-
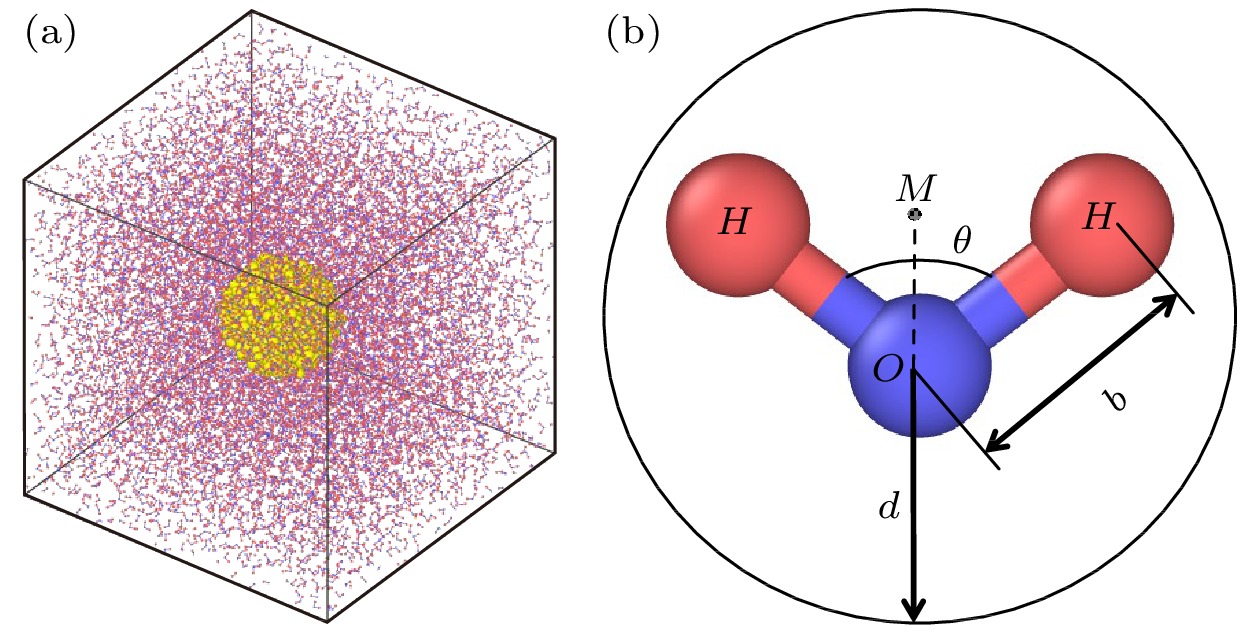
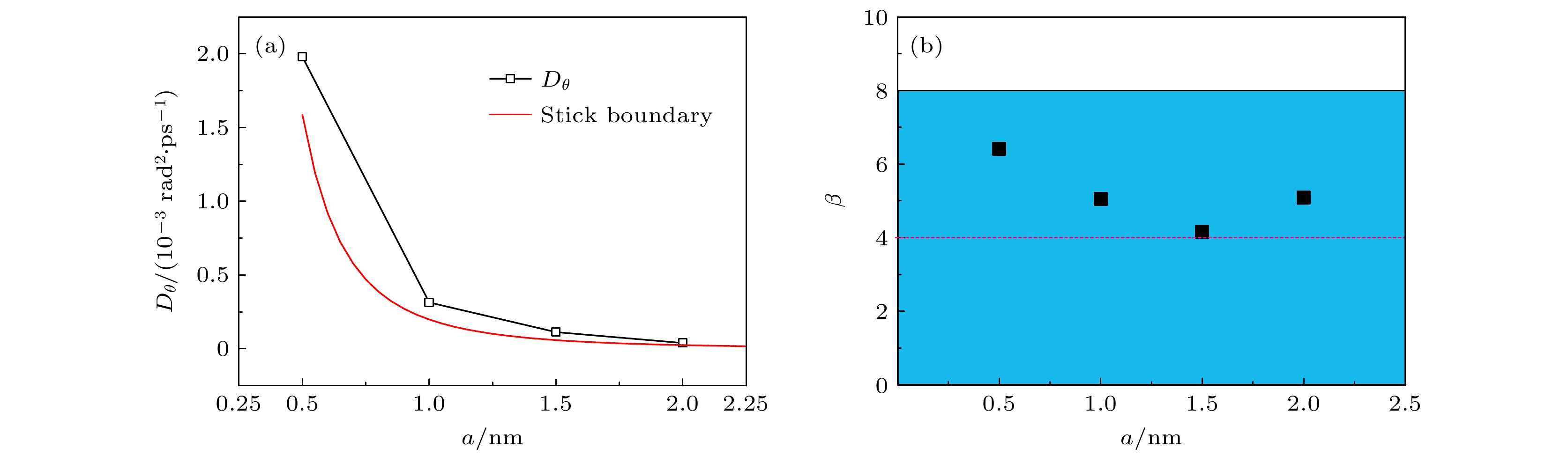
介观尺度下颗粒布朗运动的摩擦系数符合黏性流体力学边界条件, 当颗粒尺度减小至纳米级别时, 边界条件向滑移过渡; 另一方面, 随着颗粒尺度的减小, 颗粒表面溶剂分子的吸附效应对颗粒水动力学半径的影响不可忽略. 分子动力学模拟可以捕获纳米流体中颗粒与溶剂分子相互作用的微观细节且计算精度高. 以刚性TIP4P/2005水分子模型为溶剂, 建立不同大小的Cu纳米颗粒在水中扩散的全原子模型. 采用单颗粒追踪方法对颗粒的平动和转动扩散系数进行拟合, 将摩擦因子与黏性边界条件和滑移边界条件下的结果进行比较, 并研究了溶剂在颗粒表面的吸附特性. 研究发现纳米颗粒的平转动摩擦因子均在两种边界条件所预测的理论值之间, 颗粒尺寸越小, 溶剂分子的吸附越明显, 颗粒表面的水分子层会增大颗粒的水动力学半径使得摩擦因子的计算结果偏向黏性边界, 纳米颗粒尺寸约小于5倍溶剂分子尺寸时, 需要考虑颗粒表面的溶剂层对颗粒水动力半径的影响.Brownian motion refers to the endless random motion of nanometer-to-micron particles suspended in a fluid. It widely exists in nature, and is applied to energy, biology, chemical industry, environment and other industries. As the Brownian motion of the object decreases from the micron level to the nanometer level, the boundary conditions of the particle motion no longer strictly follow the stick hydrodynamic boundary conditions, but are closer to the slip boundary theory, meanwhile, the interaction between particles and solvents has increasingly important influence on particle dynamics. Molecular dynamics simulation is an important means to study nanofluids, which can not only capture the microscopic details of the interactions between particles and solvent molecules in nanofluids, but also have high potential function accuracy. In this paper, an all-atom model of the diffusion of Cu nanoparticles of different sizes in water is established by using the rigid TIP4P/2005 water molecule model as solvent, the dynamic viscosity from the TIP4P/2005 model is in good agreement with the experimental result, which is verified by the Green-Kubo formula. The FCC lattice structure is used to construct Cu particles of 0.5 nm, 1.0 nm, 1.5 nm, 2.0 nm in size, and the interaction between atoms in the particle is described by the EAM potential. The translational diffusion coefficient of particles is fitted by the single particle tracking algorithm and the least square method, the rotational diffusion coefficient of particles is obtained by quaternion transformation. The diffusion coefficient and friction factor of the particles are calculated, and the friction factor is compared with the result under the stick hydrodynamics boundary conditions and the result under the slip boundary conditions. It is found that the frictional factors of translation and rotation of nano-particles lie between the theoretical values predicted by the two boundary conditions. The radial distribution functions of water molecules around nanoparticles of different sizes are calculated, we find that the smaller the particle size, the more obvious the adsorption of solvent molecules will be, and the water molecular layer on the particle surface will increase the effective volume of particles and make the calculation result of friction factor larger. The effect of solvent adsorption on the effective hydrodynamic radius of particles cannot be ignored when calculating the friction coefficient of Brownian motion of nano-particles, especially when the particle radius is close to the solvent radius. In Brownian dynamics, viscous resistance and stochastic force are constrained by fluctuation dissipation theorem, and a reasonable selection of particle friction factor can provide theoretical basis for the improvement of Brownian dynamics.
-
Keywords:
- nanoparticles /
- diffusion coefficient /
- friction factor /
- hydrodynamic radius
[1] Brown R 1828 Philos. Mag. 4 161
 Google Scholar
Google Scholar
[2] Einstein A 1905 Ann. Phys. 17 549
[3] Bian X, Kim C, Karniadakis G E 2016 Soft Matter 12 01
 Google Scholar
Google Scholar
[4] Tao Q, Luigi G R 2016 Mathematical Analysis Probability and Applications-Plenary Lectures (New York: Springer International Publishing) p2
[5] 王亮, 卢宇源, 安立佳 2017 应用化学 34 1250
 Google Scholar
Google Scholar
Wang L, Lu Y Y, An L J 2017 Chin. J. Appl. Chem. 34 1250
 Google Scholar
Google Scholar
[6] Hu C M, Zwanzig R 1974 J. Chem. Phys. 60 4354
 Google Scholar
Google Scholar
[7] Edward J T 1970 J. Chem. Educ. 47 261
 Google Scholar
Google Scholar
[8] Richardson S 2006 J. Fluid Mech. 59 707
[9] Ollila S T T, Smith C J, Ala-Nissila T, Denniston C 2013 Multiscale Model. Simul. 11 213
 Google Scholar
Google Scholar
[10] Vargas-Lara F, Starr F W, Douglas J F 2016 AIP Publishing LLC 1736 020080
 Google Scholar
Google Scholar
[11] Nisha M R, Philip J 2013 Phys. Scr. 88 15602
 Google Scholar
Google Scholar
[12] Velasco-Velez J J, Wu C H, Pascal T A, Wan L F, Guo J, Prendergast D, Salmeron M 2014 Science 346 831
 Google Scholar
Google Scholar
[13] Vasanthi R, Ravichandran S, Bagchi B 2001 J. Chem. Phys. 114 7989
 Google Scholar
Google Scholar
[14] Vasanthi R, Bhattacharyya S, Bagchi B 2002 J. Chem. Phys. 116 1092
 Google Scholar
Google Scholar
[15] 何昱辰, 刘向军 2014 力学学报 46 871
 Google Scholar
Google Scholar
He Y C, Liu X J 2014 Acta Mech. Sin. 46 871
 Google Scholar
Google Scholar
[16] Markutsya S, Subramaniam S, Vigil R D, Fox R O 2008 Ind. Eng. Chem. Res. 47 3338
 Google Scholar
Google Scholar
[17] Motohashi R, Hanasaki I, Ooi Y, Matsuda Y 2017 Micro Nano Lett. 12 506
 Google Scholar
Google Scholar
[18] Boyer D, Dean D S, Mejia-Monasterio C, Oshanin G 2012 Phys. Rev. E 86 60101
 Google Scholar
Google Scholar
[19] Xavier M 2010 Phys. Rev. E 82 041914
 Google Scholar
Google Scholar
[20] Abascal J L, Vega C 2005 J. Chem. Phys. 123 234505
 Google Scholar
Google Scholar
[21] Arnold A, Fahrenberger F, Holm C, Lenz O, Bolten M, Sutmann G 2013 Phys. Rev. E 88 63308
 Google Scholar
Google Scholar
[22] Folies S M, Baskets M I, Daw M S 1986 Phys. Rev. B 33 7983
 Google Scholar
Google Scholar
[23] Gonzalez M A, Abascal J L F 2010 J. Chem. Phys. 132 96101
 Google Scholar
Google Scholar
[24] Harris K R, Woolf L A 2004 J. Chem. Eng. Data 49 1064
 Google Scholar
Google Scholar
[25] Li T, Raizen M G 2013 Ann. Phys. 525 281
 Google Scholar
Google Scholar
[26] Ernst D, Kohler 2013 Phys. Chem. Chem. Phys. 15 845
 Google Scholar
Google Scholar
[27] Pranami G, Lamm M H 2015 J. Chem. Theory Comput. 11 4586
 Google Scholar
Google Scholar
[28] 张世良, 戚力, 高伟, 冯士东, 刘日平 2015 燕山大学学报 39 213
 Google Scholar
Google Scholar
Zhang S L, Qi L, Gao W, Feng S D, Liu R P 2015 J. Yanshan Univ. 39 213
 Google Scholar
Google Scholar
-
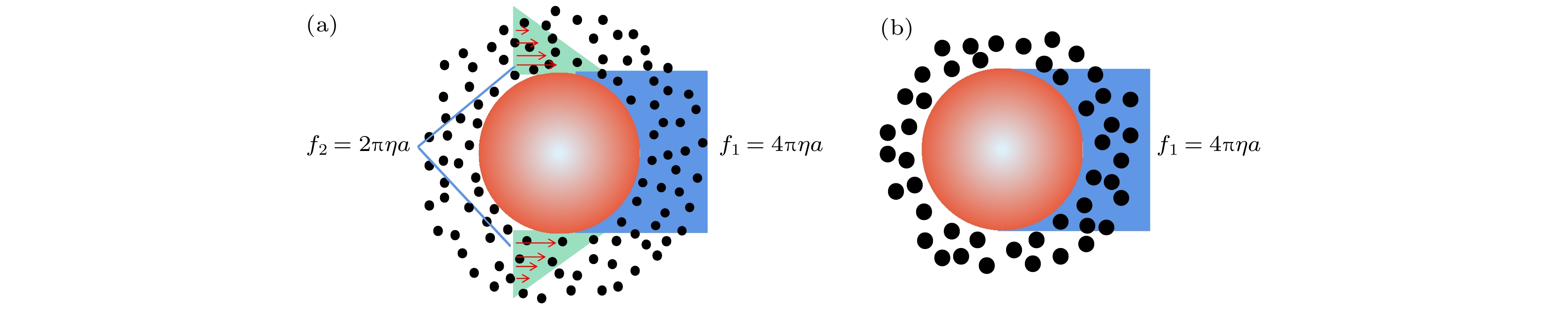
图 1 球形颗粒在不同边界条件下的平动摩擦系数, 颗粒向右运动 (a)黏性边界条件; (b)光滑边界条件
Fig. 1. Translational friction coefficients of spherical particles under different boundary conditions and the particle move to the right: (a) Translational friction coefficient for stick boundary; (b) translational friction coefficient for slip boundary.
表 1 各工况下模拟域大小与原子数目
Table 1. The size of the simulation domain and number of atoms of different working conditions.
颗粒半
径/nmCu原
子数H2O分
子数总原
子数模拟域大小
Lx × Ly × Lz /Å30.5 43 971 2956 30 × 30 × 30 1.0 351 7380 23841 60 × 60 × 60 1.5 1199 7459 23576 60 × 60 × 60 2.0 2928 14381 45972 75 × 75 × 75 表 2 Cu-H2O纳米流体势能参数
Table 2. Potential energy parameter of Cu-H2O nanofluid.
参数 取值 εo—o/eV 0.00803 σo—o/Å 3.1589 εCu—o /eV 0.052 σCu—o /Å 2.743 -
[1] Brown R 1828 Philos. Mag. 4 161
 Google Scholar
Google Scholar
[2] Einstein A 1905 Ann. Phys. 17 549
[3] Bian X, Kim C, Karniadakis G E 2016 Soft Matter 12 01
 Google Scholar
Google Scholar
[4] Tao Q, Luigi G R 2016 Mathematical Analysis Probability and Applications-Plenary Lectures (New York: Springer International Publishing) p2
[5] 王亮, 卢宇源, 安立佳 2017 应用化学 34 1250
 Google Scholar
Google Scholar
Wang L, Lu Y Y, An L J 2017 Chin. J. Appl. Chem. 34 1250
 Google Scholar
Google Scholar
[6] Hu C M, Zwanzig R 1974 J. Chem. Phys. 60 4354
 Google Scholar
Google Scholar
[7] Edward J T 1970 J. Chem. Educ. 47 261
 Google Scholar
Google Scholar
[8] Richardson S 2006 J. Fluid Mech. 59 707
[9] Ollila S T T, Smith C J, Ala-Nissila T, Denniston C 2013 Multiscale Model. Simul. 11 213
 Google Scholar
Google Scholar
[10] Vargas-Lara F, Starr F W, Douglas J F 2016 AIP Publishing LLC 1736 020080
 Google Scholar
Google Scholar
[11] Nisha M R, Philip J 2013 Phys. Scr. 88 15602
 Google Scholar
Google Scholar
[12] Velasco-Velez J J, Wu C H, Pascal T A, Wan L F, Guo J, Prendergast D, Salmeron M 2014 Science 346 831
 Google Scholar
Google Scholar
[13] Vasanthi R, Ravichandran S, Bagchi B 2001 J. Chem. Phys. 114 7989
 Google Scholar
Google Scholar
[14] Vasanthi R, Bhattacharyya S, Bagchi B 2002 J. Chem. Phys. 116 1092
 Google Scholar
Google Scholar
[15] 何昱辰, 刘向军 2014 力学学报 46 871
 Google Scholar
Google Scholar
He Y C, Liu X J 2014 Acta Mech. Sin. 46 871
 Google Scholar
Google Scholar
[16] Markutsya S, Subramaniam S, Vigil R D, Fox R O 2008 Ind. Eng. Chem. Res. 47 3338
 Google Scholar
Google Scholar
[17] Motohashi R, Hanasaki I, Ooi Y, Matsuda Y 2017 Micro Nano Lett. 12 506
 Google Scholar
Google Scholar
[18] Boyer D, Dean D S, Mejia-Monasterio C, Oshanin G 2012 Phys. Rev. E 86 60101
 Google Scholar
Google Scholar
[19] Xavier M 2010 Phys. Rev. E 82 041914
 Google Scholar
Google Scholar
[20] Abascal J L, Vega C 2005 J. Chem. Phys. 123 234505
 Google Scholar
Google Scholar
[21] Arnold A, Fahrenberger F, Holm C, Lenz O, Bolten M, Sutmann G 2013 Phys. Rev. E 88 63308
 Google Scholar
Google Scholar
[22] Folies S M, Baskets M I, Daw M S 1986 Phys. Rev. B 33 7983
 Google Scholar
Google Scholar
[23] Gonzalez M A, Abascal J L F 2010 J. Chem. Phys. 132 96101
 Google Scholar
Google Scholar
[24] Harris K R, Woolf L A 2004 J. Chem. Eng. Data 49 1064
 Google Scholar
Google Scholar
[25] Li T, Raizen M G 2013 Ann. Phys. 525 281
 Google Scholar
Google Scholar
[26] Ernst D, Kohler 2013 Phys. Chem. Chem. Phys. 15 845
 Google Scholar
Google Scholar
[27] Pranami G, Lamm M H 2015 J. Chem. Theory Comput. 11 4586
 Google Scholar
Google Scholar
[28] 张世良, 戚力, 高伟, 冯士东, 刘日平 2015 燕山大学学报 39 213
 Google Scholar
Google Scholar
Zhang S L, Qi L, Gao W, Feng S D, Liu R P 2015 J. Yanshan Univ. 39 213
 Google Scholar
Google Scholar
计量
- 文章访问数: 13778
- PDF下载量: 261
- 被引次数: 0













 下载:
下载: