-
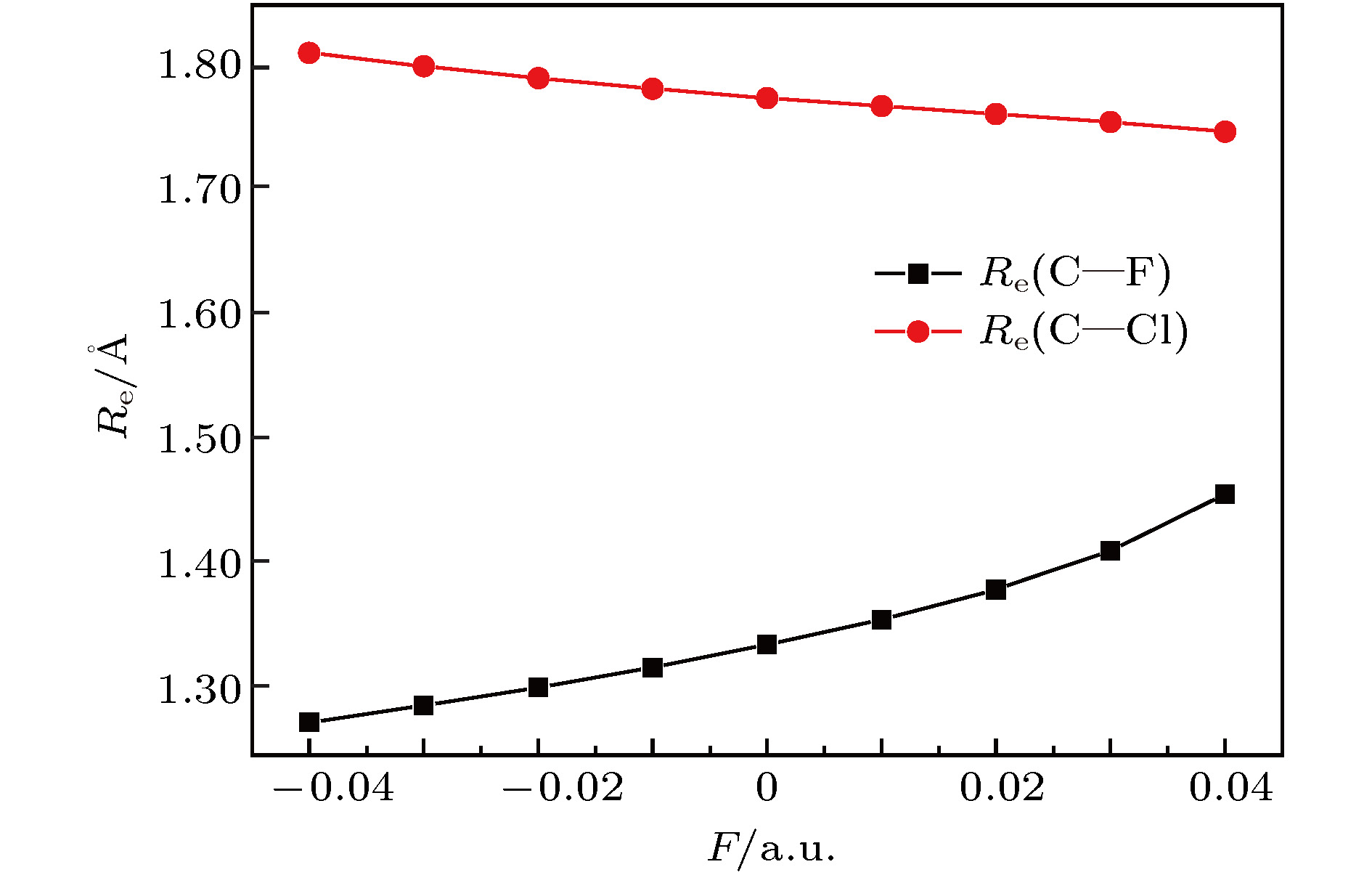
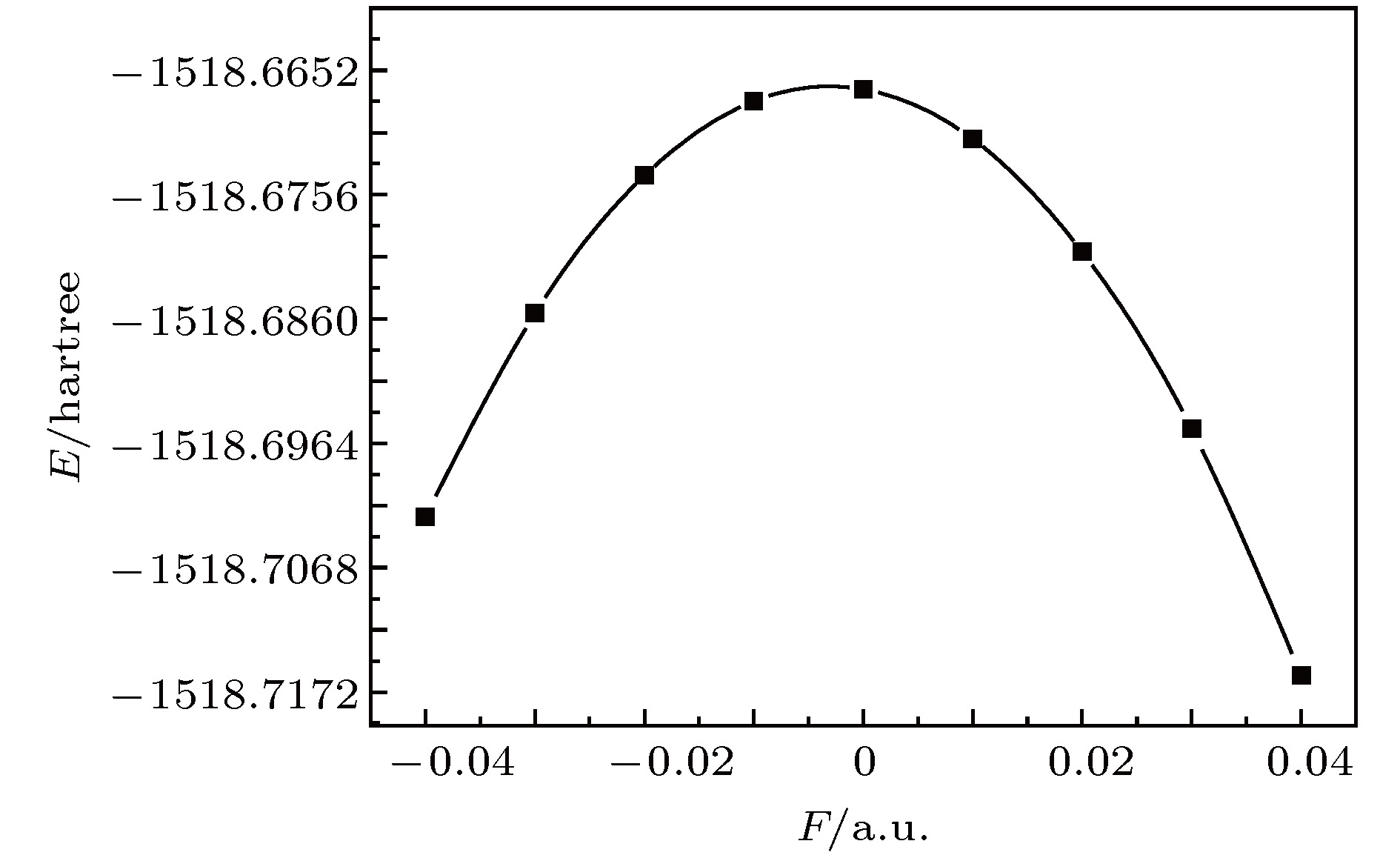
The ozone layer in the stratosphere of the earth’s atmosphere, which can be destroyed by CFC-11 molecule, plays a crucial role in human survival because it can absorb most of the harmful radiation from the sun and effectively protect the earth’s biology. Therefore, it is of evident significance to investigate the properties of CFC-11 molecule. By Motivated by this and the adoption of B3LYP complex function at a level of 6-311++g(3df, 3pd) basis set, we carry out a series of theoretical studies of the Freon material CFC-11 (CFCl3) molecules, including the studies of the equilibrium structure, electric dipole moment, total energy, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) level, energy gap, infrared and Raman spectrum, C—F dissociation characteristics of CFC-11 molecule, and the effect of the applied electric field on CFC-11 molecule as well. The results show that the maximum error between the theoretical calculation value and the experimental value is less than 2% for an optimized ground state structure; the C—F bond length and C—Cl bond length extend with the increase of electric field intensity, but the degree of change of C—F bond length is much stronger than that of C—Cl; the HOMO energy level and total energy go up and then come down as the external field rises, while the LUMO energy level goes up as the field increases. The energy gap Eg first increases and then decreases with the variation of EH and EL. The dipole moment without electric field is a minimum value, and the external electric field leads the molecular polarity to increase and the molecular activity to strengthen. The electric field influences the absorption intensity of infrared and Raman spectrum. The infrared and Raman spectrum move toward the long wave under the action of positive electric field, while they move toward the short wave under the action of negative electric field. The red- or blue-shift of infrared and Raman spectrum occur with the change of electric field. The electric field can be adopted as an auxiliary means to separate the overlapping or quasi-overlapping spectral lines. The potential well depth decreases with the increase of the reverse electric field until it vanishes, which causes the bound state ability of C—F bond of CFC-11 molecule to gradually degrade. This paper is expected to provide a feasible and effective tunable means for the final dissociation and degradation of CFC-11 molecules.
[1] Stratford J 2002 Estimating CFC Releases from Cutting of Fridges (Scottish: Scottish Environment Protection Agency) p1
[2] Molina M J, Rowland F S 1974 Nature 249 810
 Google Scholar
Google Scholar
[3] Farman J C, Gardiner B G, Shanklin J D 1985 Nature 315 207
 Google Scholar
Google Scholar
[4] Vollmer M K, Young D, Trudinger C M, Mühle J, Henne S, Rigby M, Park S, Li S, Guillevic M, Mitrevski B, Harth C M, Miller B R, Reimann S, Yao B, Steele L P, Wyss S A, Lunder C R, Arduini J, McCulloch A, Wu S H, Rhee T S, Wang R H J, Salameh P K, Hermansen O, Hill M, Langenfelds R L, Ivy D, O’Doherty S, Krummel P B , Maione M, Etheridge D M, Zhou L X, Fraser P J, Prinn R G, Weiss R F, Simmonds P G 2018 Atmos. Chem. Phys. 18 979
 Google Scholar
Google Scholar
[5] Schuck T J, Lefrancois F, Gallmann F, Wang D, Jesswein M, Hoker J, Bönisch H, Engel A 2018 Atmos. Chem. Phys. 18 16553
 Google Scholar
Google Scholar
[6] LeedhamElvidge E, Bönisch H, Brenninkmeijer C A, Engel A, Fraser P J, Gallacher E, Langenfelds R, Mühle J, Oram D E, Ray E A, Ridley A R, Röckmann T, Sturges W T, Weiss R F, Laube J C 2018 Atmos. Chem. Phys. 18 3369
 Google Scholar
Google Scholar
[7] Warner M J, Bullister J L, Wisegarver D P, Gammon R H, Weiss R F 1996 J. Geophys. Res. Oceans 101 20525
 Google Scholar
Google Scholar
[8] Willey D A, Fine R A, Sonnerup R E, Bullister J L, Smethie Jr W M, Warner M J 2004 Geophys. Res. Lett. 31 L01303
 Google Scholar
Google Scholar
[9] Font R, Fullana A, Caballero J A, Candela J, Garcıa A 2001 J. Anal. Appl. 58−59 63
 Google Scholar
Google Scholar
[10] Cullis C F, Hirschler M M 1981 The Combustion of Organic Polymers (London: Clarendon Press Oxford) p120
[11] Lattimer R P, Williams R C 2002 J. Anal. Appl. Pyroly. 63 85
 Google Scholar
Google Scholar
[12] 刘玉柱, 肖韶荣, 王俊锋, 何仲福, 邱学军, Gregor Knopp 2016 65 113301
 Google Scholar
Google Scholar
Liu Y Z, Xiao S R Wang J F, He Z F, Qiu X J, Gregor K 2016 Acta Phys. Sin. 65 113301
 Google Scholar
Google Scholar
[13] 侯健, 韩功元, 张振满, 潘循皙, 侯惠奇 1999 复旦学报 (自然科学版) 38 627
Hou J, Han G Y, Zhang Z M, Pan X X, Hou H Q 1999 J. Fudan Univ. (Natural Science)
38 627 [14] 谢安东, 谢晶, 周玲玲, 伍冬兰, 阮文, 罗文浪 2016 原子与分子 33 989
Xie A D, Xie J, Zhou L L, Wu D L, Ruan W, Luo W L 2016 Chin. J. Atom. Mol. Phys. 33 989
[15] 尹文怡, 刘玉柱, 林华, 李炳生, 秦朝朝 2018 光谱学与光谱分析 38 21
Yin W Y, Liu Y Z, Lin H, Li B S, Qin C C 2018 Spectrosc. Spect. Anal. 38 21
[16] 李亚莎, 谢云龙, 黄太焕, 徐程, 刘国成 2018 67 183101
 Google Scholar
Google Scholar
Li Y S, Xie Y L, Huang T H, Xu C, Liu G C 2018 Acta Phys. Sin. 67 183101
 Google Scholar
Google Scholar
[17] 吴永刚, 李世雄, 郝进欣, 徐梅, 孙光宇, 令狐荣锋 2015 64 153102
 Google Scholar
Google Scholar
Wu Y G, Li S X, Hao J X, Xu M, Sun G Y, Linghu R F 2015 Acta Phys. Sin. 64 153102
 Google Scholar
Google Scholar
[18] 李世雄, 张正平, 隆正文, 秦水介 2017 66 103102
 Google Scholar
Google Scholar
Li S X, Zhang Z P, Long Z W, Qin S J 2017 Acta Phys. Sin. 66 103102
 Google Scholar
Google Scholar
[19] Wu D L, Tan B, Wan H J, Xie A D, Ding D J 2015 Chin. Phys. Lett. 32 073101
 Google Scholar
Google Scholar
[20] Haynes W M 2014 CRC Handbook of Chemistry and Physics (Cleveland: CRC Press) p9
[21] Shimanouchi T 1977 J. Phys. Chem. Ref. Data 6 993
 Google Scholar
Google Scholar
[22] McDaniel A H, Cantrell C A, Davidson J A, Shetter R E, Calvert J G 1991 J. Atmos. Chem. 12 211
 Google Scholar
Google Scholar
[23] Nanes R, Silvaggio P M, Boese R W 1980 J. Quant. Spectrosc. Radiat. Transfer 23 211
 Google Scholar
Google Scholar
-
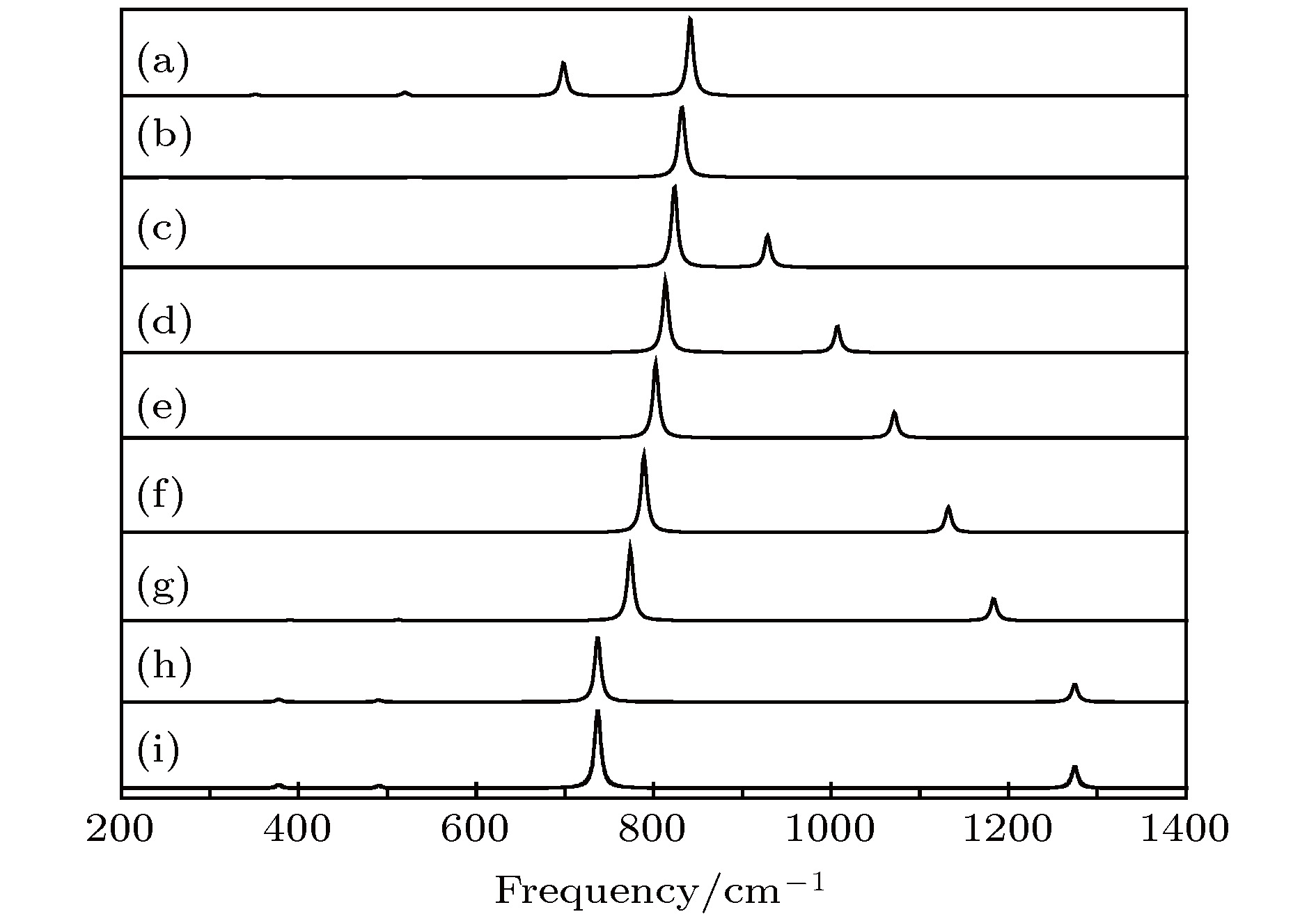
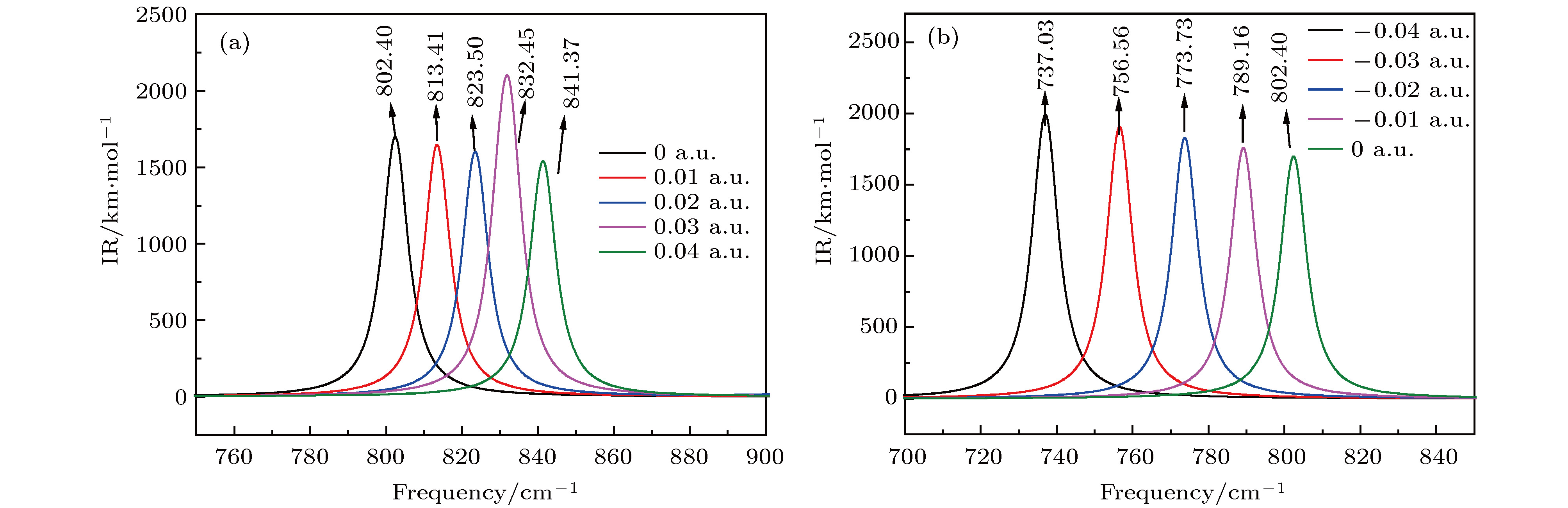
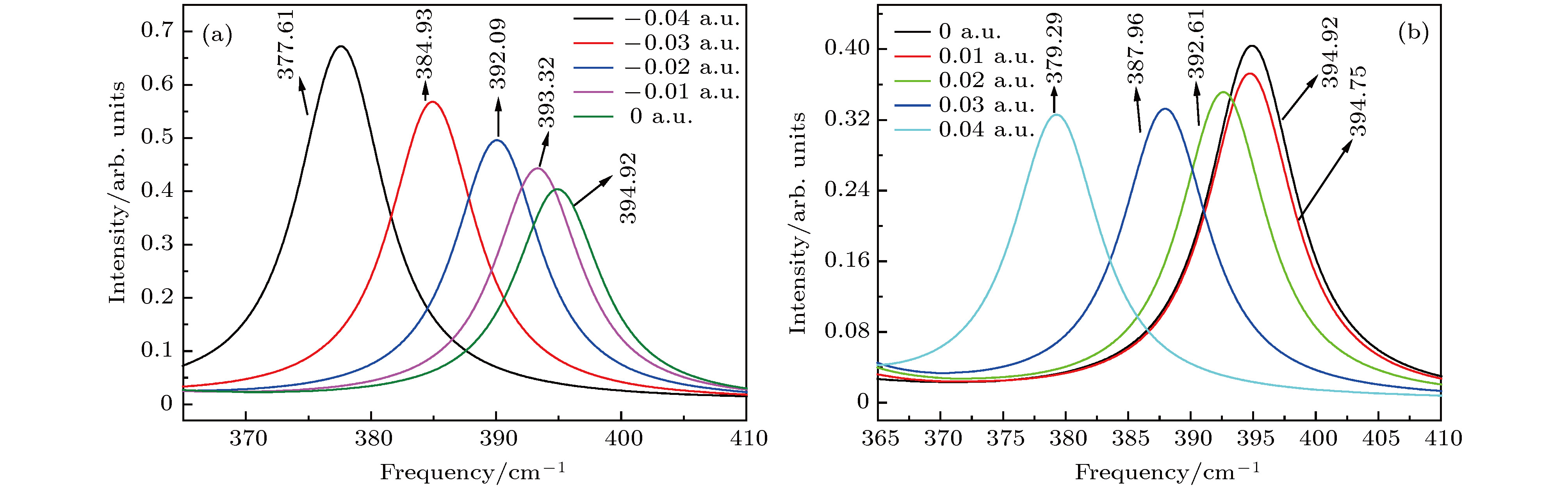
图 7 红外光谱随电场强度的变化 (a) –0.04 a.u.; (b) –0.03 a.u.; (c) –0.02 a.u.; (d) –0.01 a.u.; (e) a.u.; (f) 0.01 a.u.; (g) 0.02 a.u.; (h) 0.03 a.u.; (i) 0.04 a.u.
Figure 7. Variation of the infrared intensities with external electric field: (a) –0.04 a.u.; (b) –0.03 a.u.; (c) –0.02 a.u.; (d) –0.01 a.u.; (e) 0 a.u.; (f) 0.01 a.u.; (h) 0.02 a.u.; (g) 0.03 a.u.; (i) 0.04 a.u..
图 10 拉曼光谱随电场强度的变化 (a) –0.04 a.u.; (b) –0.03 a.u.; (c) –0.02 a.u.; (d) –0.01 a.u.; (e) 0 a.u.; (f) 0.01 a.u.; (g) 0.02 a.u.; (h) 0.03 a.u.; (i) 0.04 a.u.
Figure 10. Variation of Raman spectrum with external electric field: (a) –0.04 a.u.; (b) –0.03 a.u.; (c) –0.02 a.u.; (d) –0.01 a.u.; (e) 0 a.u.; (f) 0.01 a.u.; (h) 0.02 a.u.; (g) 0.03 a.u.; (i) 0.04 a.u..
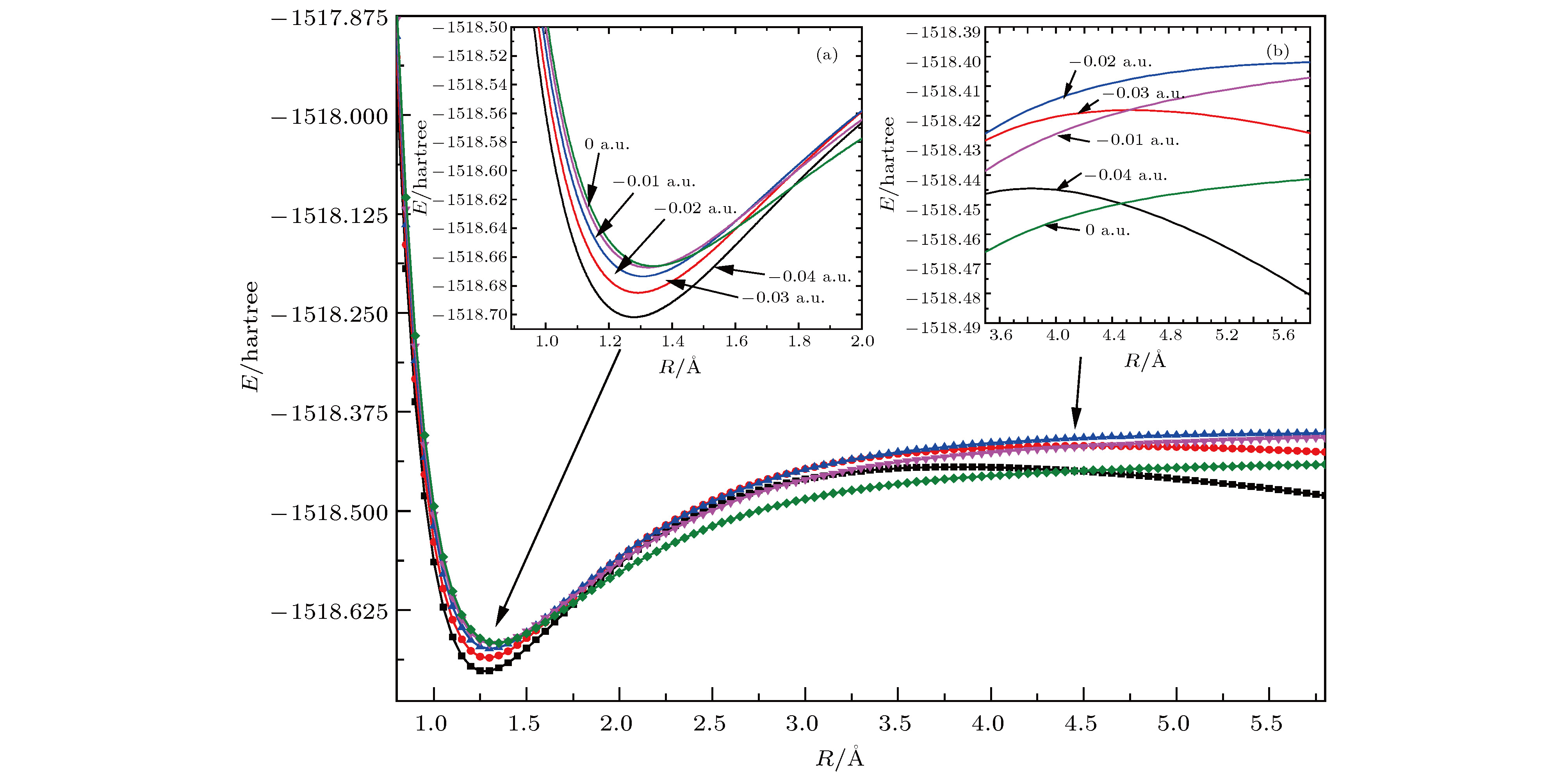
表 1 不同电场下计算得到的CFC-11分子参数(1 hartree = 110.5 × 10–21 J)
Table 1. Calculated parameters of CFC-11 molecule under different external electric field.
F/a.u. Re(C—F)/Å Re(C—Cl)/nm E/hartree $\mu$/Debye –0.04 1.2764 1.8118 –1518.7025 5.0491 –0.03 1.2898 1.8010 –1518.6855 3.6168 –0.02 1.3043 1.7916 –1518.6740 2.2462 –0.01 1.3199 1.7830 –1518.6678 0.9145 0.00 1.3385 1.7757 –1518.6668 0.4044 0.01 1.3582 1.7692 –1518.6710 1.7191 0.02 1.3825 1.7628 –1518.6803 3.0657 0.03 1.4135 1.7563 –1518.6952 4.4805 0.04 1.4587 1.7486 –1518.7158 6.0486 表 2 无电场时CFC-11分子的红外谱实验值与计算值
Table 2. Experiment data and calculated data of infrared spectrum for CFC-11 molecule without external electric field.
-
[1] Stratford J 2002 Estimating CFC Releases from Cutting of Fridges (Scottish: Scottish Environment Protection Agency) p1
[2] Molina M J, Rowland F S 1974 Nature 249 810
 Google Scholar
Google Scholar
[3] Farman J C, Gardiner B G, Shanklin J D 1985 Nature 315 207
 Google Scholar
Google Scholar
[4] Vollmer M K, Young D, Trudinger C M, Mühle J, Henne S, Rigby M, Park S, Li S, Guillevic M, Mitrevski B, Harth C M, Miller B R, Reimann S, Yao B, Steele L P, Wyss S A, Lunder C R, Arduini J, McCulloch A, Wu S H, Rhee T S, Wang R H J, Salameh P K, Hermansen O, Hill M, Langenfelds R L, Ivy D, O’Doherty S, Krummel P B , Maione M, Etheridge D M, Zhou L X, Fraser P J, Prinn R G, Weiss R F, Simmonds P G 2018 Atmos. Chem. Phys. 18 979
 Google Scholar
Google Scholar
[5] Schuck T J, Lefrancois F, Gallmann F, Wang D, Jesswein M, Hoker J, Bönisch H, Engel A 2018 Atmos. Chem. Phys. 18 16553
 Google Scholar
Google Scholar
[6] LeedhamElvidge E, Bönisch H, Brenninkmeijer C A, Engel A, Fraser P J, Gallacher E, Langenfelds R, Mühle J, Oram D E, Ray E A, Ridley A R, Röckmann T, Sturges W T, Weiss R F, Laube J C 2018 Atmos. Chem. Phys. 18 3369
 Google Scholar
Google Scholar
[7] Warner M J, Bullister J L, Wisegarver D P, Gammon R H, Weiss R F 1996 J. Geophys. Res. Oceans 101 20525
 Google Scholar
Google Scholar
[8] Willey D A, Fine R A, Sonnerup R E, Bullister J L, Smethie Jr W M, Warner M J 2004 Geophys. Res. Lett. 31 L01303
 Google Scholar
Google Scholar
[9] Font R, Fullana A, Caballero J A, Candela J, Garcıa A 2001 J. Anal. Appl. 58−59 63
 Google Scholar
Google Scholar
[10] Cullis C F, Hirschler M M 1981 The Combustion of Organic Polymers (London: Clarendon Press Oxford) p120
[11] Lattimer R P, Williams R C 2002 J. Anal. Appl. Pyroly. 63 85
 Google Scholar
Google Scholar
[12] 刘玉柱, 肖韶荣, 王俊锋, 何仲福, 邱学军, Gregor Knopp 2016 65 113301
 Google Scholar
Google Scholar
Liu Y Z, Xiao S R Wang J F, He Z F, Qiu X J, Gregor K 2016 Acta Phys. Sin. 65 113301
 Google Scholar
Google Scholar
[13] 侯健, 韩功元, 张振满, 潘循皙, 侯惠奇 1999 复旦学报 (自然科学版) 38 627
Hou J, Han G Y, Zhang Z M, Pan X X, Hou H Q 1999 J. Fudan Univ. (Natural Science)
38 627 [14] 谢安东, 谢晶, 周玲玲, 伍冬兰, 阮文, 罗文浪 2016 原子与分子 33 989
Xie A D, Xie J, Zhou L L, Wu D L, Ruan W, Luo W L 2016 Chin. J. Atom. Mol. Phys. 33 989
[15] 尹文怡, 刘玉柱, 林华, 李炳生, 秦朝朝 2018 光谱学与光谱分析 38 21
Yin W Y, Liu Y Z, Lin H, Li B S, Qin C C 2018 Spectrosc. Spect. Anal. 38 21
[16] 李亚莎, 谢云龙, 黄太焕, 徐程, 刘国成 2018 67 183101
 Google Scholar
Google Scholar
Li Y S, Xie Y L, Huang T H, Xu C, Liu G C 2018 Acta Phys. Sin. 67 183101
 Google Scholar
Google Scholar
[17] 吴永刚, 李世雄, 郝进欣, 徐梅, 孙光宇, 令狐荣锋 2015 64 153102
 Google Scholar
Google Scholar
Wu Y G, Li S X, Hao J X, Xu M, Sun G Y, Linghu R F 2015 Acta Phys. Sin. 64 153102
 Google Scholar
Google Scholar
[18] 李世雄, 张正平, 隆正文, 秦水介 2017 66 103102
 Google Scholar
Google Scholar
Li S X, Zhang Z P, Long Z W, Qin S J 2017 Acta Phys. Sin. 66 103102
 Google Scholar
Google Scholar
[19] Wu D L, Tan B, Wan H J, Xie A D, Ding D J 2015 Chin. Phys. Lett. 32 073101
 Google Scholar
Google Scholar
[20] Haynes W M 2014 CRC Handbook of Chemistry and Physics (Cleveland: CRC Press) p9
[21] Shimanouchi T 1977 J. Phys. Chem. Ref. Data 6 993
 Google Scholar
Google Scholar
[22] McDaniel A H, Cantrell C A, Davidson J A, Shetter R E, Calvert J G 1991 J. Atmos. Chem. 12 211
 Google Scholar
Google Scholar
[23] Nanes R, Silvaggio P M, Boese R W 1980 J. Quant. Spectrosc. Radiat. Transfer 23 211
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11818
- PDF Downloads: 52
- Cited By: 0















 DownLoad:
DownLoad: