-
金属有机框架材料(MOFs)是一种有机配体桥接金属离子组成的新型无机-有机杂化多孔材料, 它具有功能可调、稳定性好以及多孔性等特点, 受到人们的广泛关注. 本文利用水热法制备了高质量的锌离子掺杂的钴基[(CH3)2NH2]Co1–xZnx(HCOO3)单晶样品 (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5). 单晶衍射、摇摆曲线、能量色散X射线光谱的实验结果表明, 锌离子均匀掺杂进了钴基金属有机框架材料中, 没有出现局部团簇等现象. 低温场冷曲线和比热曲线的测量结果表明, 非磁锌离子的掺杂减弱了Co基MOFs材料中Co离子之间的长程反铁磁相互作用, 使得Co-MOF的反铁磁相变温度由纯钴的15 K变为14.2 K (x = 0.1), 12.8 K (x = 0.2). 通过对掺锌样品低温下的磁滞回线的细致研究发现, 掺锌样品相对于纯钴样品在低温下具有更大的磁滞损耗和矫顽场. 相比于纯钴样品450 Oe (1 Oe = 103/(4π) A/m)的矫顽场, 掺锌样品的矫顽场最高达到3600 Oe, 并且磁滞面积也为纯钴样品的3倍以上. 另一方面, 在DMCo0.9Zn0.1F样品的磁滞回线上发现一系列台阶, 这一台阶现象随着温度升高而逐渐消失, 类似于单分子磁体的量子隧穿现象. 以往研究表明, 在这一类钙钛矿结构的金属有机骨架材料中, 存在长程磁相互作用和磁单离子行为的竞争. 我们认为非磁锌离子的掺杂减弱了Co离子之间的长程相互作用, 使得Co离子在低温下显示出量子隧穿引起的台阶效应.Metal-organic framework (MOF) is a new type of inorganic-organic hybrid porous material composed of organic ligands bridging metal ions, and it has the characteristics of tunable functions, good stability and porosity. In this study, Zn doped Co-based metal organic frame works single-crystal samples
$\left[{(\rm{C}\rm{H}}_{3}{)}_{2}\rm{N}{\rm{H}}_{2}\right]{\rm{C}\rm{o}}_{1-x}{\rm{Z}\rm{n}}_{x} $ $ {\left[\rm{H}\rm{C}\rm{O}\rm{O}\right]}_{3}$ are synthesized by the solvothermal method with normal ratio x = 0, 0.1, 0.2, 0.3, 0.4, 0.5. Single crystal diffraction, scanning electron microscope and energy dispersive X-ray spectroscopy results show that Zn ions are uniformly doped into Co-based MOFs crystals. The field cooling curves show that antiferromagnetic phase transition temperature of Co-based MOFs decreases from 15 K for pure Co-MOF x = 0 to 12.8 K for x = 0.2. Abnormal large magnetic hysteresis is obtained for Zn doped crystals with large coercive field 3600 Oe (x = 0.3) compared with 450 Oe coercive field for pure Co-MOF (x = 0), and the hysteresis area of Zinc-doped sample is more than 3 times that of pure cobalt sample. On the other hand, we find a series of steps on the hysteresis loop of DMCo0.9Zn0.1F sample, which gradually disappears with the increase of temperature, similar to the quantum tunneling phenomenon of a single molecule magnet. Previous studies have shown that the long range magnetic interaction and the magnetic single-ion behavior competition coexist in these systems. It is believed that the doping of non-magnetic zinc ions weakens the long-range interaction between Co ions and makes Co ions show the step effect caused by quantum tunneling at low temperature.[1] Yaghi O M, Li G M, Li H L 1995 Nature 378 703
 Google Scholar
Google Scholar
[2] Schröder M 2010 Functional Metal-organic Frameworks: Gas Storage, Separation and Catalysis (Vol. 293) (Berlin: Springer) pp115–153
[3] Kuppler R J, Timmons D J, Fang Q R, Li J R, Makala T A, Young M D, Yuan D, Zhao D, Zhuang W, Zhou H C 2009 Coord. Chem. Rev. 253 3042
 Google Scholar
Google Scholar
[4] Rao C N R, Cheetham A K, Thirumurugan A 2008 J. Phys. Condens. Matter 20 083202
 Google Scholar
Google Scholar
[5] Jain P, Dalal N S, Toby B H, Kroto H W, Cheetham A K 2008 J. Am. Chem. Soc. 130 10450
 Google Scholar
Google Scholar
[6] Zhang W, Xiong R G 2012 Chem. Rev. 112 1163
 Google Scholar
Google Scholar
[7] Stroppa A, Barone P, Jain P, Perez-Mato J M, Picozzi S 2013 Adv. Mater. 25 2284
 Google Scholar
Google Scholar
[8] Hu K L, Kurmoo M, Wang Z, Gao S 2009 Chem. A Eur. J. 15 12050
 Google Scholar
Google Scholar
[9] Weng D F, Wang Z M, Gao S 2011 Chem. Soc. Rev. 40 3157
 Google Scholar
Google Scholar
[10] Fan F R, Wu H, Nabok D, Hu S B, Ren W, Draxl C, Stroppa A 2017 J. Am. Chem. Soc. 139 12883
 Google Scholar
Google Scholar
[11] Vinoda K, Deepakb C S, Sharma S, Sornaduraia D, Satyaa A T, Ravindrana T R, Sundara C S, Bharathi A 2015 RSC Adv. 5 37818
 Google Scholar
Google Scholar
[12] Jain P, Ramachandran V, Clark R J, Zhou H D, Toby B H, Dalal N S, Kroto H W, Cheetham A K 2009 J. Am. Chem. Soc. 131 13625
 Google Scholar
Google Scholar
[13] Xu G C, Z W, Ma X M, Chen Y H, Z L, Cai H L, Wang Z M, Xiong R G, Gao S 2011 J. Am. Chem. Soc. 133 14948
 Google Scholar
Google Scholar
[14] Kundys B, Lappas A, Viret M, Kapustianyk V, Rudyk V, Semak S, Simon C, Bakaimi I 2010 Phys. Rev. B 81 224434
 Google Scholar
Google Scholar
[15] Stroppa A, Jain P, Barone P, Marsman M, Perez-Mato J M, Cheetham A K, Kroto H W, Picozzi S 2011 Angew. Chem. Int. Ed. 50 5847
 Google Scholar
Google Scholar
[16] Sante D D, Stroppa A, Jain P, Picozzi S 2013 J. Am. Chem. Soc. 135 18126
 Google Scholar
Google Scholar
[17] Jain P, Stroppa A, Nabok D, Marino A, Rubano A, Paparo D, Matsubara M, Nakotte H, Fiebig M, Picozzi S, Choi E S, Cheetham A K, Drax C, Dalal N S, Zapf V S 2016 npj Quantum Mater. 1 16012
 Google Scholar
Google Scholar
[18] Gómez-Aguirre L C, Pato-Doldán B, Mira J, Castro-García S, Señarís-Rodríguez M A, Sánchez-Andújar M, Singleton J, Zapf V S 2016 J. Am. Chem. Soc. 138 1122
 Google Scholar
Google Scholar
[19] Tian Y, Shen S, Cong J, Yan L, Wang S, Sun Y 2016 J. Am. Chem. Soc. 138 782
 Google Scholar
Google Scholar
[20] Mączka M, Gągor A, Hermanowicz K, Sieradzki A, Macalik L, Pikul A 2016 J. Solid State Chem. 237 150
 Google Scholar
Google Scholar
[21] Wang X Y, Gan L, Zhang S W, Gao S 2004 Inorg. Chem. 43 4615
 Google Scholar
Google Scholar
[22] Tian Y, Wang W, Chai Y, Cong J, Shen S, Yan L, Wang S, Han X, Sun Y 2014 Phys. Rev. Lett. 112 017202
 Google Scholar
Google Scholar
[23] Pato-Dolda B, Sanchez-Andujar M, Gomez-Aguirre L C, Yanez-Vilar S, Lopez-Beceiro J, Gracia-Fernandez C, Haghighirad A A, Ritter F, Castro-Garcia D S, Senaris-Rodriguez M A 2012 Phys. Chem. Chem. Phys. 14 8498
 Google Scholar
Google Scholar
[24] Friedman J R, Sarachik M P, Tejada J, Ziolo R 1996 Phys. Rev. Lett. 76 3830
 Google Scholar
Google Scholar
-
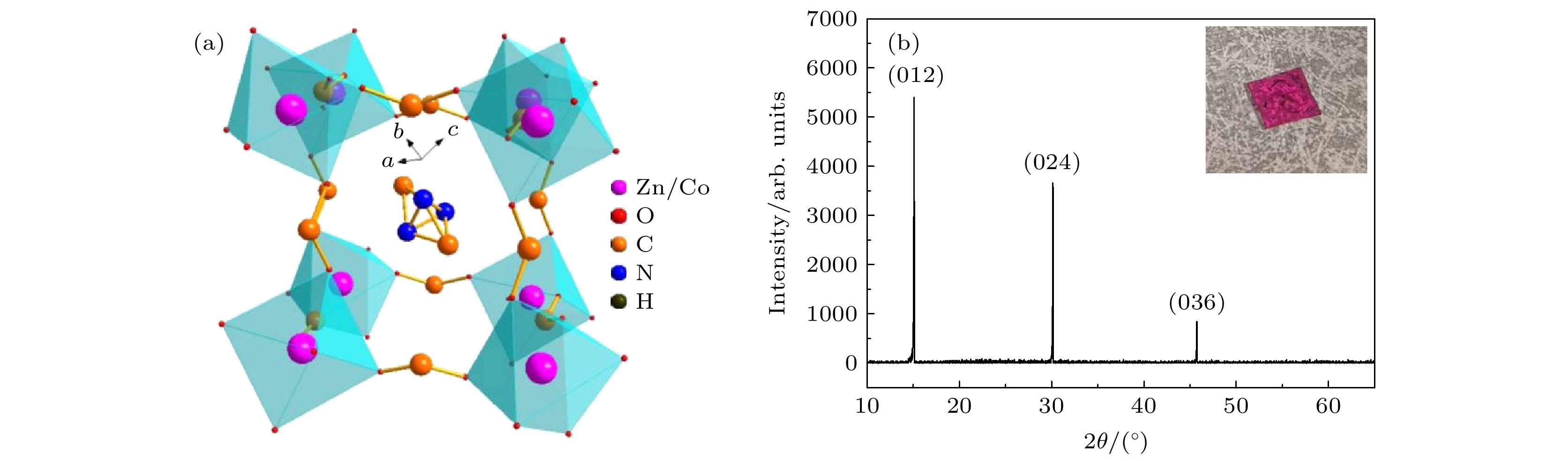
图 1 (a) 类钙钛矿型结构[(CH)3NH2]Co1–xZnx(HOOC)3的晶体框架; (b) DMCo0.9Zn0.1F 单晶样品的摇摆曲线, 插图所示为单晶样品的实物图
Fig. 1. (a) Crystal framework diagram of the perovskite-like structure [(CH)3NH2]Co1–xZnx(HOOC)3; (b) rocking curve of the DMCo0.9Zn0.1F single crystal sample, the inset shows the single crystal physical map of the sample.
图 3 (a) DMCo0.9Zn0.1F 单晶样品在不同磁场下的场冷和零场冷曲线(1 emu = 10–3 A·m2); (b) 不同掺杂比例样品在1.8 K的磁滞回线, 掺杂比例分别为x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 插图所示为由磁滞回线得到的矫顽场大小Hc随掺杂比例的变化曲线
Fig. 3. (a) Field-cooling and zero-field-cooling curves of DMCo0.9Zn0.1F single crystal samples under different magnetic fields; (b) magnetic hysteresis loops of samples with different doping ratios at 1.8 K, and the doping ratios are x = 0 , 0.1, 0.2, 0.3, 0.4, 0.5. The inset in panel (b) shows the change curve of the coercive field size obtained from the hysteresis loop with the doping ratio.
图 4 (a) DMCo0.9Zn0.1F单晶样品在不同温度下的磁滞回线; (b) DMCo0.9Zn0.1F在1.8 K时的磁滞回线和其微分曲线(反铁磁背底被扣除), 从微分曲线上可以看到, 在±0.1 T和±0.32 T附近有磁化强度随磁场变化的共振峰
Fig. 4. (a) Magnetic hysteresis loops of DMCo0.9Zn0.1F single crystal samples at different temperatures; (b) magnetic hysteresis loops and differential curves of DMCo0.9Zn0.1F at 1.8 K (the antiferromagnetic background is deduction), it can be seen on the differential curve that there are resonance peaks with magnetization varying with magnetic field near ±0.1 T and ±0.32 T.
表 1 室温和150 K温度下, [(CH)3NH2]Co0.9Zn0.1(HOOC)3单晶四圆衍射结果
Table 1. Unit cell parameters obtained by single crystal X-ray diffraction analysis of [(CH)3NH2]Co0.9Zn0.1(HOOC)3 at room temperature and 150 K.
参数 取值 Temperature/K 275 150 Formula weight/(g·mol–1) 232.66 240.72 Crystal system Trigonal Monoclinic Space group $ R\bar 3c $ C1c1 a/Å 8.158(3) 14.143(2) b/Å 8.158(3) 8.1739(13) c/Å 22.168(9) 8.7634(14) α/(°) 90 90 β/(°) 90 122.365 γ/(°) 120 90 Z 6 4 Volume/Å3 1277.7(10) 855.7(2) F(000) 692 493 hmin, max –10, 10 –18, 18 kmin, max –10, 10 –10, 10 lmin, max –29, 29 –9, 11 Reflection collected 5016 3639 Independent reflections 359[R(int) = 0.0570] 1600[R(int) = 0.0490] Data/restraints/parameters 359/0/27 1600/10/126 R(reflections) 0.0200(337) 0.0538(1562) wR2(reflections) 0.0530(359) 0.1425(1600) Final R indices [I > 2σ(I)] R1 = 0.0200, wR2 = 0.0528 R1 = 0.0538, wR2 = 0.1421 Final R indices [all data] R1 = 0.0209, wR2 = 0.0530 R1 = 0.0543, wR2 = 0.1424 Goodness-of-fit on F 2 1.188 1.137 -
[1] Yaghi O M, Li G M, Li H L 1995 Nature 378 703
 Google Scholar
Google Scholar
[2] Schröder M 2010 Functional Metal-organic Frameworks: Gas Storage, Separation and Catalysis (Vol. 293) (Berlin: Springer) pp115–153
[3] Kuppler R J, Timmons D J, Fang Q R, Li J R, Makala T A, Young M D, Yuan D, Zhao D, Zhuang W, Zhou H C 2009 Coord. Chem. Rev. 253 3042
 Google Scholar
Google Scholar
[4] Rao C N R, Cheetham A K, Thirumurugan A 2008 J. Phys. Condens. Matter 20 083202
 Google Scholar
Google Scholar
[5] Jain P, Dalal N S, Toby B H, Kroto H W, Cheetham A K 2008 J. Am. Chem. Soc. 130 10450
 Google Scholar
Google Scholar
[6] Zhang W, Xiong R G 2012 Chem. Rev. 112 1163
 Google Scholar
Google Scholar
[7] Stroppa A, Barone P, Jain P, Perez-Mato J M, Picozzi S 2013 Adv. Mater. 25 2284
 Google Scholar
Google Scholar
[8] Hu K L, Kurmoo M, Wang Z, Gao S 2009 Chem. A Eur. J. 15 12050
 Google Scholar
Google Scholar
[9] Weng D F, Wang Z M, Gao S 2011 Chem. Soc. Rev. 40 3157
 Google Scholar
Google Scholar
[10] Fan F R, Wu H, Nabok D, Hu S B, Ren W, Draxl C, Stroppa A 2017 J. Am. Chem. Soc. 139 12883
 Google Scholar
Google Scholar
[11] Vinoda K, Deepakb C S, Sharma S, Sornaduraia D, Satyaa A T, Ravindrana T R, Sundara C S, Bharathi A 2015 RSC Adv. 5 37818
 Google Scholar
Google Scholar
[12] Jain P, Ramachandran V, Clark R J, Zhou H D, Toby B H, Dalal N S, Kroto H W, Cheetham A K 2009 J. Am. Chem. Soc. 131 13625
 Google Scholar
Google Scholar
[13] Xu G C, Z W, Ma X M, Chen Y H, Z L, Cai H L, Wang Z M, Xiong R G, Gao S 2011 J. Am. Chem. Soc. 133 14948
 Google Scholar
Google Scholar
[14] Kundys B, Lappas A, Viret M, Kapustianyk V, Rudyk V, Semak S, Simon C, Bakaimi I 2010 Phys. Rev. B 81 224434
 Google Scholar
Google Scholar
[15] Stroppa A, Jain P, Barone P, Marsman M, Perez-Mato J M, Cheetham A K, Kroto H W, Picozzi S 2011 Angew. Chem. Int. Ed. 50 5847
 Google Scholar
Google Scholar
[16] Sante D D, Stroppa A, Jain P, Picozzi S 2013 J. Am. Chem. Soc. 135 18126
 Google Scholar
Google Scholar
[17] Jain P, Stroppa A, Nabok D, Marino A, Rubano A, Paparo D, Matsubara M, Nakotte H, Fiebig M, Picozzi S, Choi E S, Cheetham A K, Drax C, Dalal N S, Zapf V S 2016 npj Quantum Mater. 1 16012
 Google Scholar
Google Scholar
[18] Gómez-Aguirre L C, Pato-Doldán B, Mira J, Castro-García S, Señarís-Rodríguez M A, Sánchez-Andújar M, Singleton J, Zapf V S 2016 J. Am. Chem. Soc. 138 1122
 Google Scholar
Google Scholar
[19] Tian Y, Shen S, Cong J, Yan L, Wang S, Sun Y 2016 J. Am. Chem. Soc. 138 782
 Google Scholar
Google Scholar
[20] Mączka M, Gągor A, Hermanowicz K, Sieradzki A, Macalik L, Pikul A 2016 J. Solid State Chem. 237 150
 Google Scholar
Google Scholar
[21] Wang X Y, Gan L, Zhang S W, Gao S 2004 Inorg. Chem. 43 4615
 Google Scholar
Google Scholar
[22] Tian Y, Wang W, Chai Y, Cong J, Shen S, Yan L, Wang S, Han X, Sun Y 2014 Phys. Rev. Lett. 112 017202
 Google Scholar
Google Scholar
[23] Pato-Dolda B, Sanchez-Andujar M, Gomez-Aguirre L C, Yanez-Vilar S, Lopez-Beceiro J, Gracia-Fernandez C, Haghighirad A A, Ritter F, Castro-Garcia D S, Senaris-Rodriguez M A 2012 Phys. Chem. Chem. Phys. 14 8498
 Google Scholar
Google Scholar
[24] Friedman J R, Sarachik M P, Tejada J, Ziolo R 1996 Phys. Rev. Lett. 76 3830
 Google Scholar
Google Scholar
计量
- 文章访问数: 5751
- PDF下载量: 72
- 被引次数: 0


















 下载:
下载:



