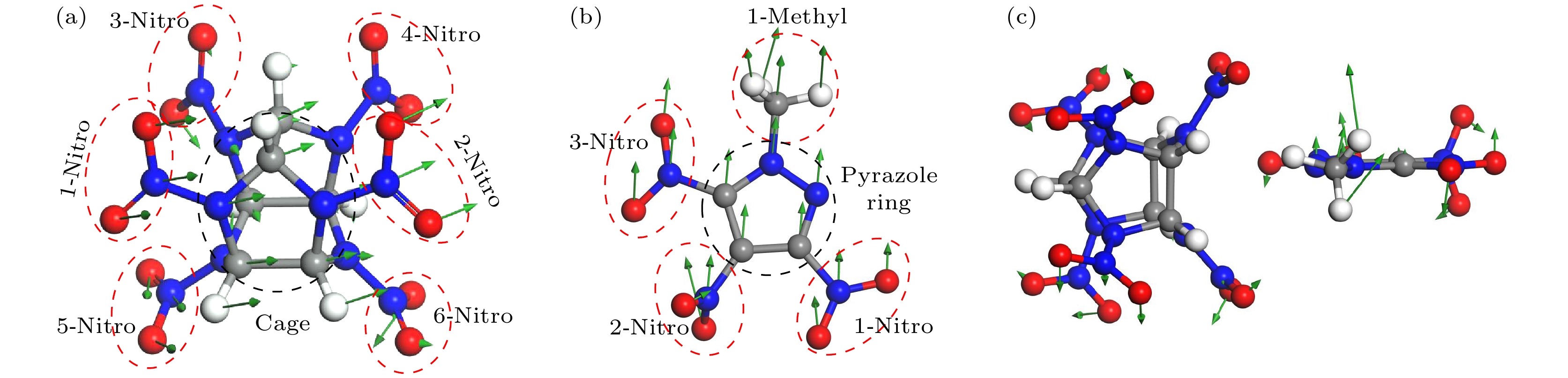
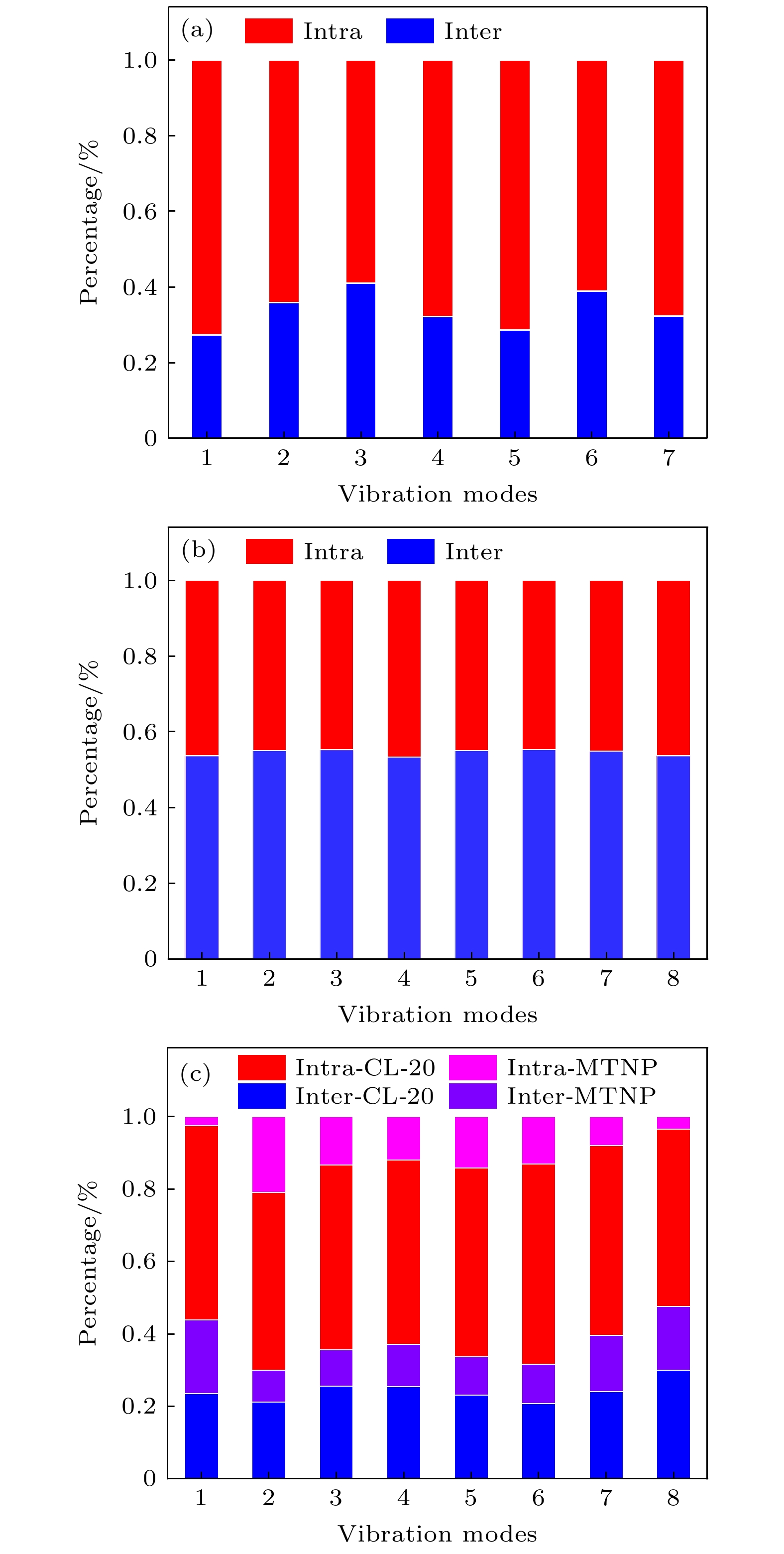
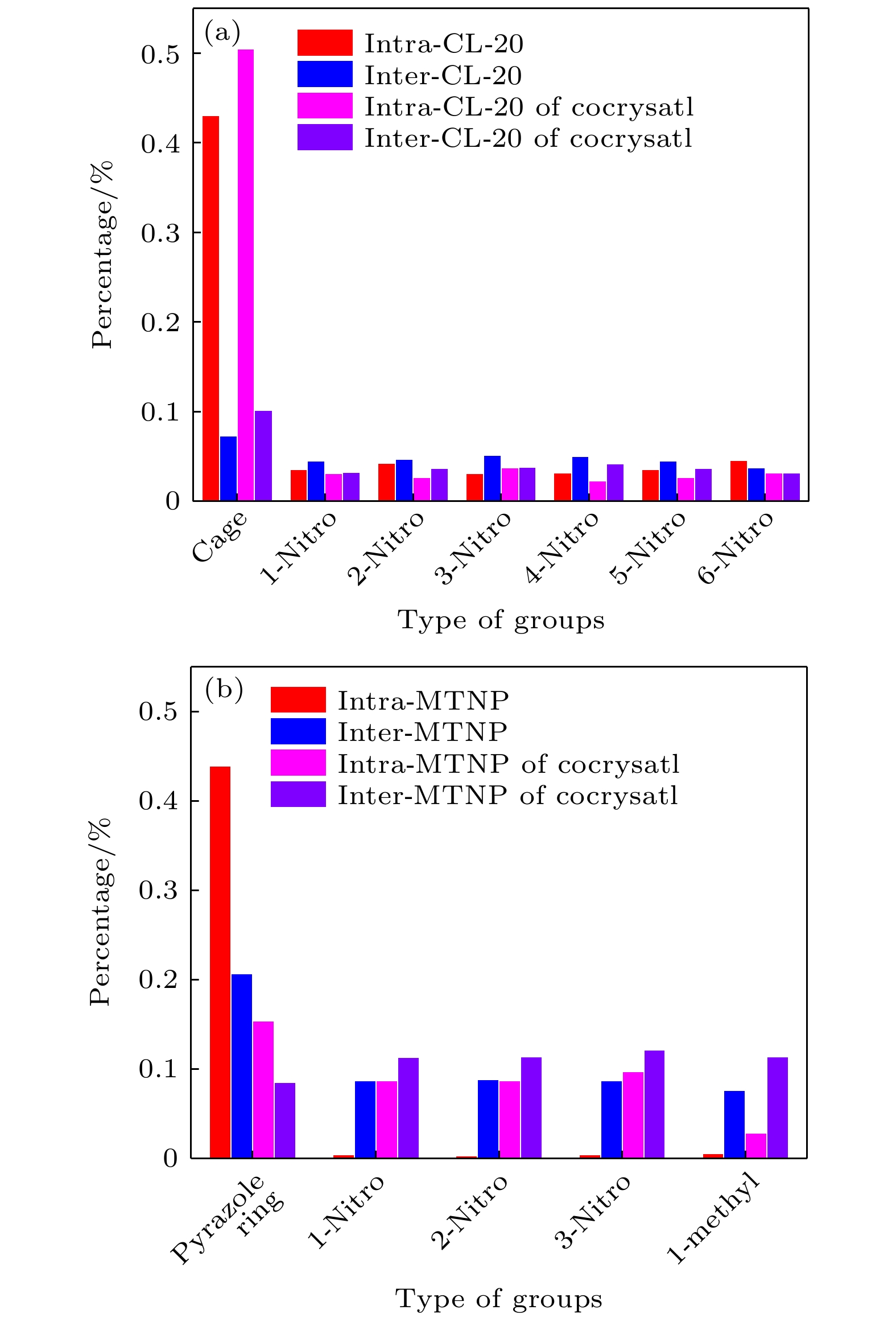
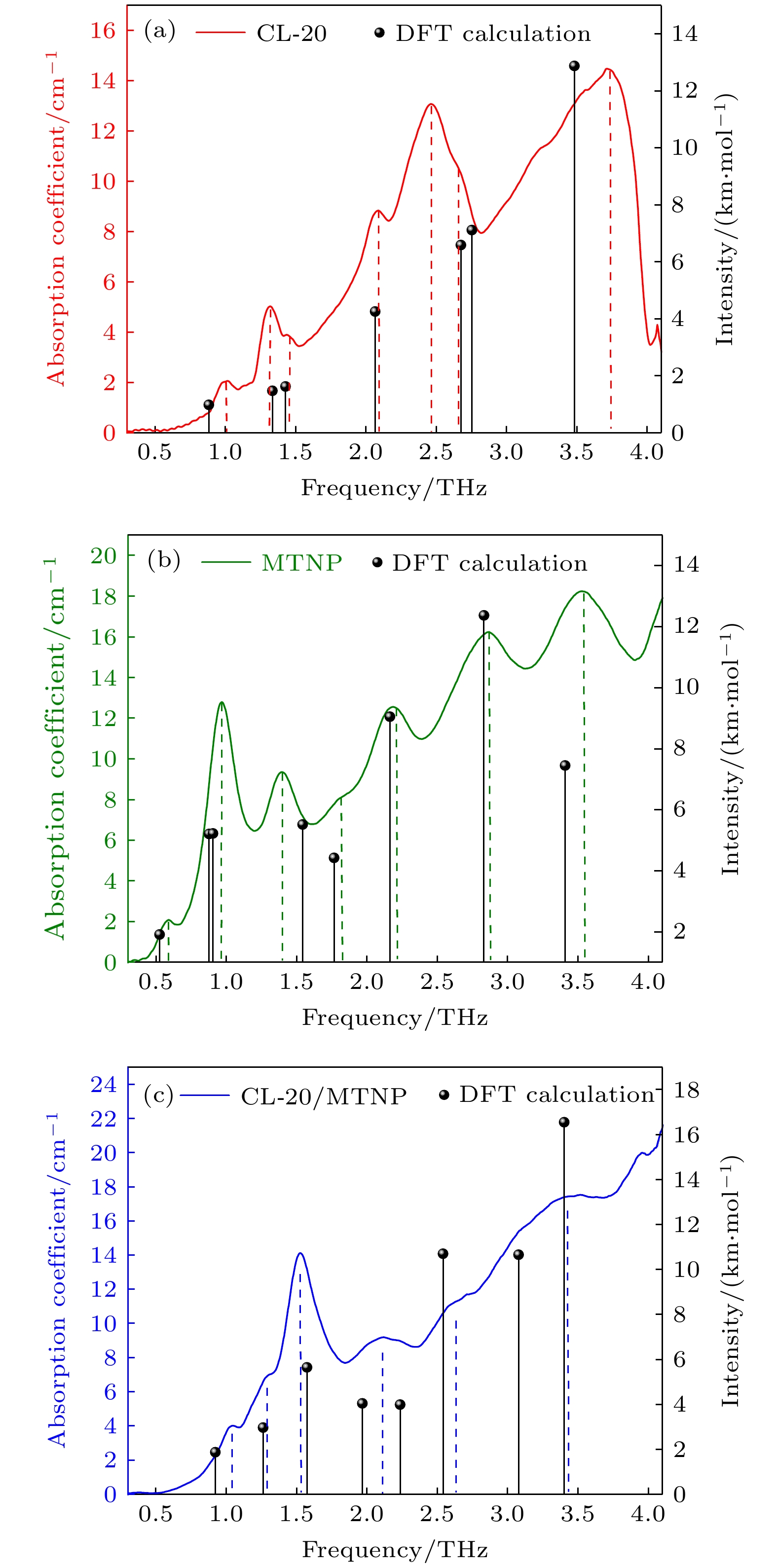
-
Cocrystals represent an effective method to manipulate the physicochemical properties of materials at a molecular level. However, understanding the relationship between their complex crystal structures and macroscopic properties is a challenge. In this paper, by using terahertz (THz) spectroscopy to characterize non-covalent interactions within crystals, the THz vibrational spectra of the CL-20/MTNP cocrystal are studied. Firstly, the THz spectra of CL-20, MTNP, and the CL-20/MTNP cocrystal are measured at room temperature. Both absorption positions and intensities of the cocrystals differ from those of their original components, confirming the unique advantage of terahertz spectroscopy in cocrystal identification. Secondly, the THz vibrational features of the three materials are calculated based on density functional theory (DFT). Then, the experimental absorptions are matched with the calculated vibrations. Furthermore, a vibrational decomposition method is employed to decompose the molecular vibrations into intermolecular and intramolecular vibrations. The vibrational variations of the cocrystal compared with its original components are analyzed. The results reveal that in the cocrystal, the intermolecular vibrational modes of both CL-20 and MTNP molecules have changed compared with their raw materials. This indicates that the non-covalent interactions in the cocrystal have changed the original intermolecular interactions of these molecules. Consequently, this enhancement promotes the heat transfer between MTNP and CL-20 molecules, thereby improving the thermal stability of the cocrystal. These findings in this study demonstrate that the THz vibrational spectroscopy technology helps establish a relationship between the molecular structure of cocrystal and its macroscopic properties. This research contributes to deepening our understanding of cocrystal systems and opens up a new way for designing and optimizing materials.
-
Keywords:
- terahertz spectroscopy /
- density functional theory /
- vibrational decomposition /
- CL-20 cocrystal
[1] Sun L J, Zhu W G, Zhang X T, Li L Q, Dong H L, Hu W P 2021 J. Am. Chem. Soc. 143 19243
 Google Scholar
Google Scholar
[2] Charpentier M D, Devogelaer J J, Tijink A, Meekes H, Tinnemans P, Vlieg E, de Gelder R, Johnston K, Ter Horst J H 2022 Cryst. Growth Des. 22 5511
 Google Scholar
Google Scholar
[3] Li X Y, Jin B, Luo L Q, Chu S J, Peng R F 2020 Thermochim. Acta 690 178665
 Google Scholar
Google Scholar
[4] Garbacz P, Wesolowski M 2020 Spectrochim. Acta Part A 234 118242
 Google Scholar
Google Scholar
[5] Zhang Y W, Ren G H, Su X Q, Meng T H, Zhao G Z 2022 Chin. Phys. B 31 103302
 Google Scholar
Google Scholar
[6] Wang C, Wang B, Wei G S, Chen J N, Wang L 2022 Chin. Phys. B 31 104201
 Google Scholar
Google Scholar
[7] Ruggiero M T 2020 J. Infrared Millim. Te. 41 491
 Google Scholar
Google Scholar
[8] Luczynska K, Druzbicki K, Runka T, Palka N, Wasicki J 2019 J. Infrared Millim. Te. 43 845
 Google Scholar
Google Scholar
[9] 郑转平, 刘榆杭, 赵帅宇, 蒋杰伟, 卢乐 2023 72 173201
 Google Scholar
Google Scholar
Zheng Z P, Liu Y H, Zhao S Y, Jiang J W, Lu L 2023 Acta Phys. Sin. 72 173201
 Google Scholar
Google Scholar
[10] Davis M P, Mohara M, Shimura K, Korter T M 2020 J. Phys. Chem. A 124 9793
 Google Scholar
Google Scholar
[11] Wang P F, Zhao J T, Zhang Y M, Zhu Z J, Liu L Y, Zhao H W, Yang X C, Yang X N, Sun X H, He M X 2022 Int. J. Pharm. 620 121759
 Google Scholar
Google Scholar
[12] Xiao Y Y, Huang H, Zhao X Y, Zou P A J, Wei L Y, Liu Y, Jin B, Peng R F, Huang S L 2023 Cryst. Growth Des. 23 6393
 Google Scholar
Google Scholar
[13] Ma Q, Jiang T, Chi Y, Chen Y, Wang J, Huang J L, Nie F D 2017 New J. Chem. 41 4165
 Google Scholar
Google Scholar
[14] Clark S J, Segallii M, Pickardii C J, Hasnipiii P J, Probertiv M 2005 Z. Kristallogr. Cryst. Mater. 220 567
 Google Scholar
Google Scholar
[15] Banks P, Burgess L, Ruggiero M 2021 Phys. Chem. Chem. Phys. 23 20038
 Google Scholar
Google Scholar
[16] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X L, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[17] Tkatchenko A, Scheffler M 2009 Phys. Rev. Lett. 102 073005
 Google Scholar
Google Scholar
[18] King M D, Buchanan W D, Korter T M 2011 Phys. Chem. Chem. Phys. 13 4250
 Google Scholar
Google Scholar
[19] Jepsen P U, Clark S J 2007 Chem. Phys. Lett. 442 275
 Google Scholar
Google Scholar
[20] Liu Q C, Deng H, Li H Z, Wang M C, Zahng Q, Kang Y, Shang L P 2022 Spectrochim. Acta A 283 121722
 Google Scholar
Google Scholar
-
图 2 三种物质的振动模式图 (a) CL-20在1.33 THz; (b) MTNP在0.88 THz; (c) CL-20/MTNP在3.08 THz. 为了清晰, 仅展示晶胞中的一个分子, 其中灰、白、红、蓝色分别表示碳、氢、氧、氮原子
Figure 2. Vibration mode of the three materails: (a) CL-20 at 1.33 THz; (b) MTNP at 0.88 THz; (c) CL-20/MTNP at 3.08 THz. For clarity, only one molecule within the unit cell is shown. Gray, white, red, and blue colors represent carbon, hydrogen, oxygen, and nitrogen atoms, respectively.
表 1 结构优化后晶格参数对比
Table 1. Comparison of lattice parameters after structural optimization.
Lattice parameters CL-20/% MTNP/% CL-20/MTNP/% Angle α/(°) 0.00 0.00 0.00 Angle β/(°) 0.71 0.00 0.06 Angle γ/(°) 0.00 0.00 0.00 Length a/Å –0.03 –0.37 –0.39 Length b/Å –0.36 –0.40 0.49 Length c/Å –0.02 0.12 0.05 Volume V/Å3 –0.81 –0.65 0.12 表 2 三种物质太赫兹吸收中心位置与DFT计算结果
Table 2. Experiment absorption center and DFT calculations of the three materials.
CL-20 MTNP CL-20/MTNP Exp. Cal. Δf Exp. Cal. Δf Exp. Cal. Δf 0.99 0.88(0.98) 0.11 0.59 0.53(1.91) 0.06 1.04 0.92(1.87) 0.12 1.31 1.33(1.47) 0.02 0.96 0.88(5.21) 0.07 1.28 1.26(2.97) 0.02 1.43 1.43(1.62) 0 0.91(5.22) 1.53 1.57(5.65) 0.04 2.08 2.07(4.25) 0.01 1.40 1.54(5.52) 0.14 2.11 1.97(4.04) 0.01 2.50 2.68(6.59) 0.18 1.81 1.77(4.42) 0.04 2.24(3.99) 2.70 2.75(7.11) 0.05 2.18 2.16(9.05) 0.02 2.62 2.54(10.70) 0.08 3.75 3.48(12.88) 0.27 2.86 2.83(12.37) 0.03 3.34 3.08(10.66) 0.10 3.53 3.41(7.45) 0.12 3.40(16.55) 注: Exp. , Experiment/THz; Cal., Calculation/THz (km · mol–1); Δf , deviation between experiment and calculation. -
[1] Sun L J, Zhu W G, Zhang X T, Li L Q, Dong H L, Hu W P 2021 J. Am. Chem. Soc. 143 19243
 Google Scholar
Google Scholar
[2] Charpentier M D, Devogelaer J J, Tijink A, Meekes H, Tinnemans P, Vlieg E, de Gelder R, Johnston K, Ter Horst J H 2022 Cryst. Growth Des. 22 5511
 Google Scholar
Google Scholar
[3] Li X Y, Jin B, Luo L Q, Chu S J, Peng R F 2020 Thermochim. Acta 690 178665
 Google Scholar
Google Scholar
[4] Garbacz P, Wesolowski M 2020 Spectrochim. Acta Part A 234 118242
 Google Scholar
Google Scholar
[5] Zhang Y W, Ren G H, Su X Q, Meng T H, Zhao G Z 2022 Chin. Phys. B 31 103302
 Google Scholar
Google Scholar
[6] Wang C, Wang B, Wei G S, Chen J N, Wang L 2022 Chin. Phys. B 31 104201
 Google Scholar
Google Scholar
[7] Ruggiero M T 2020 J. Infrared Millim. Te. 41 491
 Google Scholar
Google Scholar
[8] Luczynska K, Druzbicki K, Runka T, Palka N, Wasicki J 2019 J. Infrared Millim. Te. 43 845
 Google Scholar
Google Scholar
[9] 郑转平, 刘榆杭, 赵帅宇, 蒋杰伟, 卢乐 2023 72 173201
 Google Scholar
Google Scholar
Zheng Z P, Liu Y H, Zhao S Y, Jiang J W, Lu L 2023 Acta Phys. Sin. 72 173201
 Google Scholar
Google Scholar
[10] Davis M P, Mohara M, Shimura K, Korter T M 2020 J. Phys. Chem. A 124 9793
 Google Scholar
Google Scholar
[11] Wang P F, Zhao J T, Zhang Y M, Zhu Z J, Liu L Y, Zhao H W, Yang X C, Yang X N, Sun X H, He M X 2022 Int. J. Pharm. 620 121759
 Google Scholar
Google Scholar
[12] Xiao Y Y, Huang H, Zhao X Y, Zou P A J, Wei L Y, Liu Y, Jin B, Peng R F, Huang S L 2023 Cryst. Growth Des. 23 6393
 Google Scholar
Google Scholar
[13] Ma Q, Jiang T, Chi Y, Chen Y, Wang J, Huang J L, Nie F D 2017 New J. Chem. 41 4165
 Google Scholar
Google Scholar
[14] Clark S J, Segallii M, Pickardii C J, Hasnipiii P J, Probertiv M 2005 Z. Kristallogr. Cryst. Mater. 220 567
 Google Scholar
Google Scholar
[15] Banks P, Burgess L, Ruggiero M 2021 Phys. Chem. Chem. Phys. 23 20038
 Google Scholar
Google Scholar
[16] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X L, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[17] Tkatchenko A, Scheffler M 2009 Phys. Rev. Lett. 102 073005
 Google Scholar
Google Scholar
[18] King M D, Buchanan W D, Korter T M 2011 Phys. Chem. Chem. Phys. 13 4250
 Google Scholar
Google Scholar
[19] Jepsen P U, Clark S J 2007 Chem. Phys. Lett. 442 275
 Google Scholar
Google Scholar
[20] Liu Q C, Deng H, Li H Z, Wang M C, Zahng Q, Kang Y, Shang L P 2022 Spectrochim. Acta A 283 121722
 Google Scholar
Google Scholar
-
 19-20240944Suppl.pdf
19-20240944Suppl.pdf

Catalog
Metrics
- Abstract views: 3511
- PDF Downloads: 98
- Cited By: 0
















 DownLoad:
DownLoad: