-
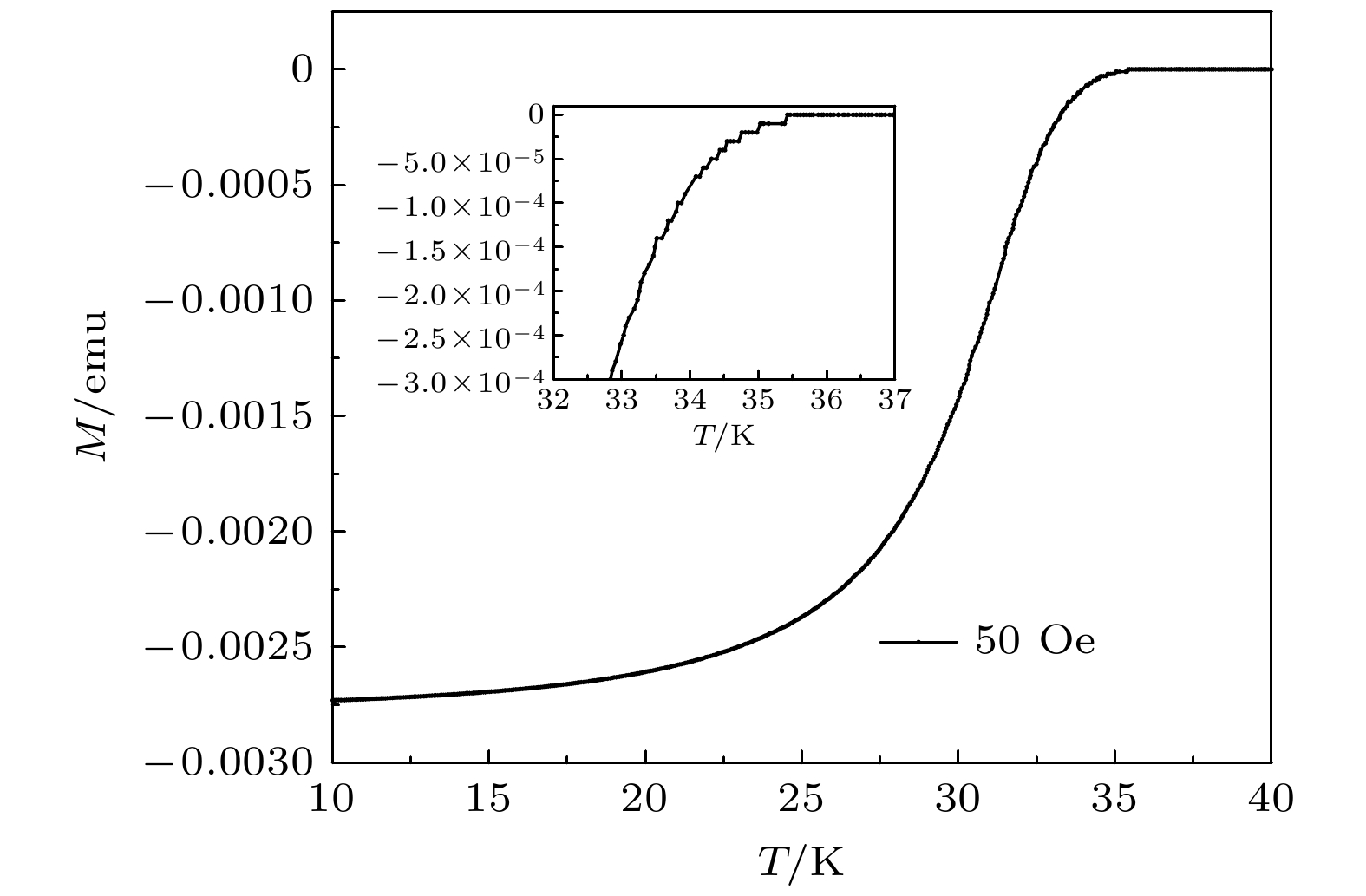
Mg(BH4)2作为优质的储氢材料, 在约300 ℃开始分解释放H2, 并最终生成MgB2. 由于Mg(BH4)2的释氢反应可以在较低的温度下获得MgB2, 使其成为了制备MgB2超导材料的一种有效途径. 本文采用了原位电阻法, 通过测量Mg(BH4)2分解过程中电阻温度曲线, 详细地研究了Mg(BH4)2分解生成MgB2的相变过程. 同时, 利用电阻温度的微分曲线, 确定了在分解过程中不同产物的成相温度(TPF). 其中, MgB2的成相温度可以低至410 ℃. 通过与粉末烧结法制备MgB2块材的成相温度对比, 估算出反应前Mg的颗粒尺寸最低可达3.4 nm. 此外, 样品的XRD分析给出了生成的MgB2晶粒在10—18 nm之间, 在SEM图像中也同样观察到了MgB2纳米纤维结构. 这表明, Mg(BH4)2分解生成的Mg与B 形成了接近原子级的混合, 从而使MgB2可以在更低的成相温度(410 ℃)、更短的反应时间内成相. 该方法为MgB2在超导应用的制备提供了新的思路, 有利于实现MgB2的工业化生产.Mg(BH4)2 was previously studied as a promising hydrogen storage material, because of its high gravimetric storage capacities for hydrogen and suitable thermodynamic properties. Mg(BH4)2 began to decompose at about 300 ℃, and formed MgB2 at the end of hydrogen desorption process with the weight content of 14.9% of hydrogen lost. Aside from the prominent hydrogen storage property, the decomposition process from Mg(BH4)2 to MgB2 can be a potential method for fabricating superconducting MgB2 at a low sintering temperature. In this paper, MgB2 bulk was prepared by an in-situ reaction, using the Mg(BH4)2 pressed block as a precursor. The resistance change of the sample was monitored during the Mg(BH4)2 decomposition process and the resistance-temperature (R-T) curve of this process was recorded. Phase of MgH2, Mg and B were formed as the block slowly release its hydrogen before MgB2 occurred. According to the R-T curve, the phase formation of MgB2 started in a relatively low temperature of 410 ℃. Because MgB2 was critically formed by Mg and B derived from Mg(BH4)2, we can compare our formation temperature with previous study on MgB2 prepared by Mg and B in different particle size. The fitting result indicated that the particle size of Mg and B harvest from Mg(BH4)2 decomposition was only 3.4 nm on average. The nearly atomic level mixture of Mg and B resulted in a high chemical reactivity, which was the main reason for low sintering temperature. X-ray diffraction results showed that the purity of MgB2 was 95.2%, and the size of MgB2 grains was 10–18 nm. SEM images showed that the MgB2 bulk had a porous structure and poor connectivity, which was caused by large amount the hydrogen release during the decomposition. MgB2 nanofibers can also be observed inside the bulk. In the superconductivity test, the superconducting transition temperature of the bulk was 35 K. After all, such in situ method to fabricate MgB2 showed a great advantage in some aspects, as its low-cost precursors, low sintering temperature, small grain-size and high superconducting transition temperature in the formed MgB2, which have the potential in industrial scale fabrication of MgB2 bulks and wires.
-
Keywords:
- MgB2 /
- Mg(BH4)2 /
- in situ resistance measurement
[1] Nakamori Y, Miwa K, Ninomiya A, Li H, Ohba N, Towata S I, Zuettel A, Orimo S I 2006 Phys. Rev. B 74 045126
 Google Scholar
Google Scholar
[2] Voss J, Hummelshøj J S, Łodziana Z, Vegge T 2008 J. Phys.: Condens. Matter 21 012203
 Google Scholar
Google Scholar
[3] Li H W, Kikuchi K, Nakamori Y, Ohba N, Miwa K, Towata S, Orimo S 2008 Acta Mater. 56 1342
 Google Scholar
Google Scholar
[4] Chlopek K, Frommen C, Leon A, Zabara O, Fichtner M 2007 J. Mater. Chem. 17 3496
 Google Scholar
Google Scholar
[5] Fujii H, Ozawa K 2011 Supercond. Sci. Technol. 24 095009
 Google Scholar
Google Scholar
[6] Luo W H, Huang Z G, Cai X W, Niu R R, Nie R J, Feng Q R, Wang F R, Gan Z Z 2019 Supercond. Sci. Technol. 32 085006
 Google Scholar
Google Scholar
[7] Yang J Z, Zhang X Z, Zheng J, Song P, Li X G 2010 Scripta Mater. 64 225
 Google Scholar
Google Scholar
[8] Yang J, Zheng J, Zhang X Z, Li Y Q, Yang R, Feng Q R, Li X G 2010 Chem. Commun. 46 7530
 Google Scholar
Google Scholar
[9] Chen L P, Zhang C, Wang Y B, Wang Y, Feng Q R, Gan Z Z, Yang J Z, Li X G 2010 Supercond. Sci. Technol. 24 015002
 Google Scholar
Google Scholar
[10] 张辰, 陈丽萍, 王银博, 吴桃李, 刘文静, 刘雨潇, 薛驰, 冯庆荣 2011 低温 33 97
 Google Scholar
Google Scholar
Zhang C, Chen L P, Wang Y B, Wu T L, Liu W J, Liu Y X, Xue C, Feng Q R 2011 Chin. J. Low Temp. Phys. 33 97
 Google Scholar
Google Scholar
[11] 郭峥山, 陈艺灵, 冯庆荣 2012 真空科学与技术学报 32 693
 Google Scholar
Google Scholar
Guo Z S, Chen Y L, Feng Q R 2012 J. Vac. Sci. Technol. 32 693
 Google Scholar
Google Scholar
[12] Chen Y L, Liao X B, Cai X Q, Yang C, Guo Z S, Niu R R, Zhang Y, Jia C Y, Feng Q R 2017 Physica C 542 34
 Google Scholar
Google Scholar
[13] Guo C, Wang H Z, Cai X W, Luo W H, Huang Z G, Zhang Y, Feng Q R, Gan Z Z 2021 Physica C 584 1353863
 Google Scholar
Google Scholar
[14] Hanada N, Chopek K, Frommen C, Lohstroh W, Fichtner M 2008 J. Mater. Chem. 18 2611
 Google Scholar
Google Scholar
[15] Zhuang C G, Liu X X, Guo T, Wang B, Li X G, Chen C P, Feng Q R 2007 Supercond. Sci. Technol. 20 1125
 Google Scholar
Google Scholar
[16] Chen C P, Zhou Z J, Li X G, Xu J, Wang Y H, Gao Z X, Feng Q R 2004 Solid State Commun. 131 275
 Google Scholar
Google Scholar
[17] DeFouw J D, Quintana J P, Dunand D C 2008 Acta Mater. 56 1680
 Google Scholar
Google Scholar
[18] 冯庆荣, 陈晋平, 徐军, 王宇昊, 陈鑫 2004 低温 26 46
 Google Scholar
Google Scholar
Feng Q R, Chen C P, Xu J, Wang Y H, Chen X 2004 Chin. J. Low Temp. Phys. 26 46
 Google Scholar
Google Scholar
[19] Yamamoto A, Shimoyama J-i, Ueda S, Katsura Y, Horii S, Kishio K 2004 Supercond. Sci. Technol. 18 116
 Google Scholar
Google Scholar
[20] Yamamoto A, Shimoyama J, Ueda S, Katsura Y, Iwayama I, Horii S, Kishio K 2006 Physica C 445-448 806
 Google Scholar
Google Scholar
-
表 1 不同颗粒度Mg粉对应的MgB2成相温度
Table 1. Phase forming temperature of MgB2 fabricated by different sized Mg powders.
样品编号 1 2 3 4 TPF/K 908 876 842 727 a/μm 100 45 15 0.04 -
[1] Nakamori Y, Miwa K, Ninomiya A, Li H, Ohba N, Towata S I, Zuettel A, Orimo S I 2006 Phys. Rev. B 74 045126
 Google Scholar
Google Scholar
[2] Voss J, Hummelshøj J S, Łodziana Z, Vegge T 2008 J. Phys.: Condens. Matter 21 012203
 Google Scholar
Google Scholar
[3] Li H W, Kikuchi K, Nakamori Y, Ohba N, Miwa K, Towata S, Orimo S 2008 Acta Mater. 56 1342
 Google Scholar
Google Scholar
[4] Chlopek K, Frommen C, Leon A, Zabara O, Fichtner M 2007 J. Mater. Chem. 17 3496
 Google Scholar
Google Scholar
[5] Fujii H, Ozawa K 2011 Supercond. Sci. Technol. 24 095009
 Google Scholar
Google Scholar
[6] Luo W H, Huang Z G, Cai X W, Niu R R, Nie R J, Feng Q R, Wang F R, Gan Z Z 2019 Supercond. Sci. Technol. 32 085006
 Google Scholar
Google Scholar
[7] Yang J Z, Zhang X Z, Zheng J, Song P, Li X G 2010 Scripta Mater. 64 225
 Google Scholar
Google Scholar
[8] Yang J, Zheng J, Zhang X Z, Li Y Q, Yang R, Feng Q R, Li X G 2010 Chem. Commun. 46 7530
 Google Scholar
Google Scholar
[9] Chen L P, Zhang C, Wang Y B, Wang Y, Feng Q R, Gan Z Z, Yang J Z, Li X G 2010 Supercond. Sci. Technol. 24 015002
 Google Scholar
Google Scholar
[10] 张辰, 陈丽萍, 王银博, 吴桃李, 刘文静, 刘雨潇, 薛驰, 冯庆荣 2011 低温 33 97
 Google Scholar
Google Scholar
Zhang C, Chen L P, Wang Y B, Wu T L, Liu W J, Liu Y X, Xue C, Feng Q R 2011 Chin. J. Low Temp. Phys. 33 97
 Google Scholar
Google Scholar
[11] 郭峥山, 陈艺灵, 冯庆荣 2012 真空科学与技术学报 32 693
 Google Scholar
Google Scholar
Guo Z S, Chen Y L, Feng Q R 2012 J. Vac. Sci. Technol. 32 693
 Google Scholar
Google Scholar
[12] Chen Y L, Liao X B, Cai X Q, Yang C, Guo Z S, Niu R R, Zhang Y, Jia C Y, Feng Q R 2017 Physica C 542 34
 Google Scholar
Google Scholar
[13] Guo C, Wang H Z, Cai X W, Luo W H, Huang Z G, Zhang Y, Feng Q R, Gan Z Z 2021 Physica C 584 1353863
 Google Scholar
Google Scholar
[14] Hanada N, Chopek K, Frommen C, Lohstroh W, Fichtner M 2008 J. Mater. Chem. 18 2611
 Google Scholar
Google Scholar
[15] Zhuang C G, Liu X X, Guo T, Wang B, Li X G, Chen C P, Feng Q R 2007 Supercond. Sci. Technol. 20 1125
 Google Scholar
Google Scholar
[16] Chen C P, Zhou Z J, Li X G, Xu J, Wang Y H, Gao Z X, Feng Q R 2004 Solid State Commun. 131 275
 Google Scholar
Google Scholar
[17] DeFouw J D, Quintana J P, Dunand D C 2008 Acta Mater. 56 1680
 Google Scholar
Google Scholar
[18] 冯庆荣, 陈晋平, 徐军, 王宇昊, 陈鑫 2004 低温 26 46
 Google Scholar
Google Scholar
Feng Q R, Chen C P, Xu J, Wang Y H, Chen X 2004 Chin. J. Low Temp. Phys. 26 46
 Google Scholar
Google Scholar
[19] Yamamoto A, Shimoyama J-i, Ueda S, Katsura Y, Horii S, Kishio K 2004 Supercond. Sci. Technol. 18 116
 Google Scholar
Google Scholar
[20] Yamamoto A, Shimoyama J, Ueda S, Katsura Y, Iwayama I, Horii S, Kishio K 2006 Physica C 445-448 806
 Google Scholar
Google Scholar
计量
- 文章访问数: 6309
- PDF下载量: 83
- 被引次数: 0














 下载:
下载: