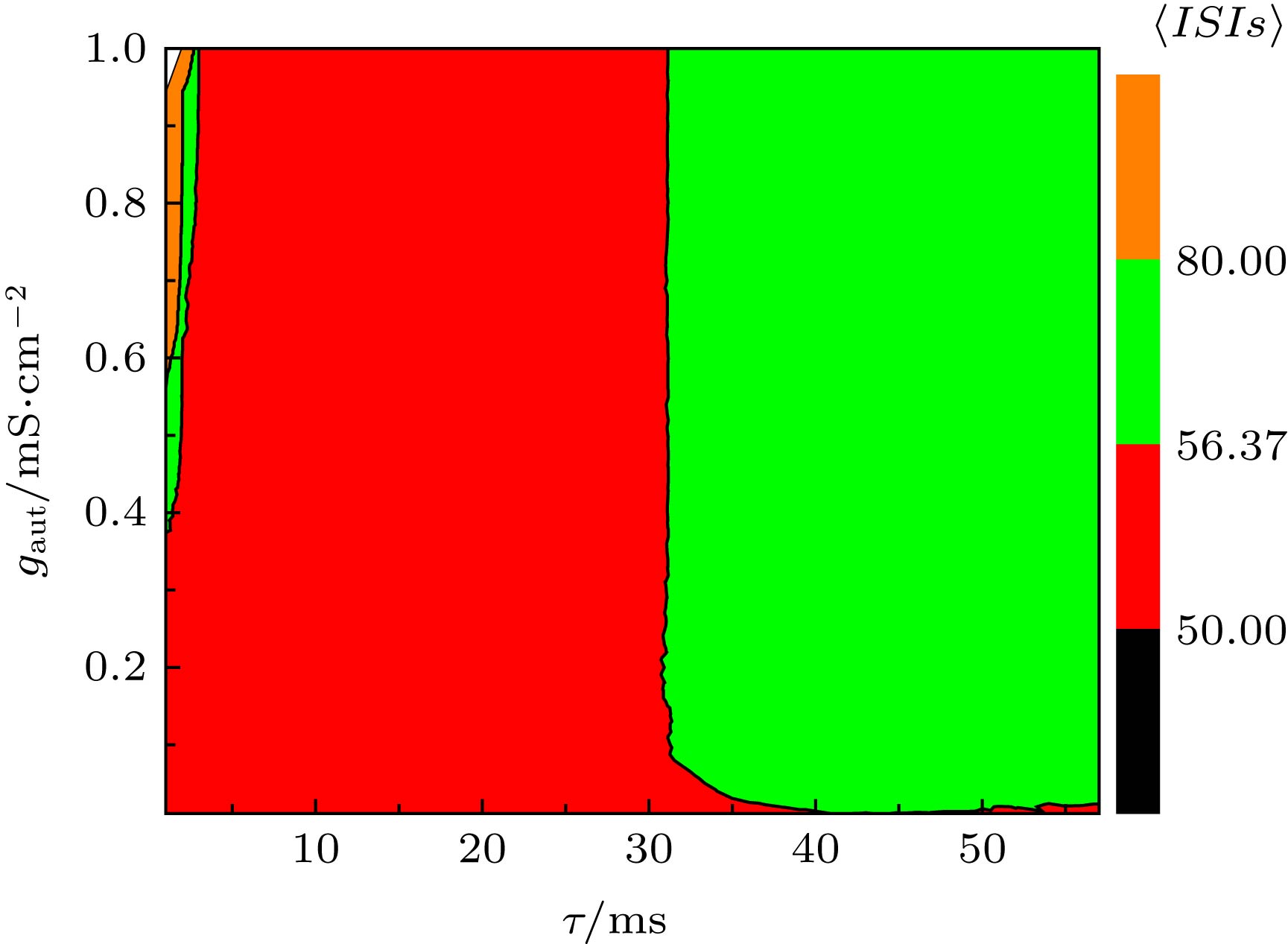
图 8 当I = 45.5 µA·cm–2, D = 0.5 µA·cm–2时, (a) ISIs的STD对时滞τ和耦合强度gaut的依赖关系, (b) ISIs的CV对时滞τ和耦合强度gaut的依赖关系
Fig. 8. (a) Dependence of standard deviation of ISIs (STD) on time delay τ and coupling strength gaut; (b) the dependence of coefficient of variation of ISIs (CV) on delay τ and coupling strength gaut. The parameter values are I = 45.5 µA·cm–2 and D = 0.5 µA·cm–2.
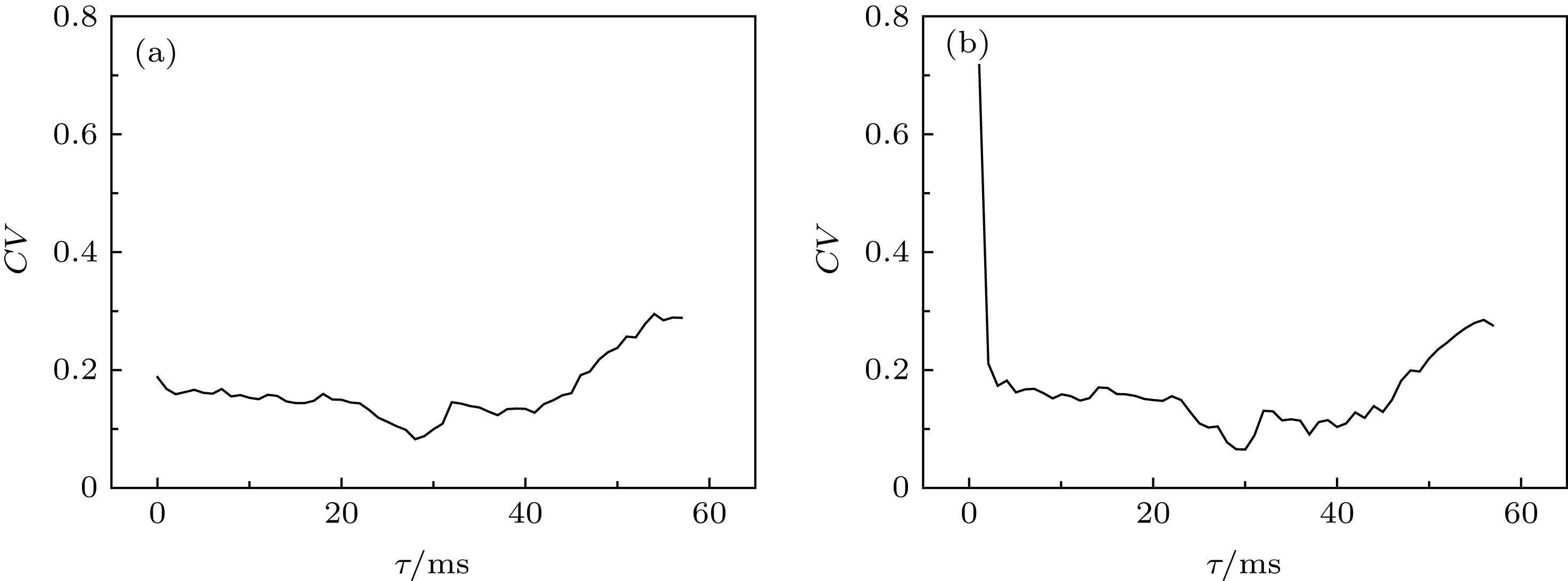
图 9 固定时滞τ在不同水平下, ISIs的CV随着耦合强度gaut的变化 (a) τ = 27 ms; (b) τ = 30 ms; (c) τ = 40 ms; (d) τ = 46 ms
Fig. 9. Changes of coefficient of variation (CV) of ISIs with respect to coupling strength gaut when time delay τ is fixed at different values: (a) τ = 27 ms; (b) τ = 30 ms; (c) τ = 40 ms; (d) τ = 46 ms.
图 11 当I = 45.5 µA·cm–2, D = 0.5 µA·cm–2, 耦合强度gaut = 0.61 mS·cm–2时, 时滞τ对神经元模型的精确放电的影响 (a) τ = 1 ms; (b) τ = 10 ms; (c) τ = 30 ms; (d) τ = 50 ms
Fig. 11. Effect of time delay τ on spike-timing precision of neuron model when I = 45.5 µA·cm–2, D = 0.5 µA·cm–2, and gaut = 0.61 mS·cm–2: (a) τ = 1 ms; (b) τ = 10 ms; (c) τ = 30 ms; (d) τ = 50 ms.
-
[1] Gu H G, Pan B B, Chen G R, Duan L X 2014 Nonlinear Dyn. 78 391
 Google Scholar
Google Scholar
[2] Gu H G, Chen S G 2014 Sci. China: Technol. Sci. 57 864
 Google Scholar
Google Scholar
[3] Gu H G, Chen S G, Li Y Y 2015 Chin. Phys. B 24 050505
 Google Scholar
Google Scholar
[4] Izhikevich E M 2000 Int. J. Bifurcat. Chaos 10 1171
 Google Scholar
Google Scholar
[5] Tateno T, Pakdaman K 2004 Chaos 14 511
 Google Scholar
Google Scholar
[6] Tateno T, Harsch A, Robinson H P C 2004 J. Neurophysiol. 92 2283
 Google Scholar
Google Scholar
[7] Ermentrout B 1996 Neural Comput. 8 979
 Google Scholar
Google Scholar
[8] Gutkin B S, Ermentrout G B, Reyes A D 2005 J. Neurophysiol. 94 1623
 Google Scholar
Google Scholar
[9] Stiefel K M, Gutkin B S, Sejnowski T J 2009 J. Comput. Neurosci. 26 289
 Google Scholar
Google Scholar
[10] 谢勇, 程建慧 2017 66 090501
 Google Scholar
Google Scholar
Xie Y, Cheng J H 2017 Acta Phys. Sin. 66 090501
 Google Scholar
Google Scholar
[11] van der Loos H, Glaser E M 1972 Brain Res. 48 355
 Google Scholar
Google Scholar
[12] Pouzat C, Marty A 1998 J. Physiol. 509 777
 Google Scholar
Google Scholar
[13] Tamas G, Buhl E H, Somogyi P 1997 J. Neurosci. 17 6352
 Google Scholar
Google Scholar
[14] Saada R, Miller N, Hurwitz I, Susswein A J 2009 Curr. Biol. 19 479
 Google Scholar
Google Scholar
[15] Bacci A, Huguenard J R 2006 Neuron 49 119
 Google Scholar
Google Scholar
[16] Bacci A, Huguenard J R, Prince D A 2003 J. Neurosci. 23 859
 Google Scholar
Google Scholar
[17] Hájos N, Pálhalmi J, Mann E O, Németh B, Paulsen O, Freund T F 2004 J. Neurosci. 24 9127
 Google Scholar
Google Scholar
[18] Vida I, Bartos M, Jonas P 2006 Neuron 49 107
 Google Scholar
Google Scholar
[19] Zhao Z G, Li L, Gu H G 2018 Front. Cell. Neurosci. 12 62
[20] Zhao Z G, Jia B, Gu H G 2016 Nonlinear Dyn. 86 1549
 Google Scholar
Google Scholar
[21] Wang H T, Ma J, Chen Y L, Chen Y 2014 Commun. Nonlinear Sci. Numer. Simul. 19 3242
 Google Scholar
Google Scholar
[22] Wang H T, Wang L F, Chen Y L, Chen Y 2014 Chaos 24 033122
 Google Scholar
Google Scholar
[23] Zhao Z G, Gu H G 2017 Sci. Rep. 7 6760
 Google Scholar
Google Scholar
[24] Han F, Gu X C, Wang Z J, Fan H, Cao J F, Lu Q S 2018 Chaos 28 106324
 Google Scholar
Google Scholar
[25] Dodla R, Svirskis G, Rinzel J 2006 J. Neurophysiol. 95 2664
 Google Scholar
Google Scholar
[26] Dodla R, Rinzel J 2006 Phys. Rev. E 73 010903
 Google Scholar
Google Scholar
[27] 丁学利, 李玉叶 2016 65 210502
 Google Scholar
Google Scholar
Ding X L, Li Y Y 2016 Acta Phys. Sin. 65 210502
 Google Scholar
Google Scholar
[28] Qin H X, Ma J, Wang C N, Wu Y 2014 PloS One 9 e100849
 Google Scholar
Google Scholar
[29] Wu Y A, Gong Y B, Wang Q 2015 Chaos 25 043113
 Google Scholar
Google Scholar
[30] Yilmaz E, Baysal V, Perc M, Ozer M 2016 Sci. China: Technol. Sci. 59 364
 Google Scholar
Google Scholar
[31] Elson R C, Selverston A I, Abarbanel H D I, Rabinovich M I 2002 J. Neurophysiol. 88 1166
 Google Scholar
Google Scholar
[32] Prinz A A, Abbott L F, Marder E 2004 Trends Neurosci. 27 218
 Google Scholar
Google Scholar
[33] Blitz D M, Nusbaum M P 2012 J. Neurosci. 32 9182
 Google Scholar
Google Scholar
[34] Hashemi M, Valizadeh A, Azizi Y 2012 Phys. Rev. E 85 021917
 Google Scholar
Google Scholar
[35] Gu H G, Zhao Z G 2015 PloS One 10 e0138593
 Google Scholar
Google Scholar
[36] Jia B, Wu Y C, He D, Guo B H, Xue L 2018 Nonlinear Dyn. 93 1599
 Google Scholar
Google Scholar
[37] 曹奔, 关利南, 古华光 2018 67 240502
 Google Scholar
Google Scholar
Cao B, Guan L N, Gu H G 2018 Acta Phys. Sin. 67 240502
 Google Scholar
Google Scholar
[38] Achuthan S, Canavier C C 2009 J. Neurosci. 29 5218
 Google Scholar
Google Scholar
[39] Goldberg J A, Atherton J F, Surmeier D J 2013 J. Neurophysiol. 110 2497
 Google Scholar
Google Scholar
[40] Smeal R M, Ermentrout G B, White J A 2010 Philos. Trans. R. Soc. Lond. B: Biol. Sci. 365 2407
 Google Scholar
Google Scholar
计量
- 文章访问数: 9846
- PDF下载量: 88
- 被引次数: 0














 下载:
下载: