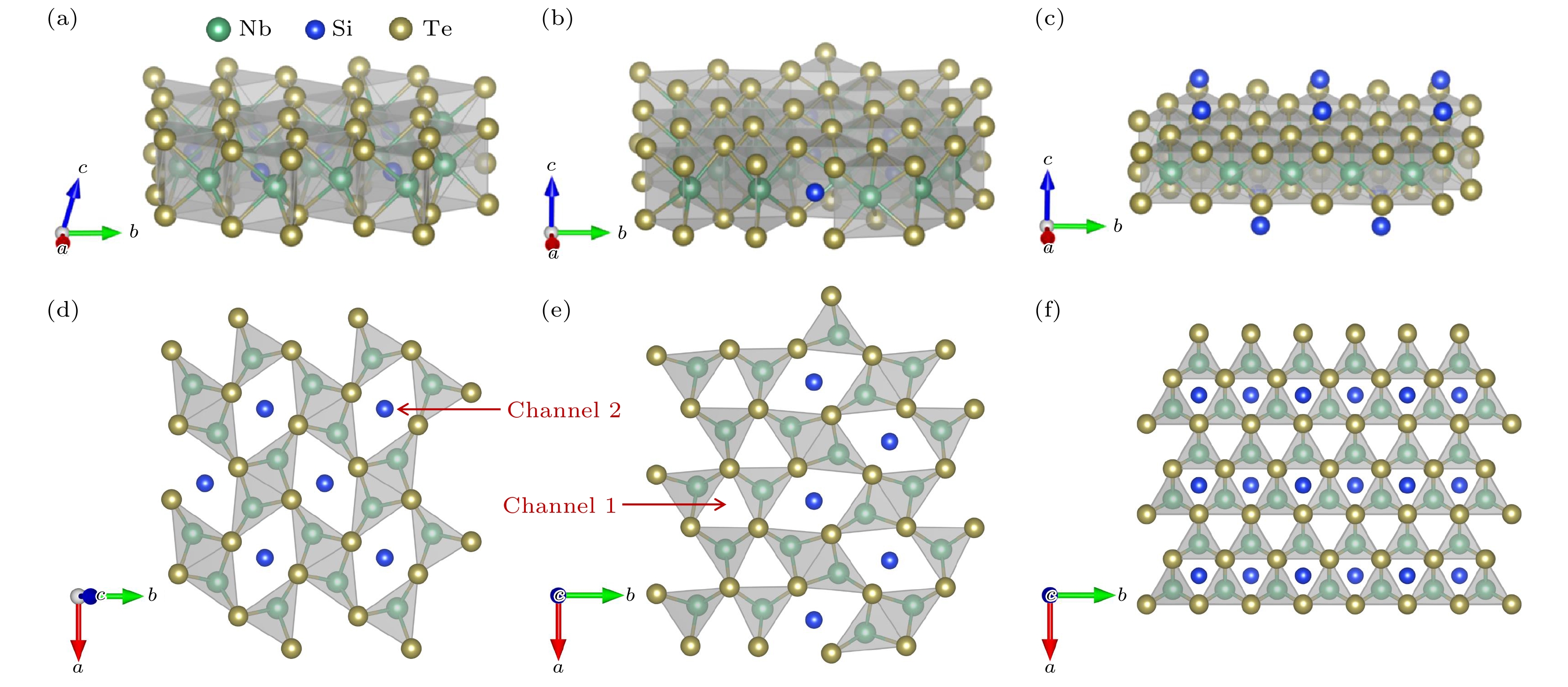
-
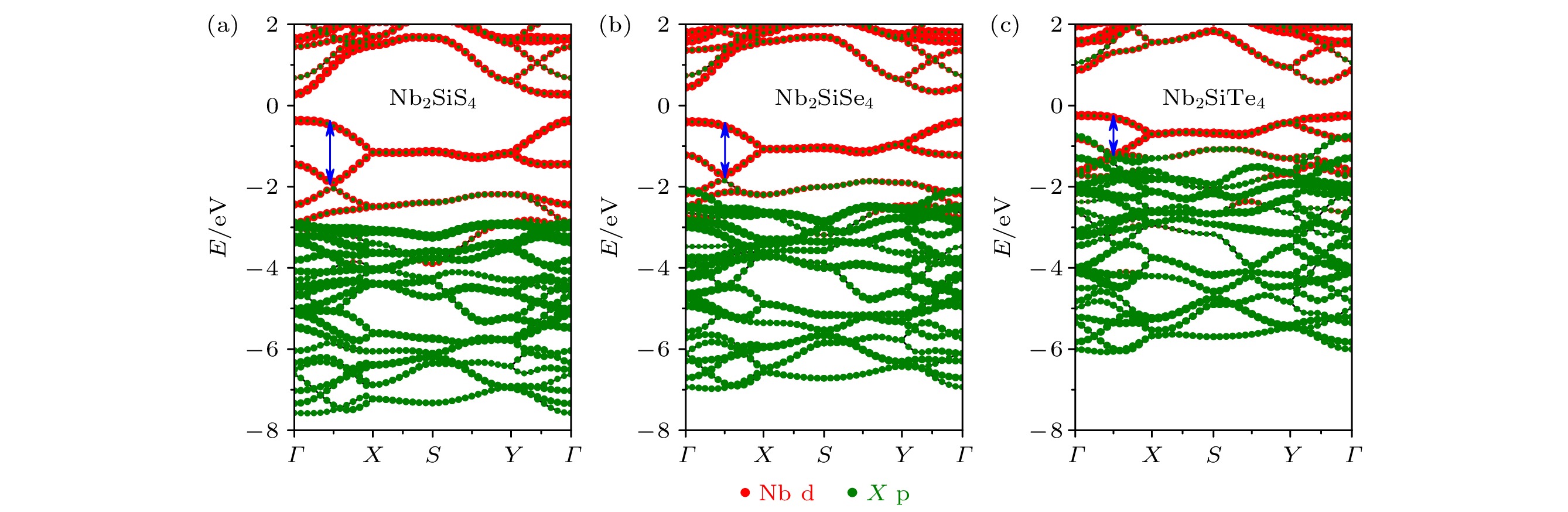
传统硫族化合物中阳离子相同时, 随着阴离子原子序数的增加, 价带顶逐渐升高, 带隙呈减小趋势. 在A2BX4基(A = V, Nb, Ta; B = Si, Ge, Sn; X = S, Se, Te)化合物中, 观察到随着阴离子原子序数增加, 其带隙呈现反常增大的现象. 为了探究其带隙异常变化的原因, 基于第一性原理计算, 对A2BX4基化合物的电子结构展开系统地研究, 包括能带结构、带边相对位置、轨道间耦合作用以及能带宽度等影响. 研究发现, Nb2SiX4基化合物中Nb原子4d轨道能量明显高于阴离子p轨道, 其价带顶和导带底主要由Nb原子4d轨道相互作用组成, 其带宽主要影响带隙大小. Nb2SiX4基化合物的带隙大小通过Nb—Nb和Nb—X键共同作用于Nb原子4d轨道的宽度来控制. 当阴离子序数增加时, Nb—Nb键长增加, 其相互作用减弱, 由Nb原子4d轨道主导的能带变宽, 带隙减小; 另一方面, Nb—X键长增加又使Nb原子4d带宽变窄, 带隙增加, 并且Nb—X键长增长占主导, 所以带隙最终呈现异常增加的趋势.Two-dimensional (2D) niobium silicon telluride (Nb2SiTe4) with good stability, a narrow band gap of 0.39 eV, high carrier mobility and superior photoresponsivity, is highly desired for applications in mid-infrared (MIR) detections, ambipolar transistors. Intensive investigations on its ferroelasticity, anisotropic carrier transport, anisotropic thermoelectric property, etc., have been reported recently. Motivated by the above prominent properties and promising applications, we systematically study the electronic properties of single-layer (SL) A2BX4 analogues (A = V, Nb, Ta; B = Si, Ge, Sn; X = S, Se, Te) and find a band-gap anomaly with respect to anion change, which differs from conventional 2D metal chalcogenide. In conventional binary chalcogenide, when cations are kept fixed, the bandgap tends to decrease as the atomic number of anions in the same group increases. However, in SL A2BX4, as atomic number of anions increases, its bandgaps tend to increase, with cations kept fixed. In order to find the underlying mechanism of such an abnormal bandgap, using first-principles calculations, we thoroughly investigate the electronic structures of Nb2SiX4 (X = S, Se, Te) surving as an example. It is found that the valance band maximum (VBM) and conduction band minimum (CBM) are mainly derived from the bonding and antibonding coupling between Nb 4d states. The bandwidth of Nb 4d states determines the relative value of the band gap in Nb2SiX4. We demonstrate that the band gap is largely influenced by the competition effect between Nb—Nb and Nb—X interactions in Nb2SiX4. As the anion atomic number increases, the Nb—Nb bond length increases, yielding an increased bandwidth of Nb 4d state and a smaller bandgap of Nb2SiX4. Meanwhile, as Nb—X bond length increases, the bandwidth of Nb 4d however decreases, yielding a larger bandgap. The interaction between Nb and X should be dominant and responsible for the overall bandgap increase of Nb2SiX4 compared with the Nb—Nb interaction.
-
Keywords:
- Nb2SiTe4 /
- band-gap anomaly /
- electronic structures /
- first-principles calculations
[1] Guo Q, Pospischil A, Bhuiyan M, Jiang H, Tian H, Farmer D, Deng B, Li C, Han S J, Wang H, Xia Q, Ma T P, Mueller T, Xia F 2016 Nano. Lett. 16 4648
 Google Scholar
Google Scholar
[2] Youngblood N, Chen C, Koester S J, Li M 2015 Nat. Photonics 9 247
 Google Scholar
Google Scholar
[3] Yuan H, Liu X, Afshinmanesh F, Li W, Xu G, Sun J, Lian B, Curto A G, Ye G, Hikita Y, Shen Z, Zhang S, Chen X, Brongersma M, Hwang H Y, Cui Y 2015 Nat. Nanotechnol. 10 707
 Google Scholar
Google Scholar
[4] Li L, Yu Y, Ye G J, Ge Q, Ou X, Wu H, Feng D, Chen X H, Zhang Y 2014 Nat. Nanotechnol. 9 372
 Google Scholar
Google Scholar
[5] Han C, Hu Z, Gomes L C, Bao Y, Carvalho A, Tan S J R, Lei B, Xiang D, Wu J, Qi D, Wang L, Huo F, Huang W, Loh K P, Chen W 2017 Nano. Lett. 17 4122
 Google Scholar
Google Scholar
[6] Tian H, Deng B, Chin M L, Yan X, Jiang H, Han S J, Sun V, Xia Q, Dubey M, Xia F, Wang H 2016 ACS Nano 10 10428
 Google Scholar
Google Scholar
[7] Castellanos-Gomez A, Vicarelli L, Prada E, Island J O, Narasimha-Acharya K L, Blanter S I, Groenendijk D J, Buscema M, Steele G A, Alvarez J V, Zandbergen H W, Palacios J J, vander Zant H S J 2014 2D Mater. 1 025001
 Google Scholar
Google Scholar
[8] Long M, Gao A, Wang P, Xia H, Ott C, Pan C, Fu Y, Liu E, Chen X, Lu W, Nilges T, Xu J, Wang X, Hu W, Miao F 2017 Sci. Adv. 3 e1700589
 Google Scholar
Google Scholar
[9] Wood J D, Wells S A, Jariwala D, Chen K S, Cho E, Sangwan V K, Liu X, Lauhon L J, Marks T J, Hersam M C 2014 Nano. Lett. 14 6964
 Google Scholar
Google Scholar
[10] Zhang T, Ma Y, Xu X, Lei C, Huang B, Dai Y 2020 J. Phys. Chem. Lett. 11 497
 Google Scholar
Google Scholar
[11] Wang W, Dai S, Li X, Yang J, Srolovitz D J, Zheng Q 2015 Nat. Commun. 6 7853
 Google Scholar
Google Scholar
[12] Zhao M, Xia W, Wang Y, Luo M, Tian Z, Guo Y, Hu W, Xue J 2019 ACS Nano 13 10705
 Google Scholar
Google Scholar
[13] Fang W Y, Li P A, Yuan J H, Xue K H, Wang J F 2020 J. Electron. Mater. 49 959
 Google Scholar
Google Scholar
[14] Ponce’S, Margine E R, Giustino F 2018 Phys. Rev. B 97 121201
 Google Scholar
Google Scholar
[15] Xu B, Xiang H, Yin J, Xia Y, Liu Z 2018 Nanoscale 10 215
 Google Scholar
Google Scholar
[16] Wu M, Zeng X 2016 Nano. Lett. 16 3236
 Google Scholar
Google Scholar
[17] Wang H, Li X, Sun J, Liu Z, Yang J 2017 2D Mater. 4 045020
 Google Scholar
Google Scholar
[18] 罗雄, 孟威威, 陈国旭佳, 管晓溪, 贾双凤, 郑赫, 王建波 2020 69 197102
 Google Scholar
Google Scholar
Luo X, Meng W W, Chen G X J, Guan X X, Jia S F, Zheng H, Wang J B 2020 Acta Phys. Sin. 69 197102
 Google Scholar
Google Scholar
[19] Carrier P, Wei S H 2005 J. Appl. Phys. 97 033707
 Google Scholar
Google Scholar
[20] Wei S H, Zunger A 1997 Phys. Rev. B 55 13605
 Google Scholar
Google Scholar
[21] Ye Z Y, Deng H X, Wu H Z, Li S S, Wei S H, Luo J W 2015 Npj Comput. Mater. 1 15001
 Google Scholar
Google Scholar
[22] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[23] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[24] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[25] Heyd J, Scuseria G E, Ernzerhof M 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
[26] Deringer V L, Tchougréeff A L, Dronskowski R 2011 J. Phys. Chem. A 115 5461
 Google Scholar
Google Scholar
[27] Maintz S, Deringer V L, Tchougréeff A L, Dronskowski R 2016 J. Comput. Chem. 37 1030
 Google Scholar
Google Scholar
[28] Wang V, Xu N, Liu J C, Tang G, Geng W T 2021 Comput. Phys. Commun. 267 108033
 Google Scholar
Google Scholar
[29] Wang F, Xu Y, Mu L, Zhang J, Xia W, Xue J, Guo Y, Yang J, Yan H 2022 ACS Nano 16 8107
 Google Scholar
Google Scholar
[30] Boucher F, Zhukov V, Evain M 1996 Inorg. Chem. 35 7649
 Google Scholar
Google Scholar
[31] Hu J, Liu X, Yue C L, Liu J Y, Zhu H W, He J B, Wei J, Mao Z Q, Antipina L Y, Popov Z I, Sorokin P B, Liu T J, Adams P W, Radmanesh S M A, Spinu L, Ji H, Natelson D 2015 Nat. Phys. 11 471
 Google Scholar
Google Scholar
[32] An L, Zhang H, Hu J, Zhu X, Gao W, Zhang J, Xi C, Ning W, Mao Z, Tian M 2018 Phys. Rev. B 97 235133
 Google Scholar
Google Scholar
-
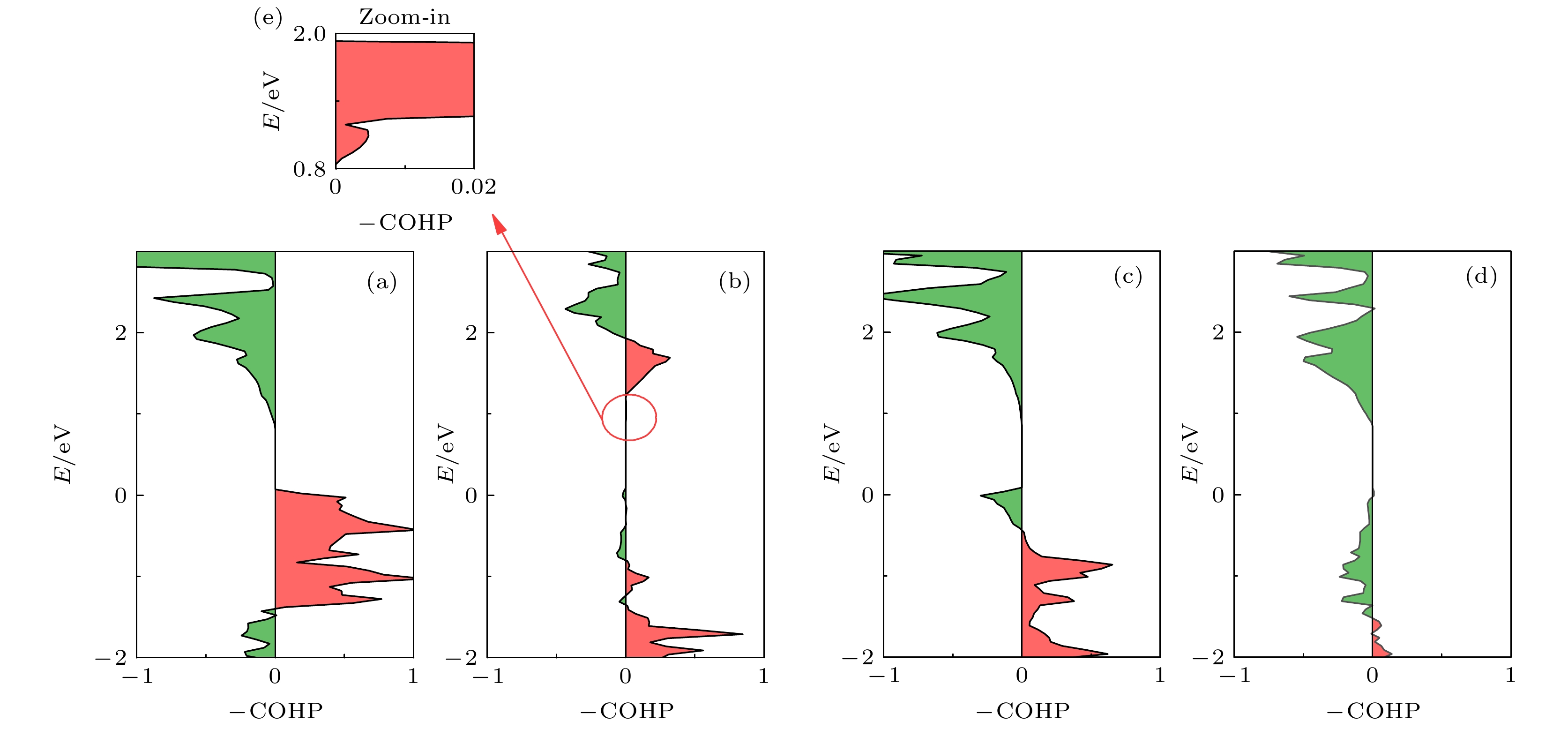
图 4 Nb2SiTe4中不同原子间哈密顿轨道布局 (a) Nb-Nb; (b) Nb-Si; (c) Nb-Te; (d) Si-Te; (e)图(b)中红色圆圈的放大部分(横坐标的正(负)表示原子轨道间为成(反)键态, 用图中红(绿)色区域表示)
Fig. 4. Crystal orbital Hamilton populations for different interatomic in Nb2SiTe4: (a) Nb-Nb; (b) Nb-Si; (c) Nb-Te; (d) Si-Te; (e) enlarged portion of the red circle in Fig. (b) (The positive (negative) of COHP represents bonding (antibonding) interaction between ions, as colored in red (green)).
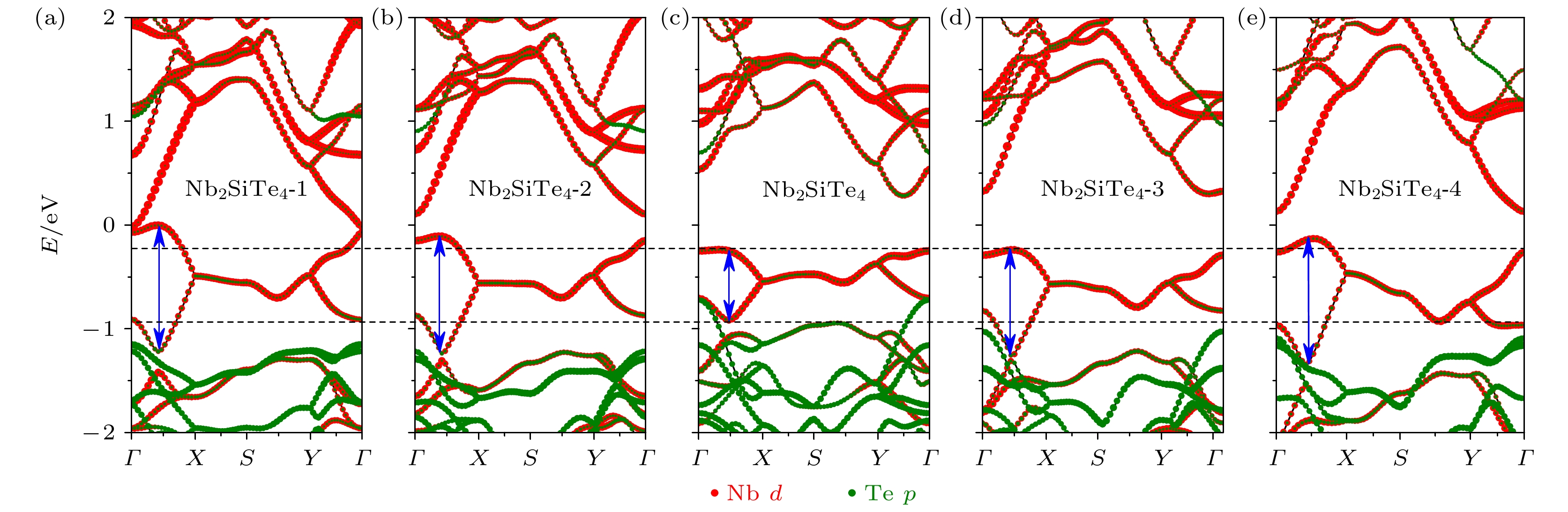
表 1 系列Nb2SiTe4构型的参数对比
Table 1. Comparisons of representative Nb2SiTe4-based compounds.
Nb—Nb键长/Å Nb—Te键长/Å Nb 4d带宽/Å 带隙Eg /eV Nb2SiTe4-1 2.91 2.72 1.23 –0.01 Nb2SiTe4-2 2.91 2.76 1.14 0.21 Nb2SiTe4 2.91 2.88 0.69 0.52 Nb2SiTe4-3 2.81 2.71 1.03 0.53 Nb2SiTe4-4 2.75 2.59 1.23 0.25 -
[1] Guo Q, Pospischil A, Bhuiyan M, Jiang H, Tian H, Farmer D, Deng B, Li C, Han S J, Wang H, Xia Q, Ma T P, Mueller T, Xia F 2016 Nano. Lett. 16 4648
 Google Scholar
Google Scholar
[2] Youngblood N, Chen C, Koester S J, Li M 2015 Nat. Photonics 9 247
 Google Scholar
Google Scholar
[3] Yuan H, Liu X, Afshinmanesh F, Li W, Xu G, Sun J, Lian B, Curto A G, Ye G, Hikita Y, Shen Z, Zhang S, Chen X, Brongersma M, Hwang H Y, Cui Y 2015 Nat. Nanotechnol. 10 707
 Google Scholar
Google Scholar
[4] Li L, Yu Y, Ye G J, Ge Q, Ou X, Wu H, Feng D, Chen X H, Zhang Y 2014 Nat. Nanotechnol. 9 372
 Google Scholar
Google Scholar
[5] Han C, Hu Z, Gomes L C, Bao Y, Carvalho A, Tan S J R, Lei B, Xiang D, Wu J, Qi D, Wang L, Huo F, Huang W, Loh K P, Chen W 2017 Nano. Lett. 17 4122
 Google Scholar
Google Scholar
[6] Tian H, Deng B, Chin M L, Yan X, Jiang H, Han S J, Sun V, Xia Q, Dubey M, Xia F, Wang H 2016 ACS Nano 10 10428
 Google Scholar
Google Scholar
[7] Castellanos-Gomez A, Vicarelli L, Prada E, Island J O, Narasimha-Acharya K L, Blanter S I, Groenendijk D J, Buscema M, Steele G A, Alvarez J V, Zandbergen H W, Palacios J J, vander Zant H S J 2014 2D Mater. 1 025001
 Google Scholar
Google Scholar
[8] Long M, Gao A, Wang P, Xia H, Ott C, Pan C, Fu Y, Liu E, Chen X, Lu W, Nilges T, Xu J, Wang X, Hu W, Miao F 2017 Sci. Adv. 3 e1700589
 Google Scholar
Google Scholar
[9] Wood J D, Wells S A, Jariwala D, Chen K S, Cho E, Sangwan V K, Liu X, Lauhon L J, Marks T J, Hersam M C 2014 Nano. Lett. 14 6964
 Google Scholar
Google Scholar
[10] Zhang T, Ma Y, Xu X, Lei C, Huang B, Dai Y 2020 J. Phys. Chem. Lett. 11 497
 Google Scholar
Google Scholar
[11] Wang W, Dai S, Li X, Yang J, Srolovitz D J, Zheng Q 2015 Nat. Commun. 6 7853
 Google Scholar
Google Scholar
[12] Zhao M, Xia W, Wang Y, Luo M, Tian Z, Guo Y, Hu W, Xue J 2019 ACS Nano 13 10705
 Google Scholar
Google Scholar
[13] Fang W Y, Li P A, Yuan J H, Xue K H, Wang J F 2020 J. Electron. Mater. 49 959
 Google Scholar
Google Scholar
[14] Ponce’S, Margine E R, Giustino F 2018 Phys. Rev. B 97 121201
 Google Scholar
Google Scholar
[15] Xu B, Xiang H, Yin J, Xia Y, Liu Z 2018 Nanoscale 10 215
 Google Scholar
Google Scholar
[16] Wu M, Zeng X 2016 Nano. Lett. 16 3236
 Google Scholar
Google Scholar
[17] Wang H, Li X, Sun J, Liu Z, Yang J 2017 2D Mater. 4 045020
 Google Scholar
Google Scholar
[18] 罗雄, 孟威威, 陈国旭佳, 管晓溪, 贾双凤, 郑赫, 王建波 2020 69 197102
 Google Scholar
Google Scholar
Luo X, Meng W W, Chen G X J, Guan X X, Jia S F, Zheng H, Wang J B 2020 Acta Phys. Sin. 69 197102
 Google Scholar
Google Scholar
[19] Carrier P, Wei S H 2005 J. Appl. Phys. 97 033707
 Google Scholar
Google Scholar
[20] Wei S H, Zunger A 1997 Phys. Rev. B 55 13605
 Google Scholar
Google Scholar
[21] Ye Z Y, Deng H X, Wu H Z, Li S S, Wei S H, Luo J W 2015 Npj Comput. Mater. 1 15001
 Google Scholar
Google Scholar
[22] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[23] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
 Google Scholar
Google Scholar
[24] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[25] Heyd J, Scuseria G E, Ernzerhof M 2003 J. Chem. Phys. 118 8207
 Google Scholar
Google Scholar
[26] Deringer V L, Tchougréeff A L, Dronskowski R 2011 J. Phys. Chem. A 115 5461
 Google Scholar
Google Scholar
[27] Maintz S, Deringer V L, Tchougréeff A L, Dronskowski R 2016 J. Comput. Chem. 37 1030
 Google Scholar
Google Scholar
[28] Wang V, Xu N, Liu J C, Tang G, Geng W T 2021 Comput. Phys. Commun. 267 108033
 Google Scholar
Google Scholar
[29] Wang F, Xu Y, Mu L, Zhang J, Xia W, Xue J, Guo Y, Yang J, Yan H 2022 ACS Nano 16 8107
 Google Scholar
Google Scholar
[30] Boucher F, Zhukov V, Evain M 1996 Inorg. Chem. 35 7649
 Google Scholar
Google Scholar
[31] Hu J, Liu X, Yue C L, Liu J Y, Zhu H W, He J B, Wei J, Mao Z Q, Antipina L Y, Popov Z I, Sorokin P B, Liu T J, Adams P W, Radmanesh S M A, Spinu L, Ji H, Natelson D 2015 Nat. Phys. 11 471
 Google Scholar
Google Scholar
[32] An L, Zhang H, Hu J, Zhu X, Gao W, Zhang J, Xi C, Ning W, Mao Z, Tian M 2018 Phys. Rev. B 97 235133
 Google Scholar
Google Scholar
计量
- 文章访问数: 6560
- PDF下载量: 116
- 被引次数: 0














 下载:
下载: